-
PDF
- Split View
-
Views
-
Cite
Cite
Vincenzo Nuzzi, Aldostefano Porcari, Marta Gigli, Francesco Zaja, Franca Dore, Rossana Bussani, Gianfranco Sinagra, Marco Merlo, A case report of isolated cardiac light chain amyloidosis without clinically overt heart failure: an under-recognized presentation, European Heart Journal - Case Reports, Volume 7, Issue 3, March 2023, ytad072, https://doi.org/10.1093/ehjcr/ytad072
Close - Share Icon Share
Abstract
Cardiac involvement in amyloid light-chain (AL) amyloidosis usually represents a brick in the wall of a multi-system disease. The presence of cardiac deposition of free light chains (FLCs) is the main determinant of survival. Isolated cardiac AL is an uncommon scenario characterized by a challenging diagnostic and therapeutic workup.
A 57-year-old asymptomatic man was presented for an incidental finding of myocardial necrosis at the electrocardiogram (ECG) performed for newly diagnosed arterial hypertension. Alongside signs of previous myocardial infarction, transthoracic echocardiography showed a severely increased left ventricular (LV) wall thickness not consistent with ECG voltages, segmental akinaesia with normal LV systolic function with ‘apical sparing’ pattern. Laboratory assessment showed an unexpectedly high level of natriuretic peptide and persistently abnormal troponin in the absence of symptoms or signs of heart failure or ongoing ischaemia. Coronary angiogram confirmed the coronary artery disease. Before revascularization, a complete diagnostic workup was carried. Serum electrophoresis detected a monoclonal gammopathy that was further investigated by serum immunofixation, revealing high lambda FLCs concentration. Fat pad, bone marrow, and salivary glands biopsies resulted negative for amyloid deposition. Finally, endomyocardial biopsy was consistent with AL amyloidosis. Urgent percutaneous revascularization was performed, and the patients was timely started on chemotherapy.
The diagnosis of isolated cardiac AL amyloidosis is challenging and carries important therapeutic implications. As the short-term prognosis might be severely compromised, an accurate diagnostic flowchart has to be systematically pursued to obtain a precise diagnosis and address the optimal, tailored management.
Isolated cardiac amyloid light-chain (AL) amyloidosis is a challenging diagnosis, especially in asymptomatic patients. In the presence of suggestive clinical scenarios, systematic assessment of potential cardiac involvement has to be regularly carried out.
Multidisciplinary approach plays a key role in the definition of the most accurate patient-tailored management.
Achieving an aetiological diagnosis of cardiac AL amyloidosis has a crucial impact on treatment strategies and patients’ outcome.
Primary specialties involved other than cardiology
Haematology, nuclear medicine, pathological anatomy, and histology
Introduction
Cardiac amyloidosis received great attention in the last two decades.1 Recognition of cardiac involvement as the leading cause of mortality in the spectrum of the multi-system infiltration allowed, firstly, to achieve the deepest knowledge of a previously largely unrecognized disease and, secondly, a better definition of the proper diagnostic and therapeutic workup.2 Amyloid light-chain (AL) amyloidosis is a systemic disease caused by misfolded monoclonal immunoglobulin light chains produced by a clonal proliferation of plasma cells in the bone marrow. Whilst cardiac AL amyloidosis almost always occurs in the context of other organs involvement, isolated cardiac involvement represents less than 5% of the cases.3 This subgroup of patients might be challenging to diagnose and may benefit from a specialty cardiologic assessment and follow-up.4 Cardiac AL amyloidosis carries usually cardiologic symptoms, such as heart failure, hypotension, and atrial and ventricular arrhythmias, and is burdened by a poor overall outcome in the short-term (median survival of 6–9 months, if untreated). We report a case of an asymptomatic patient with incident diagnosis of AL amyloidosis and an isolated cardiac involvement that significantly influenced the multidisciplinary management and the treatment strategies.
Timeline
| 2 January 2022 | Hypertension diagnosis |
| 4 January 2022 | Electrocardiogram (ECG): anterior and inferior necrosis |
| 17 January 2022 | First cardiologic assessment and subsequent suspicion of amyloidosis |
| 4 February 2022 | Full cardiologic assessment including coronary angiogram and full laboratory tests suggestive of AL amyloidosis |
| 10 February 2022 | Negative fat pad and bone marrow biopsy |
| 22 February 2022 | Negative salivary glands biopsy |
| 28 February 2022 | Endomyocardial biopsy positive for cardiac AL amyloid |
| 6 March 2022 | Coronary revascularization and subsequent initiation of chemotherapy |
| 2 January 2022 | Hypertension diagnosis |
| 4 January 2022 | Electrocardiogram (ECG): anterior and inferior necrosis |
| 17 January 2022 | First cardiologic assessment and subsequent suspicion of amyloidosis |
| 4 February 2022 | Full cardiologic assessment including coronary angiogram and full laboratory tests suggestive of AL amyloidosis |
| 10 February 2022 | Negative fat pad and bone marrow biopsy |
| 22 February 2022 | Negative salivary glands biopsy |
| 28 February 2022 | Endomyocardial biopsy positive for cardiac AL amyloid |
| 6 March 2022 | Coronary revascularization and subsequent initiation of chemotherapy |
| 2 January 2022 | Hypertension diagnosis |
| 4 January 2022 | Electrocardiogram (ECG): anterior and inferior necrosis |
| 17 January 2022 | First cardiologic assessment and subsequent suspicion of amyloidosis |
| 4 February 2022 | Full cardiologic assessment including coronary angiogram and full laboratory tests suggestive of AL amyloidosis |
| 10 February 2022 | Negative fat pad and bone marrow biopsy |
| 22 February 2022 | Negative salivary glands biopsy |
| 28 February 2022 | Endomyocardial biopsy positive for cardiac AL amyloid |
| 6 March 2022 | Coronary revascularization and subsequent initiation of chemotherapy |
| 2 January 2022 | Hypertension diagnosis |
| 4 January 2022 | Electrocardiogram (ECG): anterior and inferior necrosis |
| 17 January 2022 | First cardiologic assessment and subsequent suspicion of amyloidosis |
| 4 February 2022 | Full cardiologic assessment including coronary angiogram and full laboratory tests suggestive of AL amyloidosis |
| 10 February 2022 | Negative fat pad and bone marrow biopsy |
| 22 February 2022 | Negative salivary glands biopsy |
| 28 February 2022 | Endomyocardial biopsy positive for cardiac AL amyloid |
| 6 March 2022 | Coronary revascularization and subsequent initiation of chemotherapy |
Case description
A 57-year-old man was presented to the outpatient general cardiology clinic for a first assessment of his newly diagnosed mild arterial hypertension. He did not suffer from any chronic disease and had no past medical history. Alongside arterial hypertension, he had a family history of ischaemic heart disease and is a current light smoker. Physical examination was unremarkable. The patient was completely asymptomatic; ambulatory blood pressure was 150/95 mmHg on losartan 75 mg/day. The ECG showed regular sinus rhythm, heart rate was 67 b.p.m., and there were signs of anterior and inferior myocardial necrosis with otherwise normal QRS voltages (Figure 1). Transthoracic echocardiography was requested for suspected ischaemic heart disease and reported akinaesia of a small area of the left ventricular (LV) apex. Left ventricular ejection fraction was normal (60%), but a reduced global longitudinal strain (−11.4%, TomTec 2D Cardiac Performance Analysis software) with sparing of the apex (excluding the ischaemic segment) was noted. The LV was severely hypertrophic with a maximal tele-diastolic basal septal thickness of 20 mm, not consistent with the ECG voltages, and grade II diastolic dysfunction was noted (Figure 2, see Supplementary material online, Video S1). The patient was started on low-dose aspirin, bisoprolol 2.5 mg/day, and statin therapy and was referred to our hospital cardiovascular department for further assessment of suspected cardiac amyloidosis and ischaemic heart disease. Laboratory tests showed increased serum N-terminal B-type natriuretic peptide values (1041 pg/mL), without clinical signs and symptoms of fluid overload, together with a persistently increased high-sensitive troponin I concentration (average value 210 ng/L, normal value <18 ng/L) in the absence of clinical evidence of ongoing ischaemia. Moreover, serum electrophoresis revealed a monoclonal gammopathy. Serum immunofixation showed high lambda concentration (0.4 g/dL), with a gamma/lambda ratio of 0.04 (reference value 0.26–1.65) and a positive Bence–Jones protein urine test for lambda free light chains (FLCs). Invasive angiography showed a severe triple-vessel coronary disease with chronic occlusion of the left anterior descending (LAD) artery (revascularized by collateral vessels) and its second diagonal branch, subocclusion of the circumflex marginal branch and critical stenosis of the right coronary artery-posterior interventricular artery (see Supplementary material online, Video S2). Also, stress echocardiography showed inducible myocardial ischaemia (see Supplementary material online, Video S3). However, the decision regarding the revascularization strategy was postponed after the identification of the specific cardiac disease. Scintigraphy with bone tracer showed a mild myocardial uptake (Perugini scale 1), excluding transthyretin (TTR) amyloidosis. Cardiac magnetic resonance imaging demonstrated the presence of a relative thinning of the apex and of the mid-segments of anterior and inferior interventricular septum, consistent with ischaemic necrosis, in the absence of myocardial oedema. A diffuse subendocardial hyperintensity, with a non-coronary distribution pattern, was observed after gadolinium administration, consistent with cardiac amyloidosis, alongside atrial delayed enhancement and limited ischaemic apical necrosis. An abnormal gadolinium kinetics with the myocardium and blood nulling at the same time was noted (Figure 3). Of note, both atria were dilated with increased wall signal; these findings were consistent with cardiac amyloid deposition. Fat pad and salivary glands biopsies were performed to identify AL amyloid deposition in extra-cardiac organs, but they returned negative. The bone marrow biopsy demonstrated a 5–8% plasmacytosis without traces of amyloid. The case was discussed with the haematologist. Given the high suspicion of cardiac involvement and the major implications for treatment, as the presence of cardiac AL amyloidosis would be itself an indication to targeted chemotherapy, the patient underwent endomyocardial biopsy (EMB). The procedure was carried on the right side of the interventricular septum without any complication. Histological examination of the myocardial sample with dedicated Congo red staining and evaluation under cross-polarized light detected AL amyloid fibril (Figure 4). The case was discussed in the Heart Team: the advanced heart involvement (Mayo cardiac stage III)5 and the extent of coronary artery disease-contraindicated autologous stem cell transplant as upfront therapy. Furthermore, considering the need for urgent chemotherapy, the evidence of inducible ischaemia on stress echocardiography in the LAD territory, and the increased risk of intra- and post-operative complications with coronary bypass surgery, percutaneous coronary revascularization was deemed the best option. After revascularization, the patient underwent the first cycle of chemotherapy that was administered before discharge. After safe discharge, regular haematological and cardiologic follow-up were scheduled.
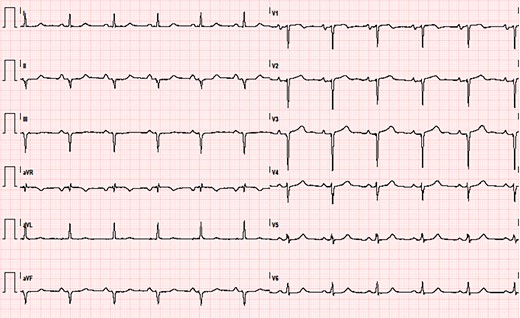
ECG showing sinus rhythm, left atrial enlargement, left anterior fascicular block, and anterior and inferior necrosis.
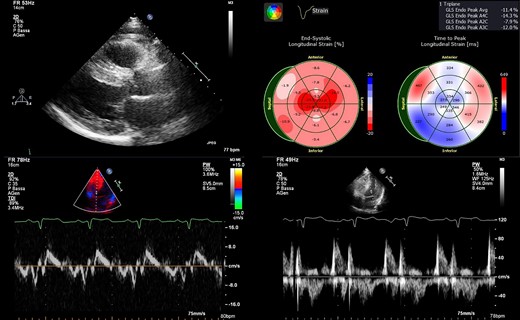
Main echocardiographic findings. (upper left) Parasternal long-axis view showing severely increased interventricular septum thickness (20 mm). (upper right) Strain analysis showing the ‘apical sparing pattern’ with the exception of the limited infarcted area. (bottom left) Tissue Doppler imaging showing a reduced E′ septal velocity. (bottom right) Diastolic trans-mitral flow showing pseudo-normal pattern.
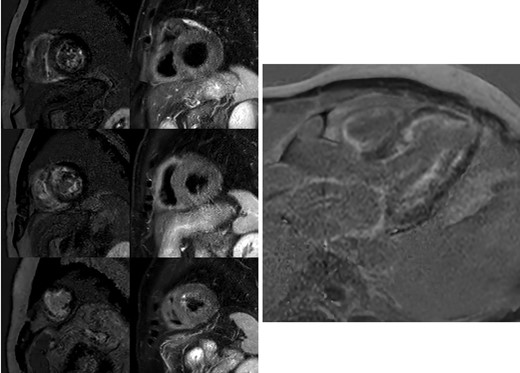
Cardiac magnetic resonance. The left side of the panel on the left shows the diffuse subendocardial late gadolinium enhancement pattern in the absence of a coronary pattern distribution in short axis images. The right side of the panel on the left shows the absence of oedema. From top to bottom: basal segments, mid segments and apical segments. The panel on the right reports the four-chamber delayed enhancement image showing the diffuse subendocardial enhancement, the atrial enhancement, and the limited apical necrosis.

Histological analysis of endomyocardial biopsy. The left side shows amyloid B immunohistochemistry (×20). The right side reports Congo red stain analysed under polarized light (×20). Histological analysis revealed the presence of abnormal protein deposition in the interstitium, producing slight green birefringence thin filaments in polarized light.
Discussion
We reported an uncommon case of an asymptomatic patient in his 50s with an incidental diagnosis of isolated cardiac AL amyloidosis. The precise and expedite diagnostic word-up had a crucial role in the definition of the optimal therapeutic pattern. Moreover, the multidisciplinary team, including different cardiology sub-specialities and a dedicated haematologist, delineated the most appropriate management of this specific patient, both from the cardiologic and haematological points of view.
The first remarkable issue of this case is the relevance of maintaining a high suspicion of AL cardiac amyloidosis in patients with suggestive findings and performing a comprehensive diagnostic workup.6 A number of inconsistencies were observed in our patient: (i) the recent-onset mild hypertension did not explain the severe LV hypertrophy; (ii) ECG voltages, despite not absolutely low, were discrepant with the degree of LV wall thickness measured at echocardiography; (iii) the ‘apical sparing’ pattern, except for the limited infarcted apical area, was another ‘red flag’; (iv) the increased serum natriuretic peptides and troponin in the absence of ongoing myocardial ischaemia or congestion.7 A cardiomyopathy-oriented interpretation of clinical and instrumental findings was crucial in the present case.8 Reaching an aetiological diagnosis of cardiac amyloidosis (i.e. TTR or AL amyloidosis) is of utmost importance to guide the management and the therapeutic options.9 Transthyretin amyloidosis was extremely unlikely in our patient because of young age, very mild uptake of bone tracer on planar scintigraphy, and the absence of neurological manifestations. Although some TTR variants or other forms may present with absent or low-grade myocardial uptake (i.e. Ser97Tyr, Phe64Leu, or ApoA-1),10,11 Amyloid light-chain amyloidosis is the most likely diagnosis in patients with Perugini grade 1 cardiac uptake at bone tracer scintigraphy and evidence of monoclonal proteins, as finally demonstrated by histological analysis of myocardial samples in our case. Amyloid light-chain amyloidosis was strongly suggested by routine laboratory tests that showed polyclonal hypergammaglobulinemia with, in particular, a high lambda FLCs serum concentration. Evidence of AL deposition in any tissue is an indication to urgent chemotherapy, especially in the presence of organ function impairment, as it was demonstrated to improve the outcome.12 The issue deserving particular attention is the absence of any extra-cardiac involvement, which did not allow a definite histological diagnosis with extra-cardiac biopsies (e.g. salivary glands, abdominal fat pad, and bone marrow). In this scenario, EMB might be required to obtain a definite diagnosis, but it is indicated only in selected cases due to the possibility of potential severe complications.13,14 Therefore, cardiac magnetic resonance showing characteristic findings of cardiac amyloidosis supported the indication to EMB. This approach is currently recommended by the Position Statement on ‘Diagnosis and treatment of cardiac amyloidosis’ from the European Society of Cardiology.2
Finally, early diagnosis of AL amyloidosis with characterization of the cardiac involvement and timely initiation of specific treatments is paramount to obtain the highest prognostic benefits.12 Of note, the goal of chemotherapy is to achieve suppression of the amyloidogenic FLCs exerting a direct toxic effect on cardiomyocytes. In recent years, proteasome inhibitors (i.e. bortezomib)15 and, more recently, human CD38-targeting antibody (i.e. daratumumab)16 for the treatment of haematological malignancies and AL amyloidosis improved patients’ overall survival compared with regimens previously used. However, these drugs, especially bortezomib, may be poorly tolerated due to cardiac complications such as development of systolic dysfunction or sudden death, especially during the first cycles.17,18 In this view, revascularization was deemed a mandatory safety procedure before the initiation of haematological therapies. This clinical decision was further supported by the presence of inducible myocardial ischaemia on stress echocardiography. The decision to schedule a percutaneous revascularization instead of an off-pump aortocoronary bypass graft surgery was deeply discussed, and the final decision was related to the increased surgical risk conferred by the underlying cardiomyopathy and to the possible longer times required for a full recovery. Indeed, the percutaneous approach allowed timely initiation of chemotherapy, which was likely to provide a prognostic benefit. Finally, dedicated cardiologic and haematological speciality follow-up must be guaranteed to observe the response to chemotherapy and to monitor possible long-term complications (heart failure, arrhythmias, and amyloidosis relapse).
Conclusions
Isolated cardiac AL amyloidosis is uncommon in clinical practice, especially in asymptomatic patients. However, it has been likely underestimated in the past. It should be systematically investigated in patients with suggestive clues (the so-called red flags) of AL amyloidosis. The diagnosis of this unusual finding has a crucial importance in the clinical management of these patients. Early initiation of specific chemotherapies should be regularly pursued.
Lead author biography
 Dr Vincenzo Nuzzi received his MD degree at the University of Rome ‘La Sapienza’, Italy. He then focused on clinical research in heart failure in Hull, UK, before starting his speciality training in cardiology at University of Trieste. Part of his training was held in Cambridge (Royal Papworth Hospital, UK) and in Pisa (cardiac MR training, Italy). He is currently working as a cardiologist in the Heart Failure Unit of the Mediterranean Institute for Transplantations and Advanced Specialized Therapies (ISMETT, Palermo, Italy).
Dr Vincenzo Nuzzi received his MD degree at the University of Rome ‘La Sapienza’, Italy. He then focused on clinical research in heart failure in Hull, UK, before starting his speciality training in cardiology at University of Trieste. Part of his training was held in Cambridge (Royal Papworth Hospital, UK) and in Pisa (cardiac MR training, Italy). He is currently working as a cardiologist in the Heart Failure Unit of the Mediterranean Institute for Transplantations and Advanced Specialized Therapies (ISMETT, Palermo, Italy).
Supplementary material
Supplementary material is available at European Heart Journal—Case Reports.
Acknowledgements
The authors acknowledge Cassa di Risparmio di Trieste (CRT) Foundation for the continuous support in research.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission and publication of this case, including images, has been obtained from the patient in line with COPE guidance.
Funding: None declared.
References
Author notes
Conflict of interest: None declared.




Comments