-
PDF
- Split View
-
Views
-
Cite
Cite
Toshihiro Nakamura, Koji Fukuzawa, Takeshi Aiba, Seiko Ohno, Case report of a ventricular fibrillation storm with a cardiac conduction disorder and HCN4 variant 18 years after ablation of atrial flutter, European Heart Journal - Case Reports, Volume 6, Issue 11, November 2022, ytac431, https://doi.org/10.1093/ehjcr/ytac431
Close - Share Icon Share
Abstract
Genetic abnormalities causing various arrhythmias including atrial arrhythmias, specialized cardiac conduction disorders, and malignant ventricular arrhythmias have been reported. However, it is sometimes difficult to diagnose and treat patients with various arrhythmias.
A 49-year-old woman who underwent ablation of typical atrial flutter (AFL) at 31 years of age visited the emergency room due to a cardiopulmonary arrest. Her 12-lead electrocardiogram during sinus rhythm after resuscitation exhibited first-degree atrioventricular block with right bundle branch block and right axis deviation. No structural heart disease was evident on standard imaging screening. An implantation of a single-chamber implantable cardioverter defibrillator (ICD) was indicated. After the ICD implantation, she then experienced multiple ventricular fibrillation (VF) episodes. Radiofrequency catheter ablation of triggered ventricular premature contractions (VPCs) was performed but failed because the clinical VPCs could not be induced during the session. Although no pathogenic variants associated with Brugada syndrome or long-QT syndrome were found, a rare HCN4 variant, c.1209+2_1209+3insGAGT (rs786205418), was identified in a gene panel analysis. Because high-frequency clinical pacing was effective for suppressing the VF, the single-chamber ICD was upgraded to a dual-chamber ICD. Thereafter, high-rate pacing successfully prevented any further ventricular arrhythmias during the follow up.
A clinical course with prominent wide QRS complexes and AFL in one’s early 30s followed by sudden onset of a VF storm about 20 years later is extremely rare. Her clinical phenotype expression was possibly associated with a rare HCN4 variant; however, further study is needed to confirm whether this variant was pathological or not.
This is a very complex case of recurrent ventricular fibrillation in a patient with a structurally normal heart, history of conduction disease, and atrial flutter.
A rare variant in HCN4, exon2c.1209+2_1209+3insGAGT (rs786205418), was found but it was of uncertain significance.
We could not rule out that the patient’s very unusual clinical course may have suggested a variety of phenotypes brought on by unknown genetic abnormalities, and further studies are needed to clarify this issue.
Introduction
Young women rarely develop atrial flutter (AFL) unless they have congenital or structural heart disease.1 Epidemiological studies have suggested that widening of the QRS complexes is a predictor of cardiac disease and sudden cardiac death.2 On the other hand, genetic abnormalities that cause atrial arrhythmias, specialized cardiac conduction disorders, and malignant ventricular arrhythmias have been reported.3 We sometimes struggle to provide a unified explanation of rare diseases that present with a wide variety of arrhythmias.
We experienced a female patient with a rare HCN4 variant who developed AFL in her 30s and developed a ventricular fibrillation (VF) storm 18 years later. In this report, we describe her clinical course in detail and address the possible factors for developing a variety of arrhythmias for the readers.
Timeline
| 2001 (age 31 years) |
|
| 2001 to December 2019 |
|
| December 2019 (age 49 years) |
|
| February to July 2021 (age 50 years) |
|
| August 2021 (age 50 years) |
|
| 2001 (age 31 years) |
|
| 2001 to December 2019 |
|
| December 2019 (age 49 years) |
|
| February to July 2021 (age 50 years) |
|
| August 2021 (age 50 years) |
|
| 2001 (age 31 years) |
|
| 2001 to December 2019 |
|
| December 2019 (age 49 years) |
|
| February to July 2021 (age 50 years) |
|
| August 2021 (age 50 years) |
|
| 2001 (age 31 years) |
|
| 2001 to December 2019 |
|
| December 2019 (age 49 years) |
|
| February to July 2021 (age 50 years) |
|
| August 2021 (age 50 years) |
|
Case presentation
A 49-year-old woman developed VF requiring resuscitation and external defibrillation, while she was waiting at the bus stop, on her way home as usual. She had no complaints in the days before the VF and took no medications. She was restored to sinus rhythm after just the first cardioversion shock. Her blood pressure and pulse rate were 106/59 mmHg and 43 beats per minute (b.p.m.), respectively. Her consciousness level was awake and alert. There were no auscultatory abnormalities of cardiac and respiratory sounds. No thyroid enlargement or leg oedema was observed. After returning to sinus rhythm, a 12-lead ECG exhibited first-degree AV block with right bundle branch block and right axis deviation (Figure 1A). Her blood tests revealed that her potassium level was 3.5 mmol/L (normal range: 3.5–5.0 mmol/L) but no other abnormalities were found including of the thyroid function. Eighteen years prior, when she was 31 years old, she was referred to our hospital to be treated for common AFL and a wide QRS complex (complete right bundle branch block; Figure 1B). AFL was documented by chance during a medical check-up. No electrolyte abnormalities were observed at that time. There was no evidence of structural heart disease and she underwent a cavo-tricuspid isthmus ablation. After that, echocardiography exhibited a normal left ventricular ejection fraction and no valvular disease (Supplementary material online, Video S1). The QRS morphology did not change even after the rhythm had returned to sinus rhythm. Unfortunately, we did not have any ECG data before the onset of the AFL. After that, she has been followed annually at our outpatient clinic with no drugs; however, no change was observed on the 12-lead ECG or cardiac ultrasound, and she did not have any symptoms during the 18 years of follow up. However, 18 years later, she suddenly developed VF.
(A) A resting 12-lead electrocardiogram exhibiting first-degree atrioventricular block with right bundle branch block and right axis deviation. The heart rate was 74 beats per minute. and the PQ, QRS, and QTc intervals were 254, 191, and 486 ms, respectively. (B) Before the cavo-tricuspid isthmus ablation, the 12-lead electrocardiogram shows a common atrial flutter with right bundle branch block and right axis deviation. (C) A 12-lead electrocardiogram showing ventricular premature contractions triggering ventricular fibrillation. (D) Example of a stored ICD electrogram. The red arrows show the ventricular premature contraction triggering ventricular fibrillation. (E) The resting 12-lead electrocardiogram after adding an atrial lead. The implantable cardioverter defibrillator was programmed to the AAI mode at 90 beats per minute.
Echocardiography exhibited a normal left ventricular ejection fraction and no valvular disease (Supplementary material online, Video S2). We also assessed her cardiac function using cardiac cine and late gadolinium enhancement magnetic resonance imaging. However, no morphological abnormalities or specific fibrosis was observed (Supplementary material online, Figure S1). The 24 h ECG recording revealed only a 0.3% monophasic VPC burden. She was implanted with a single-lead ICD for secondary prevention of VF. Also, she was prescribed potassium chloride tablets.
Following that, she was frequently hospitalized for multiple VF episodes requiring ICD shocks. On one occasion, she developed VF in the early stages of a coronavirus infection. However, no VF occurred during the week that followed when she had a fever of nearly 40°C. On the other hand, VF occurred asymptomatically, without any specific electrolyte abnormalities during the remaining time. Beta-blockers did not prevent the lethal arrhythmia, and amiodarone was not used because we were afraid of a prolongation of the QT-interval and worsening VF episodes. From the results of the ICD interrogation and 12-lead ECG, it was found that VPCs had triggered the VF (Figure 1C and D). Therefore, radiofrequency catheter ablation of the triggering VPCs was performed to reduce the ICD shock therapies. An electrophysiological study (EPS) using a CARTO system revealed no low voltage zones in the right atrium. Although transient 2:1 AV block was observed during the procedure, a detailed EPS was not performed, and the sinus node recovery time and Wenckebach AV block rate were not evaluated. Since the VPCs rarely occurred despite an isoproterenol infusion or single/double extrastimuli from the right ventricular (RV) apex using two basic cycle lengths (600 and 400 ms), a pace map was performed of the RV. Unfortunately, no good pace maps of the VPCs were obtained from the endocardial RV. In addition, a voltage map of the entire RV revealed no low voltage zones. Therefore, we had no choice but to perform an radio frequency application in the anteroseptal region of the RV where a delayed potential was observed (Figure 2). We considered that the ablation of the triggering VPC was not adequate to control the VF. For that reason, we planned to add an atrial lead to suppress the VF by high-rate pacing.
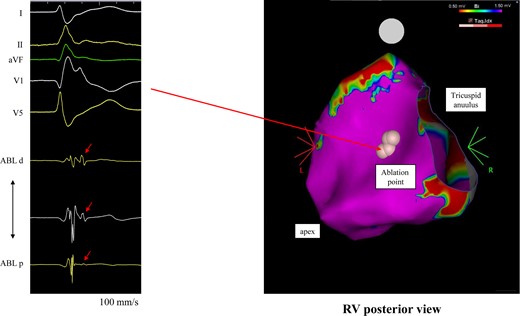
The voltage map of the right ventricle during the triggering ventricular premature contraction ablation. The pink points indicate the ablation sites where delayed potentials were observed. ABL, ablation catheter.
A week before the surgery to add the lead, she was taken to the emergency room because of a VF storm. As it was not controlled with an intravenous injection of 100 mg of lidocaine and since we were afraid of a prolongation of the QT-interval or worsening conduction disorders if other anti-arrhythmic drugs were used, she was sedated and intubated. According to the ECG during the electrical storm, it was observed that the VF was initiated by an R-on-T extrasystole. Therefore, she required high-rate pacing to prevent any malignant VPCs and underwent surgery to add the atrial lead. A setting of 90 b.p.m. with a long AV delay to avoid ventricular stimulation successfully prevented any further VF episodes (Figure 1E). After 2 months, the stimulation rate was reduced to 80 b.p.m. She is taking potassium chloride tablets and a mineralocorticoid receptor antagonist to prevent hypokalaemia. Clinical follow-up visits and device interrogations were scheduled every 3 months after the procedure, with remote device monitoring when possible, which showed a stable pacing threshold. She has been free from any VF recurrences during a 6-month follow up.
At a relatively young age, the patient’s arrhythmias raised suspicion of a possible underlying inherited arrhythmogenic condition; therefore, genetic testing and a familial evaluation were recommended. Although she underwent genetic testing targeting the KCNQ1, KCNH2, and SCN5A genes associated with Brugada syndrome and long-QT syndrome (LQTS), there were no remarkable findings among these genes. Therefore, comprehensive genetic screening targeting >60 genes (Supplementary material online, Table S1) associated with inherited arrhythmias or cardiomyopathies was performed that identified a rare variant, c.1209+2_1209+3insGAGT (rs786205418) in HCN4, which is located at a splice donor site and is reported to be a variant with uncertain significance. The details of the genetic testing are described in the Supplementary material online, File S1.
In addition, she had no family history of arrhythmias, cardiomyopathy, or sudden cardiac death. Also, her family had no history of a car accident, seizure/epilepsy, or pacemaker implantation at a young age. Unfortunately, no familial evaluation was performed due to the patient’s refusal.
Discussion
This was a very complex case of recurrent VF in a patient with a structurally normal heart, history of conduction disease, and AFL. In our case, a specific cardiac conduction system disorder was found at an early age. However, this patient not only had a cardiac conduction disorder but also ST elevation as in Brugada syndrome and QT prolongation, which lead to refractory VF. Moreover, general genetic testing revealed that there were no remarkable findings of Brugada syndrome or LQTS. Therefore, this patient’s cardiac disorder could not be classified as any of the other already-known inherited primary arrhythmia syndromes.
Of interest, a rare variant in HCN4, exon2c.1209+2_1209+3insGAGT (rs786205418), was found but it was of uncertain significance. So far, there have been only two cases that have been reported to be related to this variant from the ClinVar database.4,5 One case, which was reported to have a rare variant in an exome cohort, had an incomplete intraventricular conduction disturbance and enlarged aortic roof, but VF never occurred.4 The other case, which was in a report on the role of the HCN4 channel in preventing ventricular arrhythmias, had bradycardia-induced VF, but no cardiac conduction disorders were observed.5
Generally, the HCN4 gene is mainly expressed in atrial muscle for pacemaker currents, and its loss of function is known to be associated with bradycardia. Although she had a cardiac conduction disorder and AFL at a relatively young age, there were no typical symptoms of HCN4 syndromes, such as sick sinus syndrome,6 atrial fibrillation,7 or a left ventricular non-compaction.8
To the best of our knowledge, the clinical course of prominent wide QRS complexes and AFL in one’s early 30s followed by a sudden onset of a VF storm about 20 years later is extremely rare, and we could not confirm an adequate diagnosis that could univocally explain her clinical course. There is no evidence that this rare HCN4 variant is pathological. A functional analysis and the accumulation of cases are needed to prove the pathological significance, and it may be premature to suspect an association between these cardiac disorders and the genetic variant. However, we could not rule out that the patient’s very unusual clinical course may have suggested a variety of phenotypes brought on by unknown genetic abnormalities. As a matter of fact, we could not determine how to predict VF before it developed. Comprehensive genetic testing of asymptomatic patients is not appropriate at this time; however, we may be able to do something in the future if the quality and meaning of the genetic testing changes, and we believe that it is worth sharing this report.
Conclusion
We experienced a case of a young woman without structural cardiac disease, who developed AFL, was asymptomatic for 18 years, and suddenly developed a VF storm. There were no signs at all, and it was difficult to predict, but we could treat it appropriately by high-rate pacing. You should pay attention to an early onset of AFL and a cardiac conduction defect even in the absence of symptoms.
Lead author biography
 Dr Toshihiro Nakamura is a cardiologist with a clinical focus on interventional electrophysiology. He graduated from Kobe University graduate school of medicine in 2021. In 2020, he was a winner of the Young Investigator Award of the Japanese Heart Rhythm Society.
Dr Toshihiro Nakamura is a cardiologist with a clinical focus on interventional electrophysiology. He graduated from Kobe University graduate school of medicine in 2021. In 2020, he was a winner of the Young Investigator Award of the Japanese Heart Rhythm Society.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Acknowledgement
The authors thank Mr John Martin for his linguistic assistance.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission and publication of this case report including the images and associated text has been obtained from the patient’s relative in line with the COPE guidance.
Funding: None declared.
References
Author notes
Conflict of interest: None declared.






Comments