-
PDF
- Split View
-
Views
-
Cite
Cite
Dmytro Volkov, Dmytro Lopin, Stanislav Rybchynskyi, Dmytro Skoryi, His-optimized cardiac resynchronization therapy in a patient with heart failure and right bundle branch block: a case report, European Heart Journal - Case Reports, Volume 5, Issue 8, August 2021, ytab277, https://doi.org/10.1093/ehjcr/ytab277
Close - Share Icon Share
Abstract
Cardiac resynchronization therapy (CRT) is an option for treatment for chronic heart failure (HF) associated with left bundle branch block (LBBB). Patients with HF and right bundle branch block (RBBB) have potentially worse outcomes in comparison to LBBB. Traditional CRT in RBBB can increase mortality and HF deterioration rates over native disease progression. His bundle pacing may improve the results of CRT in those patients. Furthermore, atrioventricular node ablation (AVNA) for rate control in atrial fibrillation (AF) can be challenging in patients with previously implanted leads in His region.
We report the case of 74-year-old gentleman with a 5-year history of HF, permanent AF with a rapid ventricular response, and RBBB. He was admitted to the hospital with complaints of severe weakness and shortness of breath. Left ventricular ejection fraction (LVEF) was decreased (41%), right ventricle (RV) was dilated (41 mm), and QRS was prolonged (200 ms) with RBBB morphology. The patient underwent His-optimized CRT with further left-sided AVNA. As a result, LVEF increased to 51%, RV dimensions decreased to 35 mm with an improvement of the clinical status during a 6-month follow-up.
Patients with AF, RBBB, and HF represent the least evaluated clinical subgroup of individuals with less beneficial clinical outcomes according to CRT studies. Achieving the most effective resynchronization could require pacing fusion from sites beyond traditional with the intention to recruit intrinsic conduction pathways. This approach can be favourable for reducing RV dilatation, improving LVEF, and maximizing electrical resynchronization.
Learning points
His-optimized cardiac resynchronization therapy (HOT-CRT) could be used for an effective overcoming of profound conduction delays in a case of either left bundle branch block or right bundle branch block (RBBB).
The presence of permanent atrial fibrillation allows using of atrial channel as an additional port for His, trifocal or back-up right ventricular pacing in pacemaker-dependent patients during HOT-CRT.
The retrograde aortic approach can be considered as an initial one in patients with RBBB undergoing atrioventricular nodal radiofrequency ablation for rate control after HOT-CRT.
Introduction
Cardiac resynchronization therapy (CRT) provides biventricular (BiV) pacing to patients with heart failure (HF) in the presence of the left bundle branch block (LBBB) with prolonged QRS duration and decreased left ventricular ejection fraction (LVEF). CRT in patients with HF and non-LBBB QRS morphology thought to be less beneficial, receiving either Class IIa or IIb recommendation depending on the QRS duration.1 These recommendations are applied to patients in sinus rhythm; however, the presence of atrial fibrillation (AF) with uncontrolled rapid ventricular response (RVR) rate results in a necessity of AVNA in a case of no response or intolerance to the rate and (or) rhythm control therapy, thus making patients pacemaker (PM)-dependent.2
We are presenting the clinical case where all mentioned issues are combined in a challenging scenario with a need for the adoption of the His-optimized CRT (HOT-CRT) to provide the most favourable treatment result.
Timeline
| Past 5 years | First admission for heart failure (HF); documented left ventricular ejection fraction (LVEF)—52%; treated with amlodipine, torasemide, spironolactone, and bisoprolol |
| Admission | Presented with severe HF |
| Day 1 | Treated with additional intravenous inotropes and diuretics administration of bisoprolol, valsartan, apixaban, tiotropium inhalation, torasemide, and spironolactone |
| Day 2 | Echocardiography showed decreased LVEF 41%, severe right heart dilatation, and severe tricuspid regurgitation |
| Day 3 | His-optimized cardiac resynchronization therapy (CRT) was performed with setting the base rate to 85 beats/min to achieve partial CRT |
| Day 10 | Atrioventricular nodal radiofrequency ablation was performed |
| 1 month after implantation | Re-evaluation of pacemaker parameters and clinical status |
| 6 months after implantation | Follow-up assessment showed stable pacing parameters, CRT pacing in 99%, improvement of symptoms from New York Heart Association (NYHA) IV to NYHA II and improvement in LVEF to 51%, right ventricle dimensions to 35 mm and mild tricuspid regurgitation |
| Past 5 years | First admission for heart failure (HF); documented left ventricular ejection fraction (LVEF)—52%; treated with amlodipine, torasemide, spironolactone, and bisoprolol |
| Admission | Presented with severe HF |
| Day 1 | Treated with additional intravenous inotropes and diuretics administration of bisoprolol, valsartan, apixaban, tiotropium inhalation, torasemide, and spironolactone |
| Day 2 | Echocardiography showed decreased LVEF 41%, severe right heart dilatation, and severe tricuspid regurgitation |
| Day 3 | His-optimized cardiac resynchronization therapy (CRT) was performed with setting the base rate to 85 beats/min to achieve partial CRT |
| Day 10 | Atrioventricular nodal radiofrequency ablation was performed |
| 1 month after implantation | Re-evaluation of pacemaker parameters and clinical status |
| 6 months after implantation | Follow-up assessment showed stable pacing parameters, CRT pacing in 99%, improvement of symptoms from New York Heart Association (NYHA) IV to NYHA II and improvement in LVEF to 51%, right ventricle dimensions to 35 mm and mild tricuspid regurgitation |
| Past 5 years | First admission for heart failure (HF); documented left ventricular ejection fraction (LVEF)—52%; treated with amlodipine, torasemide, spironolactone, and bisoprolol |
| Admission | Presented with severe HF |
| Day 1 | Treated with additional intravenous inotropes and diuretics administration of bisoprolol, valsartan, apixaban, tiotropium inhalation, torasemide, and spironolactone |
| Day 2 | Echocardiography showed decreased LVEF 41%, severe right heart dilatation, and severe tricuspid regurgitation |
| Day 3 | His-optimized cardiac resynchronization therapy (CRT) was performed with setting the base rate to 85 beats/min to achieve partial CRT |
| Day 10 | Atrioventricular nodal radiofrequency ablation was performed |
| 1 month after implantation | Re-evaluation of pacemaker parameters and clinical status |
| 6 months after implantation | Follow-up assessment showed stable pacing parameters, CRT pacing in 99%, improvement of symptoms from New York Heart Association (NYHA) IV to NYHA II and improvement in LVEF to 51%, right ventricle dimensions to 35 mm and mild tricuspid regurgitation |
| Past 5 years | First admission for heart failure (HF); documented left ventricular ejection fraction (LVEF)—52%; treated with amlodipine, torasemide, spironolactone, and bisoprolol |
| Admission | Presented with severe HF |
| Day 1 | Treated with additional intravenous inotropes and diuretics administration of bisoprolol, valsartan, apixaban, tiotropium inhalation, torasemide, and spironolactone |
| Day 2 | Echocardiography showed decreased LVEF 41%, severe right heart dilatation, and severe tricuspid regurgitation |
| Day 3 | His-optimized cardiac resynchronization therapy (CRT) was performed with setting the base rate to 85 beats/min to achieve partial CRT |
| Day 10 | Atrioventricular nodal radiofrequency ablation was performed |
| 1 month after implantation | Re-evaluation of pacemaker parameters and clinical status |
| 6 months after implantation | Follow-up assessment showed stable pacing parameters, CRT pacing in 99%, improvement of symptoms from New York Heart Association (NYHA) IV to NYHA II and improvement in LVEF to 51%, right ventricle dimensions to 35 mm and mild tricuspid regurgitation |
Case presentation
A 74-year-old Caucasian man was admitted with severe weakness and shortness of breath increasing in horizontal position and during minimal physical activity (walking <100 m, a one-floor stairs climb). Profound peripheral oedema and acrocyanosis were present. His symptoms were classified as New York Heart Association (NYHA) Class IV. The patient reported onset of HF 5 years ago and presence of AF for several years, which exacerbated to AF with uncontrolled rapid heart rate with gradual increase of above-mentioned complaints during several weeks, what led to current hospitalization. His medications included amlodipine 5 mg, torasemide 10 mg, spironolactone 25 mg, and bisoprolol 2.5 mg.
A transthoracic echocardiography (TTE) demonstrated moderate left ventricular (LV) dysfunction (EF, 41% by Simpson method) and RV dilatation with severe tricuspid regurgitation (43 mm2 effective regurgitation orificae area by proximal isovelocity surface area). Electrocardiogram (ECG) showed presence of AF, HR = 115 b.p.m., right bundle branch block (RBBB), and QRS prolongation (200 ms). Attempts to provide rate control therapy were abandoned due to the development of hypotension (80/60 mmHg). Coronaroangiography excluded significant coronary artery disease in this patient, and thus, due to the presence of the permanent AF with the RVR, tachycardia-induced cardiomyopathy was the most likely aetiology of HF.
Implantation details
The following plan for the intervention was adopted: (i) implantation of LV lead; (ii) cardiac mapping of the most delayed electrical zone within RV with possible implantation of a lead in the selected area; (iii) interventricular septal (IVS) implantation of RV lead; (iv) mapping of His bundle area; implantation of His bundle pacing (HBP) lead with the aim to correct conduction disorders (especially RBBB); (v) connection of leads to appropriate PM ports based on achieved pacing results; and (vi) postponed AVNA.
Three punctures of left axillary vein were performed under local anaesthesia. The LV lead was delivered and implanted (sensing—4.1–5.8 mV, pacing threshold—1.0 V) through a posterior vein to the left posterolateral area in the mid-basal region. Endocardial activation mapping of RV was conducted, and the latest point of activation was detected in basal anterolateral area; however, reliable conventional lead implantation did not seem possible in the above-mentioned region. Thus, RV lead was implanted into the middle third of IVS (sensing—16.2–17.5 mV, pacing threshold—0.8 V) to be used as regular RV lead in a case of conventional CRT configuration. LBBB-like QRS pattern was achieved during pacing.
C315HIS delivery system and SelectSecure 3830 electrode (Medtronic, Minneapolis, MN, USA) were used to provide the mapping of His bundle area and further lead implantation in its distal part (sensing—0.8–1.2 mV, pacing threshold—1.75 V for His bundle; 0.75 V for RV) (Figure 1). Leads were connected to PM [CRT-Р Allure RF (Abbott)]. During pacing in several configurations, minimal QRS width of 120 ms was during fusion of HBP and +10 ms delayed LV pacing. Taking into consideration the minimal atrioventricular delay of this PM (30 ms)—vanishing fusion effect from LV lead depolarization in case of HBP lead connection into the atrial channel—HBP lead was connected to RV port, LV to LV port, RV to A port. In case of HBP lead dysfunction, DDI sequential BiV pacing with minimal atrioventricular delay (30 ms) and yielded acceptable 145 ms QRS width. VVI BiV pacing mode was set up with the LV lead sensing as a more reliable option.
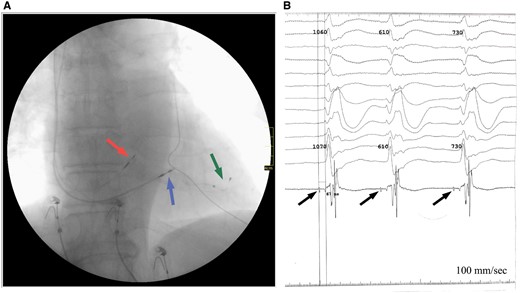
Outcomes after lead placement. (A) Final leads position according to anteroposterior projection fluoroscopy: in His bundle area (red arrow); in the middle third of interventricular septal (blue arrow); in the posterior vein of left ventricular (green arrow). (B) Potentials recorded from the His bundle (arrows) during implantation, H-V conduction time—67 ms.
AVNA using EnSite™ NavX™ was performed in 1 week after implantation. The procedure included cardiac mapping of atrioventricular node (AVN) and His bundle area with annotation of His lead implantation zones from both sides of IVS. Repeated radiofrequency ablation (RFA) attempts (up to 40 W, irrigated tip electrode) to obtain reliable AVN block from the right side with points proximally and up to the level of HBP lead’s anode were unsuccessful. Therefore, RFA (35–40 W, irrigated tip electrode) from retrograde aortic approach proximally and then distally to the projection of the HBP lead’s tip was applied with successful elimination of AV conduction with escape rhythm of 35 b.p.m. and native RBBB (Figure 2).
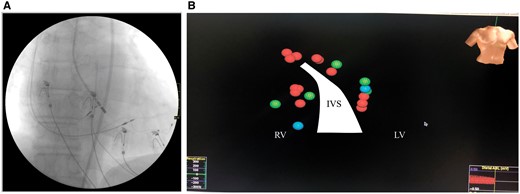
Atrioventricular nodal radiofrequency ablation. (A) Fluoroscopic view at the point of successful repeated radiofrequency ablation. (B) 3D cardiac mapping results: red spheres—repeated radiofrequency ablation applications; green—elements of conduction system; blue—zones of His bundle pacing lead projections. IVS, interventricular septal; LV, left ventricular; RV, right ventricular.
HBP lead functionality had not been affected (Figure 3). Different pacing modalities are presented in Figure 4. Stable non-selective HBP with correction of underlying RBBB was achieved with pacing amplitude above 2 V. The shortest and most ‘physiologic’ ECG pattern was obtained by fusion of HBP and 10 ms delayed LV pacing (Table 1).
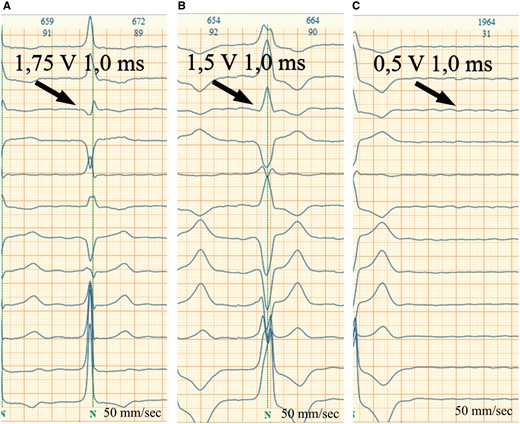
His bundle pacing lead thresholds test. (A) His bundle capture with correction of underlying right bundle branch block (1.75 V). (B) Right ventricular capture (1.5 V). (C) Loss of capture (0.5 V).
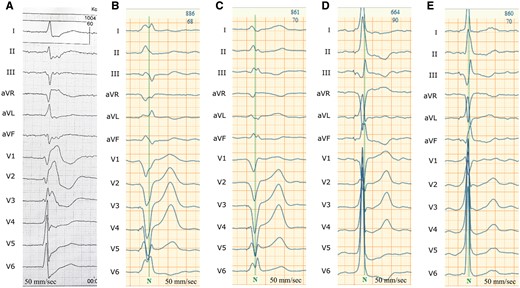
Electrocardiogram in different pacing modalities. (A) Native electrocardiogram; (B) right ventricular stimulation; (C) simultaneous biventricular stimulation; (D) His bundle pacing; (E) His bundle pacing fusion with left ventricular epicardial pacing.
EchoCG parameters on admission and 6 months after His-optimized cardiac resynchronization therapy
| Echocardiographic parameter . | On admission . | 6 months after HOT-CRT . |
|---|---|---|
| LV internal dimension | ||
| Diastolic dimension (mm) | 53.2 | 51.1 |
| Systolic dimension (mm) | 41.2 | 32.3 |
| LV volumes (biplane) | ||
| LV EDV (mL) | 135 | 127 |
| LV ESV (mL) | 80 | 62 |
| LV EF (biplane) | 41% | 51% |
| LV GLS (obtained in three apical views and averaged) | −9.7% | −11.9% |
| RV size | ||
| RV basal diameter (mm) | 41 | 35 |
| RV mid diameter (mm) | 35 | 29 |
| RV longitudinal diameter (mm) | 77 | 76 |
| RV function | ||
| TAPSE (mm) | 12 | 19 |
| Tricuspid regurgitation | ||
| EROA (mm2) by PISA | 43 | 18 |
| Echocardiographic parameter . | On admission . | 6 months after HOT-CRT . |
|---|---|---|
| LV internal dimension | ||
| Diastolic dimension (mm) | 53.2 | 51.1 |
| Systolic dimension (mm) | 41.2 | 32.3 |
| LV volumes (biplane) | ||
| LV EDV (mL) | 135 | 127 |
| LV ESV (mL) | 80 | 62 |
| LV EF (biplane) | 41% | 51% |
| LV GLS (obtained in three apical views and averaged) | −9.7% | −11.9% |
| RV size | ||
| RV basal diameter (mm) | 41 | 35 |
| RV mid diameter (mm) | 35 | 29 |
| RV longitudinal diameter (mm) | 77 | 76 |
| RV function | ||
| TAPSE (mm) | 12 | 19 |
| Tricuspid regurgitation | ||
| EROA (mm2) by PISA | 43 | 18 |
EDV, End-diastolic volume; EF, ejection fraction; EROA, effective regurgitation orificae area; ESV, End-systolic volume; GLS, Global longitudinal strain; HOT-CRT, His-optimized cardiac resynchronization therapy; LV, left ventricular; PISA, proximal isovelocity surface area; TAPSE, Tricuspid annular plane systolic excursion.
EchoCG parameters on admission and 6 months after His-optimized cardiac resynchronization therapy
| Echocardiographic parameter . | On admission . | 6 months after HOT-CRT . |
|---|---|---|
| LV internal dimension | ||
| Diastolic dimension (mm) | 53.2 | 51.1 |
| Systolic dimension (mm) | 41.2 | 32.3 |
| LV volumes (biplane) | ||
| LV EDV (mL) | 135 | 127 |
| LV ESV (mL) | 80 | 62 |
| LV EF (biplane) | 41% | 51% |
| LV GLS (obtained in three apical views and averaged) | −9.7% | −11.9% |
| RV size | ||
| RV basal diameter (mm) | 41 | 35 |
| RV mid diameter (mm) | 35 | 29 |
| RV longitudinal diameter (mm) | 77 | 76 |
| RV function | ||
| TAPSE (mm) | 12 | 19 |
| Tricuspid regurgitation | ||
| EROA (mm2) by PISA | 43 | 18 |
| Echocardiographic parameter . | On admission . | 6 months after HOT-CRT . |
|---|---|---|
| LV internal dimension | ||
| Diastolic dimension (mm) | 53.2 | 51.1 |
| Systolic dimension (mm) | 41.2 | 32.3 |
| LV volumes (biplane) | ||
| LV EDV (mL) | 135 | 127 |
| LV ESV (mL) | 80 | 62 |
| LV EF (biplane) | 41% | 51% |
| LV GLS (obtained in three apical views and averaged) | −9.7% | −11.9% |
| RV size | ||
| RV basal diameter (mm) | 41 | 35 |
| RV mid diameter (mm) | 35 | 29 |
| RV longitudinal diameter (mm) | 77 | 76 |
| RV function | ||
| TAPSE (mm) | 12 | 19 |
| Tricuspid regurgitation | ||
| EROA (mm2) by PISA | 43 | 18 |
EDV, End-diastolic volume; EF, ejection fraction; EROA, effective regurgitation orificae area; ESV, End-systolic volume; GLS, Global longitudinal strain; HOT-CRT, His-optimized cardiac resynchronization therapy; LV, left ventricular; PISA, proximal isovelocity surface area; TAPSE, Tricuspid annular plane systolic excursion.
| Mode . | Upper heart rate . | Lower heart rate . | Ventricular pacing . |
|---|---|---|---|
| VVIR | 125 b.p.m. | 70 b.p.m. | RV -> LV, 10 ms |
| Mode . | Upper heart rate . | Lower heart rate . | Ventricular pacing . |
|---|---|---|---|
| VVIR | 125 b.p.m. | 70 b.p.m. | RV -> LV, 10 ms |
LV, left ventricular; RV, right ventricular; VVIR, Ventricular pacing, ventricular sensing, inhibition response and rate-adaptive pacing mode.
| Mode . | Upper heart rate . | Lower heart rate . | Ventricular pacing . |
|---|---|---|---|
| VVIR | 125 b.p.m. | 70 b.p.m. | RV -> LV, 10 ms |
| Mode . | Upper heart rate . | Lower heart rate . | Ventricular pacing . |
|---|---|---|---|
| VVIR | 125 b.p.m. | 70 b.p.m. | RV -> LV, 10 ms |
LV, left ventricular; RV, right ventricular; VVIR, Ventricular pacing, ventricular sensing, inhibition response and rate-adaptive pacing mode.
During TTE, HBP lead was visualized in the deep layers of the membranous—initial muscular part of IVS with tip located close to the left side of IVS. M-mode parasternal long-axis view revealed elimination of significant RV and IVS wall motion delay relatively to the LV posterior wall (Figure 5).
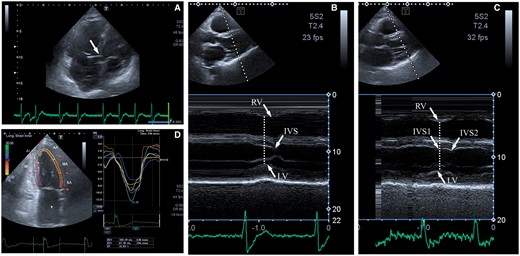
EchoCG. (A) His bundle pacing lead visualization; (B) M-mode—parasternal long-axis view—native rhythm, delay of right ventricular, and interventricular septal contraction peaks relatively to the left ventricular posterior wall. (C) M-mode parasternal long-axis view—pacing with the synchronicity and gaining of wall motion (interventricular septal 1—first peak and interventricular septal 2—second peak as signs of residual minor left ventricular dyssynchrony). IVS, interventricular septal; LV, left ventricular; RV, right ventricular.
Clinical follow-up
Pacing parameters remained stable at a 6-month follow-up. The patient noted resolution of shortness of breath in a horizontal position after HOT-CRT and AVNA and an increase in physical activity with acrocyanosis and peripheral oedema absence within 1 month. By TEE assessment, LVEF increased up to 51%, RV dimensions decreased to 35 mm and tricuspid regurgitation decreased to mild (Table 2). NYHA improved to Class II.
Discussion
Patients with pharmacologically refractory RVR are referred for AVNA to provide AV block, which is achieved successfully in 95% of cases through a right-sided approach.3 In such settings AVNA has a Class IIa (B) recommendation.2 Reasons for adoption of the left-sided approach include the presence of structural heart disease, inability to reach a stable catheter position, aortic root dilatation, or intramural AVN location.4,5 However, the left-sided approach may be considered as an initial one for AVNA in patients with RBBB undergoing HOT-CRT due to mechanical safety and remoteness from HBP lead.
According to the ESC 2016 HF guidelines, CRT rather than RV pacing is recommended for patients with HFrEF (LVEF < 40%) and high degree AV block to reduce morbidity, including patients with AF.1 The cut-off value for permanent pacing in AV block falls in different range, remaining beneficial in patients with LVEF <50% who require ventricular pacing more than 40% of the time.6,7 LVEF of 41% and relevant structural heart disease (left atrial enlargement) fits this patient in the HFmrEF (Heart failure with mid-range ejection fraction) category. Taking into account LVEF marginal value according to the criteria of HFrEF and the patient’s choice to adopt CRT, we decided to provide CRT. In this particular case it can be considered Class I recommendation.1
Non-LBBB CRT implantation remains an area of debate, leaving the question of RBBB correction open for discussion. Although the presence of RBBB was initially thought to be an occasional finding, further research revealed increased 4-year mortality in patients with RBBB in comparison to LBBB or without conduction disorders.8,9 Patients with RBBB also had higher mortality rate and increased progression of HF compared to patients with LBBB while receiving CRT.10,11 Considering successful experience in the use of HOT-CRT in patients with HF and RBBB, we decided to accept this approach.12 Nevertheless, there is limited evidence regarding its safety. Thus, we decided to implant a pacing lead into IVS besides the HBP lead and LV lead. It creates an additional opportunity to preserve the BiV pacing using DDI can mode in case of the HBP lead failure, which can be back-up option in this patient (Figure 6).
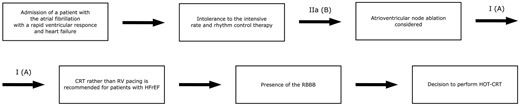
Diagram of step-by-step therapeutic approaches to the patient. CRT, cardiac resynchronization therapy; HOT-CRT, cardiac resynchronization therapy; HFrEF, Heart failure with reduced ejection fraction; RBBB, right bundle branch block; RV, right ventricular.
Conclusion
HOT-CRT + AVNA provide a flexible opportunity for maximum possible correction of underlying conduction disorders and facilitate mechanical improvement by means of dedicated lead placement connection and strict rate control.
Lead author biography
Dr Dmytro Volkov completed his medical degree in Ukraine in 1996. He completed his foundation and core training in Kharkiv, Ukraine. He got PhD degree in 2004. Currently, he serves as a chief of the department for ultrasound and instrumental diagnostics with EP Lab at the Zaycev V.T. Institute of General and Urgent Surgery of National Academy of Medical Science of Ukraine. He undertook several EP and cardiac pacing trainings locally and abroad including Institute for Clinical and Experimental Medicine, IKEM, Prague. He passed EHRA ECDS I and II examinations in 2013 and 2015, respectively.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.





Comments