-
PDF
- Split View
-
Views
-
Cite
Cite
Alex Moseley, Wojciech Mazur, Saad Ahmad, Cardiac magnetic resonance imaging in unclear cases of ventricular interdependence: a case series, European Heart Journal - Case Reports, Volume 5, Issue 7, July 2021, ytab223, https://doi.org/10.1093/ehjcr/ytab223
Close - Share Icon Share
Abstract
Diagnosis of constrictive pericarditis requires demonstration of interventricular interdependence which can prove difficult even with invasive haemodynamics. Its treatment requires invasive surgical procedures prior to which diagnostic certainty is necessary. Cardiac magnetic resonance imaging (MRI) is an underutilized tool for identification of this pathology.
We present two cases of heart failure due to interventricular interdependence with inconclusive invasive haemodynamic. Prior to recommending invasive surgical treatment, confirmation of the diagnosis was required. This was achieved using cardiac MRI leading to pericardiectomy followed by clinical improvement.
These cases demonstrate the clinical utility, sensitivity, and specificity of cardiac MRI for ventricular interdependence.
Learning points
Describe the epidemiology, pathophysiology, and treatment for ventricular interdependence
Demonstrate the use of cardiac magnetic resonance imaging to identify aetiology and diagnostic pathophysiology in ventricular interdependence
Introduction
In the cardiac cycle, diastolic filling involves lateral displacement of the free ventricular walls. Under normal physiology the filling of the left and right ventricle is influenced by intrathoracic pressure variation during breathing. Constriction leads to dissociation of intrathoracic and intracardiac pressures.1 In inspiration, negative intrathoracic pressure causes increased venous return to the right ventricle and increased capacitance in the pulmonary circulation, thus decreasing the volume returning to the left ventricle. Due to inability of the free wall to shift outwards to accommodate this additional volume the interventricular septum shifts to the left. The opposite is true in expiration, when the septum displaces to the right.2 This pathophysiology explains why patients present with classic signs of right heart failure in constrictive pericarditis.3 The most common cause of constrictive pericarditis is idiopathic,4 and carries significant mortality when left untreated.5
Transthoracic echocardiogram is the initial imaging modality of choice.6 Echocardiographic features supporting constrictive physiology and ventricular interdependence are septal bounce, early diastolic mitral inflow variation of 25% or greater with respiration, early diastolic tricuspid inflow variation of 40% or greater with respiration, diastolic hepatic vein flow reversal, annulus paradoxus, and a plethoric inferior vena cava. When present, combinations of these features support a diagnosis of constriction with sensitivity of 87% and specificity of 91%.7 Cardiac computed tomography is also useful. The presence of an effusion in addition to the thickness and contour of the pericardium can support pericardial constriction.8
The gold standard for diagnosis remains simultaneous right and left heart catheterization demonstrating ventricular interdependence. When observed, discordant left ventricular (LV) and right ventricular (RV) pressure have a sensitivity of 96% and specificity of 95%.2 Definitive treatment for constrictive pericarditis and ventricular interdependence is pericardiectomy.9 When invasive haemodynamics and initial imaging are not consistent, further diagnostic testing may need to be pursued prior to pursuing a surgical procedure.10
We present cases of two patients with heart failure despite history and clinical course suggestive of constrictive physiology with ventricular interdependence, invasive haemodynamics alone did not narrow the diagnosis. In each case, advanced, multimodal imaging with cardiac magnetic resonance imaging (MRI) helped confirm the diagnosis, leading to treatment.
Timeline
Case A
A 69-year-old male with a past medical history of myocardial infarction 1 year prior to admission and atrial fibrillation was admitted for recurrent shortness of breath, anasarca, and transaminitis. His vitals on admission included heart rate of 110 b.p.m., blood pressure of 94/68 mmHg, respiratory rate of 18 breaths per minute, oxygen saturation on room air of 96%, and temperature of 36.4°C. He was ill appearing on physical examination with bilateral inspiratory crackles in the lower half of the lung fields, ascites, lower extremity oedema, and jugular venous distention. He was tachycardic with a regular rhythm, normal S1, S2 and no murmurs, rubs, or gallops.
He had two similar admissions in the prior 6 months during which he was treated with intravenous diuretics with only modest improvement in his symptoms prior to discharge. Echocardiogram at the time of admission showed a moderate pericardial effusion, normal tissue Dopplers despite paradoxical septal motion, and a plethoric inferior vena cava (Figure 1A–C). There was no evidence of significant mitral and tricuspid inflow variability with the respiratory cycle (Figure 1D and E). Initial simultaneous right and left heart catheterization was performed with elevated filling pressures and low cardiac output and index of 3.67 L/min and 1.99 L/min m2, respectively. It did not show evidence of ventricular interdependence. He underwent pericardiocentesis followed by repeat invasive haemodynamic study which again did not demonstrate discordance between simultaneous left- and right-sided pressures.
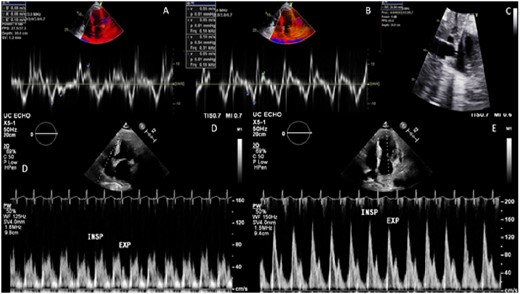
Patient A echocardiography. (A) Lateral annulus tissue Doppler with normal value. (B) Septal annulus tissue Doppler with normal value. (C) Inferior vena cava showing dilation. (D) Pulse wave Doppler tricuspid inflow with inconclusive respiratory variation. (E) Pulse wave Doppler mitral inflow with inconclusive respiratory variation.
The patient continued to experience clinical heart failure with elevated filling pressures, worsening hypotension, and renal function with attempted diuresis. A trial of dobutamine failed to improve symptoms or aid in diuretic response. Another simultaneous right and left heart catheterization was performed with a fluid challenge with no discordance noted (Figure 2A). Due to high index of suspicion, a cardiac MRI was performed to further evaluate his pathology (Figure 2B). LV and RV volumes were normal with mild reduction of RV ejection fraction (EF) at 41% and moderate reduction of LV EF to 32% and global hypokinesis. Pericardial thickening with delayed gadolinium enhancement and exaggerated right to left septal deviation during inspiration was present, supporting constrictive pericarditis (Figure 3A–C). Based on information from the cardiac MRI and clinical signs and symptoms, the patient successfully underwent pericardiectomy. After relieving the constriction, patient was eventually discharged at euvolemic state with resolution of symptoms. Surgical pathology showed thickened, fibrinous pericardial tissue. At most recent follow-up, the patient continued to maintain euvolemia without diuretics and is participating in physical therapy.
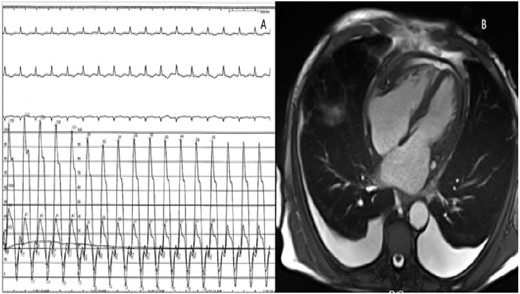
(A) Invasive haemodynamic measurement of right and left ventricular pressure with inconclusive interventricular interdependence. (B) Four-chamber cardiac magnetic resonance imaging image demonstrating moderate pericardial effusion and thickening of the pericardium.
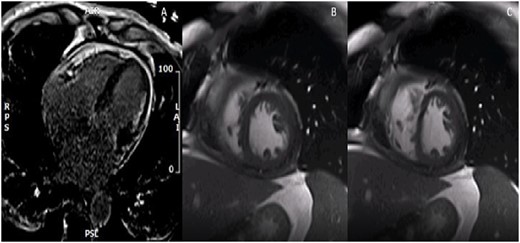
Patient A cardiac magnetic resonance imaging. (A) Four-chamber delayed gadolinium image with diffuse pericardial enhancement and thickening. (B) Two-chamber free-breathing sequence in expiration with exaggerated rightward shift of the interventricular septum. (C) Two-chamber free-breathing sequence in inspiration with exaggerated leftward shift of the interventricular septum.
Case B
A 29-year-old female with remote history of pericarditis at age 14, presented with weight gain, shortness of breath, and anasarca to her primary care physician. Vitals signs included blood pressure of 101/64 mmHg, heart rate of 90 b.p.m., 20 respirations per minute, oxygen saturation of 97%, and temperature of 36.1°C. On physical examination, she was noted to have a regular heart rate with distant S1 and S2 without murmurs, rubs, or gallops. There was decreased bilateral breath sounds with scattered wheezing and massive pitting oedema in the lower extremities with chronic lipodermatosclerosis. Transthoracic and transoesophageal echocardiograms were performed showing paradoxical septal motion with a possible pericardial mass compressing the lateral wall of the left ventricle, and a plethoric inferior vena cava (Figure 4A and B). Tissue Dopplers and mitral and tricuspid inflow were unremarkable (Figure 5A–D). The inferior vena cava was dilated with blunted respiratory changes. Cardiac MRI was performed to characterize the cardiac mass. The left and right ventricles were normal in size and function with EF of 56% and 51%, respectively, and the mass was identified as a loculated pericardial effusion (Figure 6A). The study also noted diffuse enhancement of the pericardium suggesting pericarditis and exaggerated respiratory septal excursion was present, supporting constrictive physiology (Figure 6B–D). LV and RV strain analysis were in the normal range, ruling out intrinsic RV and LV pathology (Figure 7A). She received intravenous diuretics with weight loss of ∼100 pounds followed by simultaneous right and left heart catheterization. Elevated filling pressures and ventricular interdependence were shown (Figure 7B). Based on the haemodynamic data and supportive findings on cardiac MRI, the patient underwent surgical pericardiectomy. The patient experienced clinical recovery postoperatively. Surgical pathology showed fibrosis of the pericardium with caseous inflammatory material in the loculations (Figure 7C). At most recent follow-up, the patient was euvolemic without diuretics and reported improving exercise tolerance.
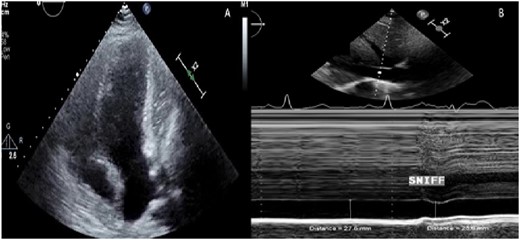
Patient B echocardiography. (A) Apical four-chamber image showing a mass compressing the lateral wall of the left ventricle. (B) M mode of the inferior vena cava showing dilation and blunted respiratory changes.
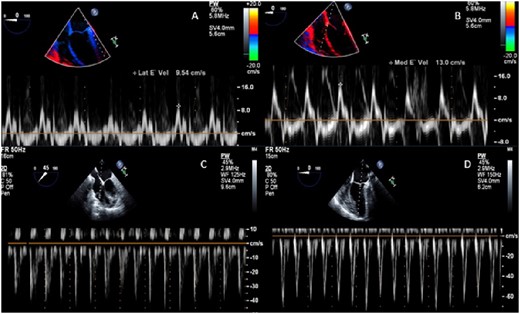
Patient B transoesophageal echocardiography. (A) Lateral annulus tissue Doppler with normal value. (B) Septal annulus tissue Doppler with normal value. (C) Pulse wave Doppler tricuspid inflow with inconclusive respiratory variation. (D) Pulse wave Doppler mitral inflow with inconclusive respiratory variation.
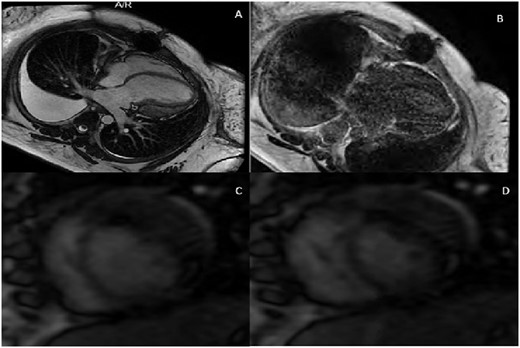
Patient B cardiac magnetic resonance imaging. (A) Four-chamber cardiac magnetic resonance imaging image showing a loculated pericardial effusion compressing the lateral wall of the left ventricle. (B) Four-chamber delayed gadolinium image with diffuse pericardial enhancement and thickening. (C) Two-chamber free-breathing sequence in expiration with exaggerated rightward shift of the interventricular septum. (D) Two-chamber free-breathing sequence in inspiration with exaggerated leftward shift of the interventricular septum.
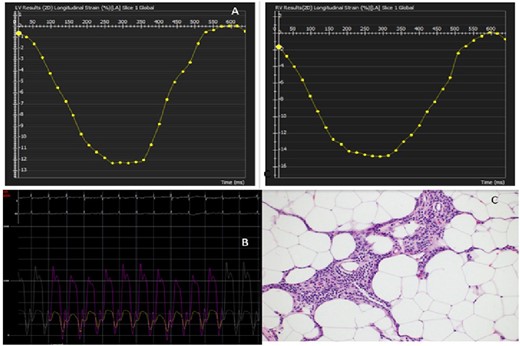
(A) Left pane: left ventricular strain analysis with normal pattern. Right pane: right ventricular strain analysis with normal pattern. (B) Invasive haemodynamic measurement of right and left ventricular pressure with interventricular interdependence. (C) Surgical pathology of the excised pericardium with fibrosis and caseous inflammatory material.
Discussion
Cardiac MRI is used as a supplemental test to diagnose structural heart disease when other diagnostic modalities are inconsistent or provide unclear data. Specifically, in cases of ventricular interdependence, interventricular septal excursion with respiratory variation and evidence of thickened pericardium may be observed.8 When the aforementioned parameters are present, it has a sensitivity and specificity of 98% and 90%, respectively.6 Detection of fluid, gas, or tumour in the pericardial space can provide aetiologic information as well, especially in less classic presentations.6
In addition to diagnostic information, cardiac MRI is helpful in guiding anti-inflammatory therapy. Pericardial thickening of >4 mm and extensive delayed gadolinium enhancement have been previously associated with reversible constrictive physiology and favourable response to anti-inflammatory therapy.11 It is also supported by the European Society of Cardiology (ESC) guideline as a Class IC recommendation for diagnosis of pericardial disease and constrictive pericarditis as a second level imaging technique. In cases such as these, where first line testing is supportive, but more information is required, it is considered the preferred imaging modality to optimally assess pericardial disease.12
In Patient A, constrictive pericarditis was supported by cardiac MRI with inconclusive haemodynamic testing. Due to his prolonged course of heart failure and his ongoing decompensation, the patient had significant shortness of breath and deconditioning limiting his participation in deep breathing during his invasive haemodynamics. This may have resulted in an inability to generate intrathoracic pressure variation. Additionally, high fidelity manometric catheters were not available while performing the patient’s invasive haemodynamics. In cases of surgically confirmed constriction compared to other causes of heart failure, these catheters demonstrated a sensitivity of 100% and specificity of 95% for detecting constriction when interventricular interdependence is demonstrated.13 The combination of patient participation and using routine standard pigtail and end hole Swan-Ganz catheter, and tubing length most likely explain the inconclusive haemodynamic studies.
In each case, the MRI also led to more rapid surgical intervention. It demonstrated mild to moderate pericardial enhancement and thickening in Case A (<4 mm). These data in addition to his prolonged symptoms predicted a poor response to anti-inflammatories, and therefore a need for surgery. In Case B, characterization of the mass by MRI was essential as it required surgical intervention for effective treatment rather than a trial of anti-inflammatory therapy.
Cardiac MRI led to treatment for patients with chronic illness secondary to heart failure due to constriction. These cases support the use of cardiac MRI as a non-invasive modality for assessment of ventricular dependence especially in the face of diagnostic uncertainty.
Lead author biography
Dr Alex Moseley graduated from The Ohio State University College of Medicine in 2015 and completed his internal medicine residency at Rush University Medical Center in Chicago, IL. He is currently Chief Fellow in the University of Cincinnati Cardiovascular Health and Diseases fellowship.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case series including image(s) and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.






Comments