-
PDF
- Split View
-
Views
-
Cite
Cite
Akinori Matsumoto, Ryo Ogawa, Masafumi Maeda, Aya Inakami, A left ventricular lead implantation at the latest site based on four-dimensional computed tomography: a case report, European Heart Journal - Case Reports, Volume 4, Issue 2, June 2020, Pages 1–5, https://doi.org/10.1093/ehjcr/ytaa033
Close - Share Icon Share
Abstract
Cardiac resynchronization therapy (CRT) could be an effective therapy for patients suffering from severe heart failure (HF) despite optimal medical therapy. However, it has been reported that about 30% of patients receive ineffective results even if CRT has been performed. In a recent study, four-dimensional computed tomography (4DCT) was shown to be useful for pre-operative planning in transcatheter aortic valve intervention. The 4DCT is reconstructed with 10% increments over the cardiac cycle so that the displacement of the myocardium can be evaluated over time. From the above, we considered that the most delayed site where we would implant the left ventricular (LV) lead could be recognized by 4DCT.
A 55-year-old man with a recurrent admission for HF indicated for CRT was referred to our hospital. In this patient, the 12-lead electrocardiogram (ECG) showed a relatively narrow QRS complex with a left bundle branch block pattern. An echocardiography demonstrated severe LV dysfunction. Although no dyssynchrony was detected, the LV lead was inserted into the most delayed site based on the 4DCT. Three-month later, the ejection fraction increased and the cardiothoracic ratio obviously shortened.
We experienced a case in which we could evaluate the effective implantation site for the LV lead based on the 4DCT even though the effective site was not detected by echocardiography, and we could implant the LV lead at that effective site. The 4DCT may be useful for implanting LV leads in effective sites.
Four-dimensional computed tomography (4DCT) has been capable not only of planning transcatheter aortic valve intervention but also of evaluating the myocardial displacement over time.
Four-dimensional computed tomography might express the most delayed site in detail.
We experienced a case in which we could evaluate the effective implantation site of the left ventricular lead based on 4DCT.
Introduction
Four-dimensional computed tomography (4DCT) has been used not only for planning transcatheter aortic valve intervention procedures but also for enabling the assessment of myocardial displacement.1,2 Moreover, 4DCT can evaluate myocardial displacement over time. We considered that the most delayed site, which would be the most effective site to implant left ventricular (LV) lead, could be recognized by 4DCT.
Timeline
| Time . | Event . |
|---|---|
| Past 2 years | First admission for heart failure (HF). Treated with intravenous diuretics. |
| Admission | Presented with severe HF |
| Day 1 | Treated with additional intravenous inotropes and diuretics |
| Day 10 | Multi-detector computed tomography was conducted to obtain the anatomical information |
| 4D motion analysis was performed to evaluate the most delayed site | |
| Day 14 | Cardiac resynchronization therapy (CRT) device was implanted |
| One month after implantation | Re-evaluation of left ventricular motion by four-dimensional computed tomography |
| Three months after implantation | Assessment of the effect of the CRT |
| Time . | Event . |
|---|---|
| Past 2 years | First admission for heart failure (HF). Treated with intravenous diuretics. |
| Admission | Presented with severe HF |
| Day 1 | Treated with additional intravenous inotropes and diuretics |
| Day 10 | Multi-detector computed tomography was conducted to obtain the anatomical information |
| 4D motion analysis was performed to evaluate the most delayed site | |
| Day 14 | Cardiac resynchronization therapy (CRT) device was implanted |
| One month after implantation | Re-evaluation of left ventricular motion by four-dimensional computed tomography |
| Three months after implantation | Assessment of the effect of the CRT |
| Time . | Event . |
|---|---|
| Past 2 years | First admission for heart failure (HF). Treated with intravenous diuretics. |
| Admission | Presented with severe HF |
| Day 1 | Treated with additional intravenous inotropes and diuretics |
| Day 10 | Multi-detector computed tomography was conducted to obtain the anatomical information |
| 4D motion analysis was performed to evaluate the most delayed site | |
| Day 14 | Cardiac resynchronization therapy (CRT) device was implanted |
| One month after implantation | Re-evaluation of left ventricular motion by four-dimensional computed tomography |
| Three months after implantation | Assessment of the effect of the CRT |
| Time . | Event . |
|---|---|
| Past 2 years | First admission for heart failure (HF). Treated with intravenous diuretics. |
| Admission | Presented with severe HF |
| Day 1 | Treated with additional intravenous inotropes and diuretics |
| Day 10 | Multi-detector computed tomography was conducted to obtain the anatomical information |
| 4D motion analysis was performed to evaluate the most delayed site | |
| Day 14 | Cardiac resynchronization therapy (CRT) device was implanted |
| One month after implantation | Re-evaluation of left ventricular motion by four-dimensional computed tomography |
| Three months after implantation | Assessment of the effect of the CRT |
Case presentation
A 55-year-old man with chronic hypertension who was diagnosed with heart failure (HF) 2 years previously experienced dyspnoea on exertion and was referred to our hospital for the treatment of HF associated with New York Heart Association (NYHA) Class III. His blood pressure was 168/96 mmHg, heart rate 125 b.p.m., and oxygen saturation 85%. The patient had coarse crackles but no leg oedema. The medications on admission were amlodipine 2.5 mg, olmesartan 20 mg, bisoprolol 2.5 mg, azosemide 60 mg, and spironolactone 25 mg. His dyspnoea disappeared after treatment with intravenous inotropes and diuretics and afterwards, his symptoms stabilized after being treated with azosemide 60 mg in addition to the medications on admission. After treatment, his electrocardiogram (ECG) exhibited a relatively narrow QRS complex (129 ms) with a left bundle branch block (LBBB) (Figure 1A). The LBBB pattern was diagnosed with QRS duration of >120 ms, absence of q waves in leads I, V5, and V6, and QS/rS pattern in lead V1.3 Echocardiography demonstrated severe LV dysfunction [ejection fraction (EF), 27% evaluated by Simpson method). Although LV motion was assessed by echocardiographic speckle-tracking two-dimensional radial strain imaging, no dyssynchrony was detected. He had no significant coronary stenosis, valvular disease, or specific tissue abnormalities. The hypertension and dilated cardiomyopathy, of course, might also have been associated with HF, however, we considered that his HF was mainly derived from LV dysfunction caused by LBBB because no specific tissue abnormalities existed by biopsy. We decided to implant a CRT device. The CRT therapy, in this case, was a Class I recommendation by Japanese Circulation Society 2018 guidelines4 and European Society of Cardiology 2013 guidelines,5 however, it was a Class III recommendation by ESC 2016 guidelines.6 This difference was considered to be due to the smaller heart size in general between Japanese and Europeans.
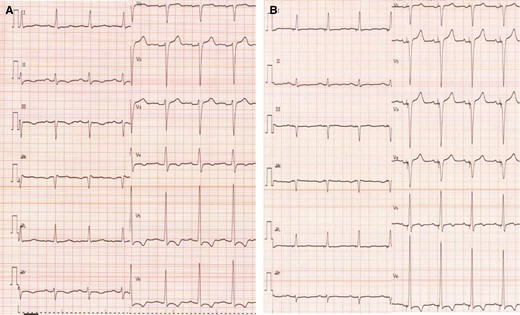
Electrocardiogram before/after cardiac resynchronization therapy implantation. (A) Pre-implantation and (B) post-implantation.
Multi-detector computed tomography (MDCT) scans were conducted to obtain the anatomical information before implantation. The MDCT was performed with a 128-detector CT scanner (Brilliance 64, Philips, Best, The Netherlands) using an axial gating protocol. With 4DCT, dynamic data obtained by MDCT with equally spaced reconstructions from 10 phases by electrocardiographic synchronization was complemented with 30 phases using PhyZiodynamics (PhyZiodynamics, 4D motion analysis; Ziosoft Inc., Tokyo, Japan), and then a 4D motion analysis was performed. In brief, the 4DCT image was what was reconstructed from raw data using a 4D motion analysis after CT scan. In the 4D motion analysis, reference time phase was set as R on ECG and colours were set so that red was displayed as a good dynamic area and the other colours were displayed as low dynamic areas. The mechanism of 4DCT involved considering a site where the voxel of myocardium had moved by more than 3 mm as a good dynamic site. From the above, the area that glowed red at the end was an area that satisfied both the most delayed area and good dynamic area (Figure 2A1–3). In this case, the area glowing red at the end was at the middle portion of posterolateral site. In other words, that area was considered to be the most effective site for LV lead implantation (Figure 2A3).
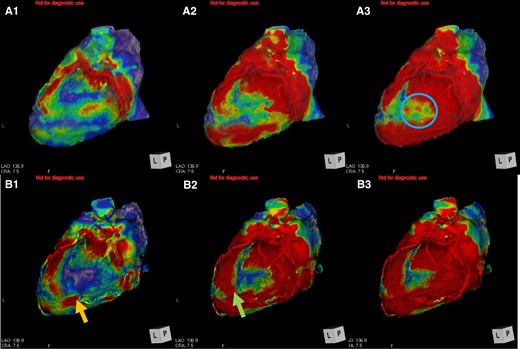
The moving pattern observed by four-dimensional computed tomography. (A) Pre-implantation. The red site gradually shifts over time (A1→A3). The area glowing at the end was in the middle of lateral left ventricular (A3, blue circle). (B) Post-implantation. The red site shifts gradually over time (B1→B3). B1: the left ventricular pacing site moved along with movement pattern of A1 (B1, orange arrow). B2: The more distal area of left ventricular pacing site moved (B2, green arrow).
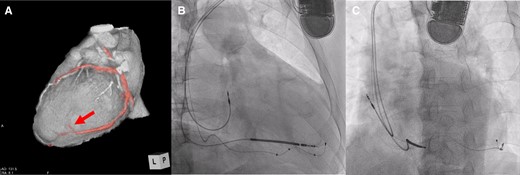
Cardiac resynchronization therapy implantation. (A) The left ventricular lead was implanted at the most delayed site evaluated by four-dimensional computed tomography (red arrow). (B and C) Fluoroscopic view (right anterior oblique 30° and left anterior oblique 45°).
The posterolateral vein bifurcated around the middle portion of posterolateral site and then was directed anteriorly and to apex (Figure 3A). In this case, the most delayed site evaluated by 4DCT was located at the end of posterolateral vein, which was directed anteriorly. The LV lead was inserted into the most delayed site (Figure 3B and C). The QLV timing was 115 ms, 102 ms, 105 ms, and 113 ms, respectively (distal→proximal). Left ventricular pacing was performed with distal electrode. As a result, QRS duration narrowed to 109 ms (Figure 1B). One month later, coincidentally, we re-evaluated the LV movement pattern by 4DCT. The site of LV pacing lead became earlier (Figure 2B1, orange arrow). Subsequently, the delayed site (Figure 2A2) started moving earlier at a more distal part of LV lead before CRT implantation (Figure 2B2, green arrow). The movement in the area glowing at the end (Figure 2A3, blue circle) become earlier (Figure 2B3). The movement pattern changed (Figure 2B1–3). The LVEF increased (27%→35%) and the cardiothoracic ratio had shortened (61%→50%) (Figure 4A and B). The CRT implantation improved symptoms from NYHA Class III to I. Further, LVEF increased to 38% by 3 months after implantation, and thus we considered that the improvement was maintained.
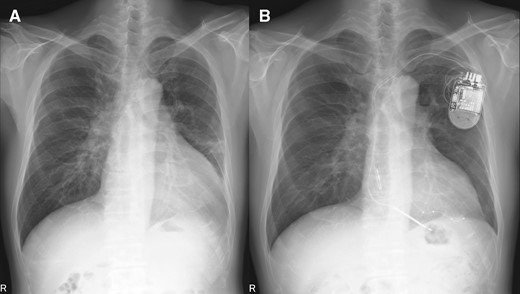
Chest X-ray before/after cardiac resynchronization therapy implantation. (A) pre-implantation and (B) post-implantation.
Discussion
The previous studies suggested that LV leads placed in apical or anterior position are associated with worse outcomes.7,8 Moreover, it has also been reported that better outcomes are demonstrated in patients with LV lead concordant with the latest site using echocardiography.9 Although MDCT has the ability to provide detailed anatomical information,10 there have been few reports on CRT effects. Recently, it has been reported that anatomy, scar area, latest site, and dyssynchrony evaluated by cardiac magnetic resonance (CMR) results in an improved CRT response.11 However, CMR has a higher temporal resolution but poorer spatial resolution.12 Echocardiography is a highly user-dependent modality.13 On the other hand, 4DCT imaging provides a higher spatial and temporal resolution compared to the other non-invasive modalities. Further, 4DCT can reveal not only anatomy but also movement pattern.
In this case, the CRT may have been ineffective from the viewpoint of echocardiography and ECG.14 However, implanting CRT based on 4DCT was effective.
Conclusions
We experienced a case in which we evaluated the effective site using 4DCT and in which CRT was effective owing to LV lead having been implanted at that site. The 4DCT may be useful for implanting LV leads at effective sites.
Lead author biography
Dr Akinori Matsumoto is a cardiologist in Akashi Medical Center, Hyogo, Japan. He graduated from the Cardiovascular Internal Medicine, Kobe University of Japan, in 2016 and obtained the PhD at Kobe University Graduate School of Medicine. He completed training in cardiology and specializes in arrhythmia in Kobe, Japan.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
Group JCSJW. 2018 JCS/JHRS Guideline on Non-Pharmacotherapy of Cardiac Arrhythmias. http://www.j-circ.or.jp/guideline/pdf/JCS2018_kurita_nogami.pdf(7 October 2019).
European Society of Cardiology, European Heart Rhythm Association,





Comments