-
PDF
- Split View
-
Views
-
Cite
Cite
Mario Meier, Jasper Boeddinghaus, Thomas Nestelberger, Luca Koechlin, Pedro Lopez-Ayala, Desiree Wussler, Joan Elias Walter, Tobias Zimmermann, Patrick Badertscher, Karin Wildi, Maria Rubini Giménez, Christian Puelacher, Noemi Glarner, Jan Magni, Òscar Miró, Francisco Javier Martin-Sanchez, Damian Kawecki, Dagmar I Keller, Danielle M Gualandro, Raphael Twerenbold, Christian H Nickel, Roland Bingisser, Christian Mueller, for the APACE investigators, Comparing the utility of clinical risk scores and integrated clinical judgement in patients with suspected acute coronary syndrome, European Heart Journal. Acute Cardiovascular Care, Volume 12, Issue 10, October 2023, Pages 693–702, https://doi.org/10.1093/ehjacc/zuad081
Close - Share Icon Share
Abstract
The utility of clinical risk scores regarding the prediction of major adverse cardiac events (MACE) is uncertain. We aimed to directly compare the prognostic performance of five established clinical risk scores as well as an unstructured integrated clinical judgement (ICJ) of the treating emergency department (ED) physician.
Thirty-day MACE including all-cause death, life-threatening arrhythmia, cardiogenic shock, acute myocardial infarction (including the index event), and unstable angina requiring urgent coronary revascularization were centrally adjudicated by two independent cardiologists in patients presenting to the ED with acute chest discomfort in an international multicentre study. We compared the prognostic performance of the HEART score, GRACE score, T-MACS, TIMI score, and EDACS, as well as the unstructured ICJ of the treating ED physician (visual analogue scale to estimate the probability of acute coronary syndrome, ranging from 0 to 100). Among 4551 eligible patients, 1110/4551 patients (24.4%) had at least one MACE within 30 days. Prognostic accuracy was high and comparable for the HEART score, GRACE score, T-MACS, and ICJ [area under the receiver operating characteristic curve (AUC) 0.85–0.87] but significantly lower and only moderate for the TIMI score (AUC 0.79, P < 0.001) and EDACS (AUC 0.74, P < 0.001), resulting in sensitivities for the rule-out of 30-day MACE of 93–96, 87 (P < 0.001), and 72% (P < 0.001), respectively.
The HEART score, GRACE score, T-MACS, and unstructured ICJ of the treating physician, not the TIMI score or EDACS, performed well for the prediction of 30-day MACE and may be considered for routine clinical use.
ClinicalTrials.gov number NCT00470587
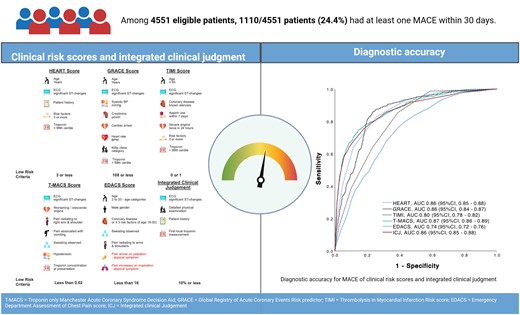
Comparing the utility of clinical risk scores and integrated clinical judgement in patients with suspected acute coronary syndrome. T-MACS, Troponin only Manchester Acute Coronary Syndrome Decision Aid; GRACE, Global Registry of Acute Coronary Events Risk predictor; TIMI, Thrombolysis in Myocardial Infarction; EDACS, Emergency Department Assessment of Chest Pain Score; ICJ, integrated clinical judgement.
In line with the Journal's conflict of interest policy, this paper was handled by Borja Ibanez.
Introduction
Chest pain is one of the most common complaints among patients presenting to the emergency department (ED), accounting for ∼15 million visits per year in Europe and North America.1–3 Among patients presenting with acute chest pain, early and accurate risk stratification in patients with acute coronary syndrome (ACS) in general, and acute myocardial infarction (AMI) in particular, is of critical importance.4–9 At a time when only cardiac troponin (cTn) assays with poor sensitivity were available, clinical risk scores were developed to aid clinicians in formal risk stratification, e.g. in the selection of low-risk patients eligible for discharge from the ED and outpatient management.10–15 Some clinical risk scores were developed specifically for use in patients presenting with acute chest pain to the ED such as the History, ECG, Age, Risk factors, and Troponin (HEART) score, the Troponin only Manchester Acute Coronary Syndrome Decision Aid (T-MACS), and the Emergency Department Assessment of Chest Pain Score (EDACS), while others were derived from patients with documented ACS such as the Global Registry of Acute Coronary Events Risk predictor (GRACE) and the Thrombolysis in Myocardial Infarction (TIMI) risk score.10–15
Due to ongoing controversies regarding the benefit of formal risk stratification, the complexity and inconvenience of using many of these scores, as well as uncertainty about which of the scores provide the best performance, substantial heterogeneity exists regarding their use from country to country and from institution to institution.3,16,17 The controversy regarding clinical risk scores was intensified by the clinical introduction of high-sensitivity cTn (hs-cTn) assays and hs-cTn-based rapid triage algorithms, which allow identifying low-risk patients even without the additional use of formal clinical risk scores.18,19
We therefore aimed to compare the performance of five established clinical risk scores as well as the informal integrated clinical judgement (ICJ) of the treating ED physician within a large multicentre study.
Methods
Study design and population
This was a secondary analysis from a prospective international multicentre study including 12 centres in five countries aiming to advance the early diagnosis of AMI (ClinicalTrials.gov registry number NCT0047058720–22). Adult patients presenting to the ED with acute chest discomfort were recruited. While enrolment was independent of renal function, we excluded patients with terminal kidney failure on chronic dialysis. For this analysis, patients in whom the diagnosis remained unknown even after final adjudication and who had at least one elevated hs-cTn concentration, possibly indicating AMI, patients with chest pain onset/maximum >12 h, and patients with missing components of the scores were excluded (Figure 1).
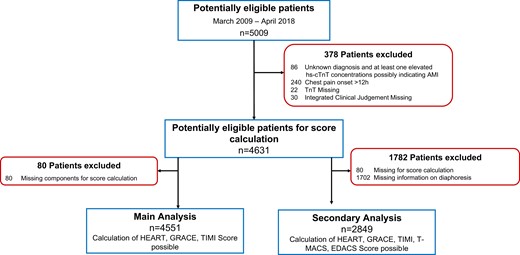
Patient flow. hs-cTnT, high-sensitivity cardiac troponin T; cTn, cardiac troponin; TIMI, Thrombolysis in Myocardial Infarction; T-MACS, Troponin only Manchester Acute Coronary Syndrome Decision Aid; EDACS, Emergency Department Assessment of Chest Pain Score; HEART, History, ECG, Age, Risk factors, and Troponin; GRACE, Global Registry of Acute Coronary Events Risk predictor.
The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees. Written informed consent was obtained from all patients. The authors designed the study, gathered and analysed the data according to the STARD guideline s23 for studies of diagnostic accuracy (see Supplementary material online, Table S1), vouch for the data and analysis, wrote the paper, and decided to submit the paper for publication.
Routine clinical assessment
Patients underwent standardized clinical assessment that included medical history, physical examination, standard blood tests including serial measurements of local (h)s-cTn, 12-lead electrocardiography (ECG), chest radiography, continuous ECG rhythm monitoring, and pulse oximetry. Management of patients was left to the discretion of the attending physician. None of the recruiting sites used a formal clinical risk score as part of the standard of care.
Follow-up and central adjudication of major adverse cardiac events
Patients were contacted at 3, 12, and 24 months after discharge by telephone calls or in written form. We obtained information regarding death during follow-up from the patient’s hospital records, the family physician’s records, and the national death registry.
Two independent cardiologists performed the central adjudication of major adverse cardiac events (MACE) including all-cause death, cardiac arrest, ventricular tachyarrhythmia, higher-degree atrioventricular block, cardiogenic shock, AMI including the index event, and unstable angina requiring urgent coronary revascularization. Acute myocardial infarction was adjudicated according to the universal definition of AMI and current guidelines (see Supplementary material online).24 The primary prognostic endpoint was the endpoint for which most of the clinical risk scores were optimized to ensure fair and unbiased comparison: the composite of 30-day MACE; the secondary prognostic endpoint was 720-day MACE. The primary diagnostic endpoint was index AMI. In a sensitivity analysis, we have assessed 30-day MACE without index AMI. Central adjudication used two sets of data: first, all available medical records obtained during clinical care including history, physical examination, results of laboratory testing including serial clinical (h)s-cTn levels, radiologic testing, ECG, echocardiography, cardiac exercise testing, lesion severity and morphology in coronary angiography, and cardiac magnetic resonance imaging—pertaining to the patient from the time of ED presentation to 720-day follow-up; and second, study-specific assessments including detailed chest pain characteristics using 34 predefined criteria, serial hs-cTnT blood concentrations obtained from study samples, and clinical follow-up by telephone and/or mail. In situations of disagreement about the diagnosis, cases were reviewed and adjudicated in conjunction with a third cardiologist.
Clinical risk scores
The clinical risk scores were applied as recommended (Figure 2; for details, see Supplementary material online).10–15 In brief, the HEART score included medical history (symptoms), ECG, age, cardiovascular risk factors, and hs-cTn, each assigned 0–2 points according to the magnitude of the corresponding risk. Patients were classified as high risk (≥7 points), intermediate risk (4–6 points), or low risk (0–3 points). The GRACE score included age, heart rate, systolic blood pressure, serum creatinine, cardiac arrest, ST-segment deviation in the ECG, elevated hs-cTn, and Killip class. Patients were classified as low risk (1–108), intermediate risk (109–140), or high risk (141–372). The TIMI risk score included age, cardiovascular risk factors, known coronary artery disease (CAD), current aspirin use, severe angina, ST-segment changes, and hs-cTn concentrations at presentation. Patients were classified as high risk (≥2 points) and low risk (0–1 points). The T-MACS included signs of ischaemia in the ECG (ST-segment depression, ST-segment elevation, or Q-wave inversion), worsening or crescendo angina, pain radiating to the right arm or shoulder, pain associated with vomiting, diaphoresis observed, hypotension (systolic blood pressure < 100 mmHg), and hs-cTnT concentration on arrival to the ED. Patients were classified as high risk (≥0.95), moderate risk (≥0.05–<0.95), low risk (≥0.02–<0.05), and very low risk (<0.02). The EDACS included age, gender, known CAD or ≥3 cardiovascular risk factors, diaphoresis, pain radiation to the shoulder/arm/neck or jaw, pain in association with breathing, and pain reproducible by palpation. Patients were classified as high risk (≥16 points) and low risk (<16 points).
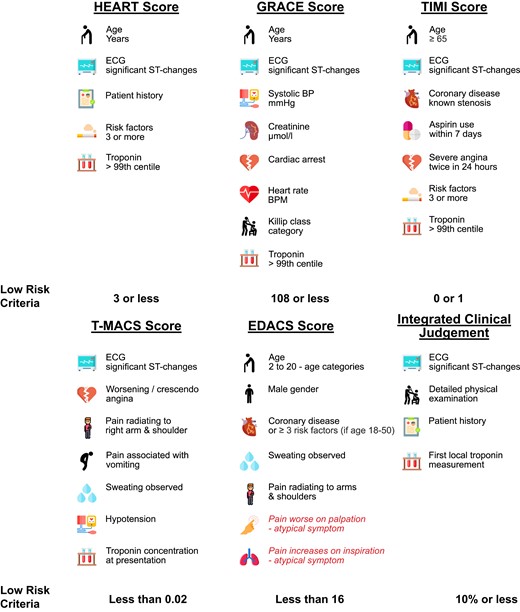
Overview of the scores and the corresponding low-risk criteria. ECG, electrocardiogram; BP, blood pressure; BPM, beats per minute. Variables in red deduct points from the score.
Integrated clinical judgement
The ICJ for the likelihood that ACS is the cause of acute chest discomfort was quantified by the treating ED physician (mostly residents) prospectively at the bed site in the ED using a visual analogue scale ranging from 0 to 100% at 90 min after the patient’s presentation to the ED. At this time, the ED physician had obtained the patient history, physical examination including vital signs, the 12-lead ECG at presentation, and the first local (h)s-cTn measurement. Patients were classified as low risk (≤10%), intermediate risk (11–79%), and high risk (≥80%). Emergency department physicians in participating centres did not receive any study-specific training to best reflect routine clinical patient care.
Statistical analysis
The prognostic accuracy and diagnostic accuracy of clinical risk scores and ICJ were quantified by the area under the receiver operating characteristic curve (AUC). In the main analysis, we compared the three scores not containing diaphoresis (HEART, GRACE, and TIMI) among each other and with ICJ, as diaphoresis was prospectively recorded only from April 2010. Variables, chest pressure, nausea, angina, pain on inspiration, body mass index (BMI), pulmonary auscultation, course of pain intensity, systolic blood pressure, diastolic blood pressure, jugular vein status, previous angina, creatinine, heart rate, and active smoker, were imputed. Detailed information is given in Supplementary material online. In a secondary analysis, we compared ICJ to all five risk scores among each other and with ICJ. The AUCs were compared as recommended by DeLong et al.25 Safety was quantified by the negative predictive value (NPV) and the sensitivity for MACE at 30 days, index AMI, or MACE without index AMI among those patients eligible for rule-out by each score. The efficacy was the percentage of patients classified as low risk. In a secondary analysis, the performance of ICJ was compared in patients in whom hs-cTn was used clinically vs. patients in whom conventional cTn was used clinically to verify the hypothesis that the use of hs-cTn would result in an improvement of the accuracy of ICJ over ICJ incorporating conventional cTn. The incidence of MACE over 2 years was plotted using Kaplan–Meier curves, and the log-rank test was used to assess differences in survival between groups. Continuous variables are described as median with interquartile range (IQR) and categorical variables by counts and percentages. Differences in baseline characteristics between patients with and without MACE as well as between patients with and without AMI were assessed using the Mann–Whitney U test for continuous variables and the Pearson χ2 test or Fisher’s exact test for categorical variables, as appropriate.
All hypothesis testing was two-tailed, and P-values < 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics for MAC, version 28.0 (SPSS Inc., Chicago, IL, USA) and MedCalc 17.6 (MedCalc Software, Ostend, Belgium).
Results
Study population
From January 2009 to April 2018, 5009 patients were enrolled, of which 4551 were eligible for the main analysis and 2849 patients for the secondary analysis with calculation of all five risk scores (Figure 1). Baseline characteristics of all patients for the main analysis according to the primary prognostic endpoint are shown in Table 1 (see Supplementary material online, Table S2 for the secondary endpoint). Median age was 60 years (IQR, 48–73), and 34% of patients were women. Median follow-up was 788 days (IQR, 735–1059), and 99.3 and 94.3% of patients had complete follow-up at 30 days and 2 years, respectively. Overall, 1110 patients (24.4%) had at least one MACE within 30 days, including 34 deaths, 12 life-threatening arrhythmias, 25 cardiogenic shocks, 966 AMIs (including the index event), and 632 unstable angina requiring urgent coronary revascularization.
| . | All patients n = 4551 . | No 30-day MACE n = 3441 . | 30-day MACE n = 1110 . | P-value . |
|---|---|---|---|---|
| Age, years | 60.0 (48.0–73.0) | 58.0 (45.0–71.0) | 68.0 (59.0–78.0) | <0.001 |
| Women, n (%) | 1543 (34) | 1228 (36) | 315 (28) | <0.001 |
| Vital signs | ||||
| Heart rate, beats per minute | 76.0 (66.0–89.0) | 76.0 (67.0–89.0) | 76.0 (66.0–90.0) | 0.854 |
| Systolic blood pressure, mmHg | 139.0 (125.0–155.0) | 138.0 (124.0–154.0) | 142.0 (126.0–160.0) | <0.001 |
| Diastolic blood pressure, mmHg | 80.0 (70.0–90.0) | 80.0 (71.0–90.0) | 80.0 (70.0–90.0) | 0.750 |
| Risk factors | ||||
| Hypertension, n (%) | 2656 (58) | 1817 (53) | 839 (76) | <0.001 |
| Hyperlipidaemia, n (%) | 2103 (46) | 1423 (41) | 680 (61) | <0.001 |
| Diabetes mellitus, n (%) | 788 (17) | 495 (14) | 293 (26) | <0.001 |
| Active smoker, n (%) | 1137 (25) | 878 (26) | 259 (23) | 0.144 |
| Past smoker, n (%) | 1691 (37) | 1229 (36) | 462 (42) | <0.001 |
| History of | ||||
| CAD, n (%) | 1408 (31) | 934 (27) | 474 (43) | <0.001 |
| MI, n (%) | 1022 (23) | 672 (20) | 350 (32) | <0.001 |
| PCI, n (%) | 1088 (24) | 726 (21) | 362 (33) | <0.001 |
| Stroke, n (%) | 234 (5) | 155 (5) | 79 (7) | <0.001 |
| Family history of CAD, n (%) | 1428 (31) | 1050 (31) | 378 (34) | 0.027 |
| Pulmonary embolism, n (%) | 102 (2) | 75 (2) | 27 (2) | 0.621 |
| PAD, n (%) | 232 (5) | 120 (4) | 112 (10) | <0.001 |
| BMI, kg/m2 | 26.4 (23.8–29.6) | 26.3 (23.6–29.4) | 26.8 (24.4–30.1) | 0.002 |
| Medication on admission | ||||
| Aspirin, n (%) | 1576 (35) | 1044 (30) | 532 (48) | <0.001 |
| Phenprocoumon, n (%) | 455 (10) | 340 (10) | 115 (10) | 0.643 |
| Beta-blocker, n (%) | 1478 (33) | 1015 (30) | 463 (42) | <0.001 |
| Calcium antagonist, n (%) | 688 (15) | 447 (13) | 241 (22) | <0.001 |
| Nitroglycerine, n (%) | 407 (9) | 258 (8) | 149 (13) | <0.001 |
| Statin, n (%) | 1567 (34) | 1059 (31) | 508 (46) | <0.001 |
| ACEi or ARB, n (%) | 1777 (39) | 1188 (35) | 589 (53) | <0.001 |
| ECG findings | ||||
| ST-segment elevation, n (%) | 406 (9) | 117 (3) | 289 (26) | <0.001 |
| ST-segment depression, n (%) | 195 (4) | 71 (2) | 124 (11) | <0.001 |
| T-wave inversion, n (%) | 570 (13) | 294 (9) | 276 (25) | <0.001 |
| Laboratory | ||||
| eGFR, mL/min/1.732 | 87.1 (70.0–101.1) | 90.1 (74.3–103.5) | 77.8 (60.4–92.3) | <0.001 |
| . | All patients n = 4551 . | No 30-day MACE n = 3441 . | 30-day MACE n = 1110 . | P-value . |
|---|---|---|---|---|
| Age, years | 60.0 (48.0–73.0) | 58.0 (45.0–71.0) | 68.0 (59.0–78.0) | <0.001 |
| Women, n (%) | 1543 (34) | 1228 (36) | 315 (28) | <0.001 |
| Vital signs | ||||
| Heart rate, beats per minute | 76.0 (66.0–89.0) | 76.0 (67.0–89.0) | 76.0 (66.0–90.0) | 0.854 |
| Systolic blood pressure, mmHg | 139.0 (125.0–155.0) | 138.0 (124.0–154.0) | 142.0 (126.0–160.0) | <0.001 |
| Diastolic blood pressure, mmHg | 80.0 (70.0–90.0) | 80.0 (71.0–90.0) | 80.0 (70.0–90.0) | 0.750 |
| Risk factors | ||||
| Hypertension, n (%) | 2656 (58) | 1817 (53) | 839 (76) | <0.001 |
| Hyperlipidaemia, n (%) | 2103 (46) | 1423 (41) | 680 (61) | <0.001 |
| Diabetes mellitus, n (%) | 788 (17) | 495 (14) | 293 (26) | <0.001 |
| Active smoker, n (%) | 1137 (25) | 878 (26) | 259 (23) | 0.144 |
| Past smoker, n (%) | 1691 (37) | 1229 (36) | 462 (42) | <0.001 |
| History of | ||||
| CAD, n (%) | 1408 (31) | 934 (27) | 474 (43) | <0.001 |
| MI, n (%) | 1022 (23) | 672 (20) | 350 (32) | <0.001 |
| PCI, n (%) | 1088 (24) | 726 (21) | 362 (33) | <0.001 |
| Stroke, n (%) | 234 (5) | 155 (5) | 79 (7) | <0.001 |
| Family history of CAD, n (%) | 1428 (31) | 1050 (31) | 378 (34) | 0.027 |
| Pulmonary embolism, n (%) | 102 (2) | 75 (2) | 27 (2) | 0.621 |
| PAD, n (%) | 232 (5) | 120 (4) | 112 (10) | <0.001 |
| BMI, kg/m2 | 26.4 (23.8–29.6) | 26.3 (23.6–29.4) | 26.8 (24.4–30.1) | 0.002 |
| Medication on admission | ||||
| Aspirin, n (%) | 1576 (35) | 1044 (30) | 532 (48) | <0.001 |
| Phenprocoumon, n (%) | 455 (10) | 340 (10) | 115 (10) | 0.643 |
| Beta-blocker, n (%) | 1478 (33) | 1015 (30) | 463 (42) | <0.001 |
| Calcium antagonist, n (%) | 688 (15) | 447 (13) | 241 (22) | <0.001 |
| Nitroglycerine, n (%) | 407 (9) | 258 (8) | 149 (13) | <0.001 |
| Statin, n (%) | 1567 (34) | 1059 (31) | 508 (46) | <0.001 |
| ACEi or ARB, n (%) | 1777 (39) | 1188 (35) | 589 (53) | <0.001 |
| ECG findings | ||||
| ST-segment elevation, n (%) | 406 (9) | 117 (3) | 289 (26) | <0.001 |
| ST-segment depression, n (%) | 195 (4) | 71 (2) | 124 (11) | <0.001 |
| T-wave inversion, n (%) | 570 (13) | 294 (9) | 276 (25) | <0.001 |
| Laboratory | ||||
| eGFR, mL/min/1.732 | 87.1 (70.0–101.1) | 90.1 (74.3–103.5) | 77.8 (60.4–92.3) | <0.001 |
Numbers are presented as median (IQR) or numbers (%). MACE, major adverse cardiac events; CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; PAD, peripheral artery disease; BMI, body mass index; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate.
| . | All patients n = 4551 . | No 30-day MACE n = 3441 . | 30-day MACE n = 1110 . | P-value . |
|---|---|---|---|---|
| Age, years | 60.0 (48.0–73.0) | 58.0 (45.0–71.0) | 68.0 (59.0–78.0) | <0.001 |
| Women, n (%) | 1543 (34) | 1228 (36) | 315 (28) | <0.001 |
| Vital signs | ||||
| Heart rate, beats per minute | 76.0 (66.0–89.0) | 76.0 (67.0–89.0) | 76.0 (66.0–90.0) | 0.854 |
| Systolic blood pressure, mmHg | 139.0 (125.0–155.0) | 138.0 (124.0–154.0) | 142.0 (126.0–160.0) | <0.001 |
| Diastolic blood pressure, mmHg | 80.0 (70.0–90.0) | 80.0 (71.0–90.0) | 80.0 (70.0–90.0) | 0.750 |
| Risk factors | ||||
| Hypertension, n (%) | 2656 (58) | 1817 (53) | 839 (76) | <0.001 |
| Hyperlipidaemia, n (%) | 2103 (46) | 1423 (41) | 680 (61) | <0.001 |
| Diabetes mellitus, n (%) | 788 (17) | 495 (14) | 293 (26) | <0.001 |
| Active smoker, n (%) | 1137 (25) | 878 (26) | 259 (23) | 0.144 |
| Past smoker, n (%) | 1691 (37) | 1229 (36) | 462 (42) | <0.001 |
| History of | ||||
| CAD, n (%) | 1408 (31) | 934 (27) | 474 (43) | <0.001 |
| MI, n (%) | 1022 (23) | 672 (20) | 350 (32) | <0.001 |
| PCI, n (%) | 1088 (24) | 726 (21) | 362 (33) | <0.001 |
| Stroke, n (%) | 234 (5) | 155 (5) | 79 (7) | <0.001 |
| Family history of CAD, n (%) | 1428 (31) | 1050 (31) | 378 (34) | 0.027 |
| Pulmonary embolism, n (%) | 102 (2) | 75 (2) | 27 (2) | 0.621 |
| PAD, n (%) | 232 (5) | 120 (4) | 112 (10) | <0.001 |
| BMI, kg/m2 | 26.4 (23.8–29.6) | 26.3 (23.6–29.4) | 26.8 (24.4–30.1) | 0.002 |
| Medication on admission | ||||
| Aspirin, n (%) | 1576 (35) | 1044 (30) | 532 (48) | <0.001 |
| Phenprocoumon, n (%) | 455 (10) | 340 (10) | 115 (10) | 0.643 |
| Beta-blocker, n (%) | 1478 (33) | 1015 (30) | 463 (42) | <0.001 |
| Calcium antagonist, n (%) | 688 (15) | 447 (13) | 241 (22) | <0.001 |
| Nitroglycerine, n (%) | 407 (9) | 258 (8) | 149 (13) | <0.001 |
| Statin, n (%) | 1567 (34) | 1059 (31) | 508 (46) | <0.001 |
| ACEi or ARB, n (%) | 1777 (39) | 1188 (35) | 589 (53) | <0.001 |
| ECG findings | ||||
| ST-segment elevation, n (%) | 406 (9) | 117 (3) | 289 (26) | <0.001 |
| ST-segment depression, n (%) | 195 (4) | 71 (2) | 124 (11) | <0.001 |
| T-wave inversion, n (%) | 570 (13) | 294 (9) | 276 (25) | <0.001 |
| Laboratory | ||||
| eGFR, mL/min/1.732 | 87.1 (70.0–101.1) | 90.1 (74.3–103.5) | 77.8 (60.4–92.3) | <0.001 |
| . | All patients n = 4551 . | No 30-day MACE n = 3441 . | 30-day MACE n = 1110 . | P-value . |
|---|---|---|---|---|
| Age, years | 60.0 (48.0–73.0) | 58.0 (45.0–71.0) | 68.0 (59.0–78.0) | <0.001 |
| Women, n (%) | 1543 (34) | 1228 (36) | 315 (28) | <0.001 |
| Vital signs | ||||
| Heart rate, beats per minute | 76.0 (66.0–89.0) | 76.0 (67.0–89.0) | 76.0 (66.0–90.0) | 0.854 |
| Systolic blood pressure, mmHg | 139.0 (125.0–155.0) | 138.0 (124.0–154.0) | 142.0 (126.0–160.0) | <0.001 |
| Diastolic blood pressure, mmHg | 80.0 (70.0–90.0) | 80.0 (71.0–90.0) | 80.0 (70.0–90.0) | 0.750 |
| Risk factors | ||||
| Hypertension, n (%) | 2656 (58) | 1817 (53) | 839 (76) | <0.001 |
| Hyperlipidaemia, n (%) | 2103 (46) | 1423 (41) | 680 (61) | <0.001 |
| Diabetes mellitus, n (%) | 788 (17) | 495 (14) | 293 (26) | <0.001 |
| Active smoker, n (%) | 1137 (25) | 878 (26) | 259 (23) | 0.144 |
| Past smoker, n (%) | 1691 (37) | 1229 (36) | 462 (42) | <0.001 |
| History of | ||||
| CAD, n (%) | 1408 (31) | 934 (27) | 474 (43) | <0.001 |
| MI, n (%) | 1022 (23) | 672 (20) | 350 (32) | <0.001 |
| PCI, n (%) | 1088 (24) | 726 (21) | 362 (33) | <0.001 |
| Stroke, n (%) | 234 (5) | 155 (5) | 79 (7) | <0.001 |
| Family history of CAD, n (%) | 1428 (31) | 1050 (31) | 378 (34) | 0.027 |
| Pulmonary embolism, n (%) | 102 (2) | 75 (2) | 27 (2) | 0.621 |
| PAD, n (%) | 232 (5) | 120 (4) | 112 (10) | <0.001 |
| BMI, kg/m2 | 26.4 (23.8–29.6) | 26.3 (23.6–29.4) | 26.8 (24.4–30.1) | 0.002 |
| Medication on admission | ||||
| Aspirin, n (%) | 1576 (35) | 1044 (30) | 532 (48) | <0.001 |
| Phenprocoumon, n (%) | 455 (10) | 340 (10) | 115 (10) | 0.643 |
| Beta-blocker, n (%) | 1478 (33) | 1015 (30) | 463 (42) | <0.001 |
| Calcium antagonist, n (%) | 688 (15) | 447 (13) | 241 (22) | <0.001 |
| Nitroglycerine, n (%) | 407 (9) | 258 (8) | 149 (13) | <0.001 |
| Statin, n (%) | 1567 (34) | 1059 (31) | 508 (46) | <0.001 |
| ACEi or ARB, n (%) | 1777 (39) | 1188 (35) | 589 (53) | <0.001 |
| ECG findings | ||||
| ST-segment elevation, n (%) | 406 (9) | 117 (3) | 289 (26) | <0.001 |
| ST-segment depression, n (%) | 195 (4) | 71 (2) | 124 (11) | <0.001 |
| T-wave inversion, n (%) | 570 (13) | 294 (9) | 276 (25) | <0.001 |
| Laboratory | ||||
| eGFR, mL/min/1.732 | 87.1 (70.0–101.1) | 90.1 (74.3–103.5) | 77.8 (60.4–92.3) | <0.001 |
Numbers are presented as median (IQR) or numbers (%). MACE, major adverse cardiac events; CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; PAD, peripheral artery disease; BMI, body mass index; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate.
Main analysis: diagnostic performance of clinical risk scores and integrated clinical judgement for the rule-out of MACE at 30 days and index AMI
| HEART–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1624 | 1034 | 93.2 (91.5–94.5) | 96.0 (95.0–96.8) |
| Rule-Out + | 1817 | 76 | ||
| HEART–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1745 | 913 | 94.5 (92.9–95.8) | 97.2 (96.4–97.9) |
| Rule-Out + | 1840 | 53 | ||
| GRACE–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1978 | 1064 | 95.9 (94.5–96.9) | 97.0 (96.0–97.7) |
| Rule-Out + | 1463 | 46 | ||
| GRACE–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1506 | 954 | 98.4 (97.5–99.1) | 99.0 (98.4–99.4) |
| Rule-Out + | 2105 | 15 | ||
| TIMI–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1433 | 961 | 86.6 (84.4–88.5) | 93.1 (91.9–94.1) |
| Rule-Out + | 2008 | 149 | ||
| TIMI–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1545 | 849 | 87.9 (85.7–89.8) | 94.6 (93.5–95.5) |
| Rule-Out + | 2040 | 117 | ||
| ICJ–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1775 | 1033 | 93.1 (91.4–94.4) | 95.6 (94.5–96.5) |
| Rule-Out + | 1666 | 77 | ||
| ICJ–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1900 | 908 | 94.0 (92.3–95.3) | 96.7 (95.7–97.4) |
| Rule-Out + | 1685 | 58 |
| HEART–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1624 | 1034 | 93.2 (91.5–94.5) | 96.0 (95.0–96.8) |
| Rule-Out + | 1817 | 76 | ||
| HEART–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1745 | 913 | 94.5 (92.9–95.8) | 97.2 (96.4–97.9) |
| Rule-Out + | 1840 | 53 | ||
| GRACE–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1978 | 1064 | 95.9 (94.5–96.9) | 97.0 (96.0–97.7) |
| Rule-Out + | 1463 | 46 | ||
| GRACE–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1506 | 954 | 98.4 (97.5–99.1) | 99.0 (98.4–99.4) |
| Rule-Out + | 2105 | 15 | ||
| TIMI–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1433 | 961 | 86.6 (84.4–88.5) | 93.1 (91.9–94.1) |
| Rule-Out + | 2008 | 149 | ||
| TIMI–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1545 | 849 | 87.9 (85.7–89.8) | 94.6 (93.5–95.5) |
| Rule-Out + | 2040 | 117 | ||
| ICJ–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1775 | 1033 | 93.1 (91.4–94.4) | 95.6 (94.5–96.5) |
| Rule-Out + | 1666 | 77 | ||
| ICJ–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1900 | 908 | 94.0 (92.3–95.3) | 96.7 (95.7–97.4) |
| Rule-Out + | 1685 | 58 |
AMI, acute myocardial infarction; MACE, major adverse cardiac events; NPV, negative predictive value; CI, confidence interval; HEART, History, ECG, Age, Risk factors, and Troponin; GRACE, Global Registry of Acute Coronary Events Risk predictor; TIMI, Thrombolysis in Myocardial Infarction; ICJ, integrated clinical judgement.
Main analysis: diagnostic performance of clinical risk scores and integrated clinical judgement for the rule-out of MACE at 30 days and index AMI
| HEART–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1624 | 1034 | 93.2 (91.5–94.5) | 96.0 (95.0–96.8) |
| Rule-Out + | 1817 | 76 | ||
| HEART–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1745 | 913 | 94.5 (92.9–95.8) | 97.2 (96.4–97.9) |
| Rule-Out + | 1840 | 53 | ||
| GRACE–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1978 | 1064 | 95.9 (94.5–96.9) | 97.0 (96.0–97.7) |
| Rule-Out + | 1463 | 46 | ||
| GRACE–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1506 | 954 | 98.4 (97.5–99.1) | 99.0 (98.4–99.4) |
| Rule-Out + | 2105 | 15 | ||
| TIMI–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1433 | 961 | 86.6 (84.4–88.5) | 93.1 (91.9–94.1) |
| Rule-Out + | 2008 | 149 | ||
| TIMI–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1545 | 849 | 87.9 (85.7–89.8) | 94.6 (93.5–95.5) |
| Rule-Out + | 2040 | 117 | ||
| ICJ–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1775 | 1033 | 93.1 (91.4–94.4) | 95.6 (94.5–96.5) |
| Rule-Out + | 1666 | 77 | ||
| ICJ–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1900 | 908 | 94.0 (92.3–95.3) | 96.7 (95.7–97.4) |
| Rule-Out + | 1685 | 58 |
| HEART–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1624 | 1034 | 93.2 (91.5–94.5) | 96.0 (95.0–96.8) |
| Rule-Out + | 1817 | 76 | ||
| HEART–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1745 | 913 | 94.5 (92.9–95.8) | 97.2 (96.4–97.9) |
| Rule-Out + | 1840 | 53 | ||
| GRACE–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1978 | 1064 | 95.9 (94.5–96.9) | 97.0 (96.0–97.7) |
| Rule-Out + | 1463 | 46 | ||
| GRACE–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1506 | 954 | 98.4 (97.5–99.1) | 99.0 (98.4–99.4) |
| Rule-Out + | 2105 | 15 | ||
| TIMI–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1433 | 961 | 86.6 (84.4–88.5) | 93.1 (91.9–94.1) |
| Rule-Out + | 2008 | 149 | ||
| TIMI–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1545 | 849 | 87.9 (85.7–89.8) | 94.6 (93.5–95.5) |
| Rule-Out + | 2040 | 117 | ||
| ICJ–MACE | MACE at 30 days − | MACE at 30 days + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1775 | 1033 | 93.1 (91.4–94.4) | 95.6 (94.5–96.5) |
| Rule-Out + | 1666 | 77 | ||
| ICJ–AMI | Index AMI − | Index AMI + | Sensitivity (95% CI) | NPV (95% CI) |
| Rule-Out − | 1900 | 908 | 94.0 (92.3–95.3) | 96.7 (95.7–97.4) |
| Rule-Out + | 1685 | 58 |
AMI, acute myocardial infarction; MACE, major adverse cardiac events; NPV, negative predictive value; CI, confidence interval; HEART, History, ECG, Age, Risk factors, and Troponin; GRACE, Global Registry of Acute Coronary Events Risk predictor; TIMI, Thrombolysis in Myocardial Infarction; ICJ, integrated clinical judgement.
Main analysis (n = 4551): HEART vs. GRACE vs. TIMI vs. integrated clinical judgement
Prognostic accuracy for 30-day major adverse cardiac events
Prognostic accuracy as quantified by the AUC was 0.85 [95% confidence interval (CI), 0.84–0.87] for the HEART score, 0.85 (95% CI, 0.84–0.87) for the GRACE score, 0.79 (95% CI, 0.77–0.80) for the TIMI score, and 0.87 (95% CI, 0.85–0.88) for the ICJ (Figure 3; Supplementary material online, Table S3A). In Addition we calculated the AUC for MACE without index AMI (Supplementary material online, Figure S6).The prognostic accuracy of the different scores overall was comparable in the countries contributing to this study (see Supplementary material online, Table S4).
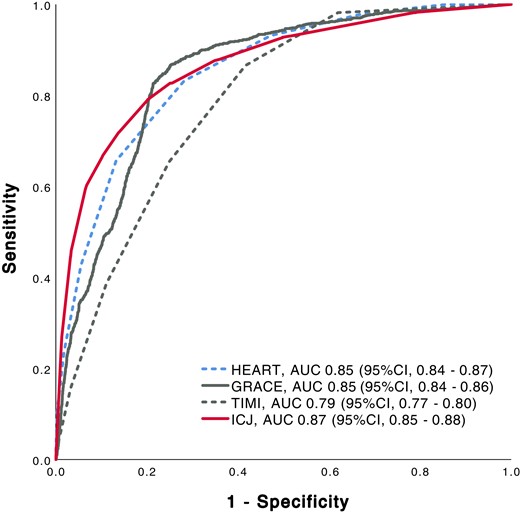
Receiver operating characteristic curve for major adverse cardiac events at 30 days. Prognostic accuracy for major adverse cardiac events at 30 days of clinical risk scores and integrated clinical judgement. HEART, History, ECG, Age, Risk factors, and Troponin; GRACE, Global Registry of Acute Coronary Events Risk predictor; TIMI, Thrombolysis in Myocardial Infarction; ICJ, integrated clinical judgement; AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Identification of patients at low risk
The HEART score identified 1893/4551 (41.6%) patients as low risk, 1992/4551 (43.8%) as intermediate risk, and 666/4551 (14.6%) as high risk (Figure 4A). For the rule-out of MACE at 30 days, the NPV was 96.0% (95% CI, 95.0–96.8) and the sensitivity was 93.2% (95% CI, 91.5–94.5) (Table 2). The GRACE score identified 1542/4551 (33.9%) patients as low risk, 1168/4551 (25.7%) as intermediate risk, and 1841/4551 (40.5%) as high risk (Figure 4B). For the rule-out of MACE at 30 days, the NPV was 97.0% (95% CI, 96.0–97.7) and the sensitivity was 95.9% (95% CI, 94.5–96.9). The TIMI score identified 2157/4551 (47.4%) patients as low risk and 2394/4551 (52.6%) as high risk (Figure 4C). For the rule-out of MACE at 30 days, the NPV was 93.1% (95% CI, 91.9–94.1) and the sensitivity was 86.6% (95% CI, 84.4–88.5). The ICJ identified 1743/4551 (38.3%) patients as low risk, 1926/4551 (42.3%) as intermediate risk, and 882/4551 (19.4%) as high risk (Figure 4D). For the rule-out of MACE at 30 days, the NPV was 95.6% (95% CI, 94.5–96.5) and the sensitivity was 93.1% (95% CI, 91.4–94.4).
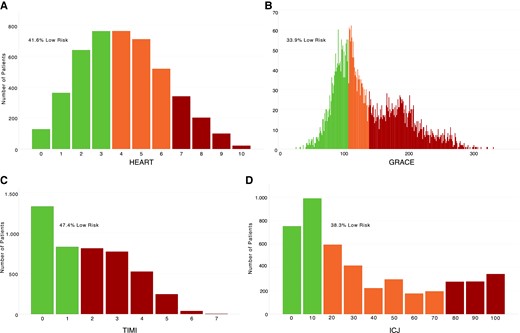
(A, B) Risk classification of patients according to the HEART score and GRACE score. (C, D) Risk classification of patients according to the TIMI risk score and the integrated clinical judgement. HEART, History, ECG, Age, Risk factors, and Troponin; GRACE, Global Registry of Acute Coronary Events Risk predictor; TIMI, Thrombolysis in Myocardial Infarction; ICJ, integrated clinical judgement.
Diagnostic accuracy for index acute myocardial infarction
The adjudicated final diagnosis was AMI in 966/4551 patients (21.2%). The diagnostic accuracy for index AMI was 0.88 (95% CI, 0.86–0.88) for the HEART score, 0.88 (95% CI, 0.86–0.89) for the GRACE score, 0.79 (95% CI, 0.77–0.80) for the TIMI score, and 0.87 (95% CI, 0.85–0.88) for the ICJ (see Supplementary material online, Figure S1 and Table S3B).
Long-term follow-up
Median follow-up time was 788 days (IQR, 735–1059) with 267 deaths occurring within 2 years. Triage according to the formal risk scores as well as according to the ICJ was also a strong predictor for long-term survival (see Supplementary material online, Figure S2A–D).
Accuracy of integrated clinical judgement according to high-sensitivity cardiac troponin
The prognostic accuracy of ICJ for 30-day MACE was significantly higher in patients in whom hs-cTn vs. conventional cTn was used clinically [AUC 0.87 (95% CI, 0.86–0.88) vs. 0.83 (95% CI, 0.81–0.85); P = 0.003; Supplementary material online, Figure S3A]. Also, the diagnostic accuracy of ICJ for index AMI was higher [AUC 0.87 (95% CI, 0.86–0.88)] in patients in whom hs-cTn was used clinically vs. patients in whom conventional cTn was used clinically [0.82 (95% CI, 0.80–0.84); P < 0.001; Supplementary material online, Figure S3B].
Secondary analysis (n = 2849): HEART vs. GRACE vs. TIMI vs. T-MACS vs. EDACS vs. integrated clinical judgement
Prognostic accuracy for 30-day major adverse cardiac events
Prognostic accuracy as quantified by the AUC was 0.86 (95% CI, 0.85–0.88) for the HEART score, 0.86 (95% CI, 0.84–0.87) for the GRACE score, 0.80 (95% CI, 0.78–0.82) for the TIMI score, 0.87 (95% CI, 0.86–0.89) for the T-MACS, 0.74 (95% CI, 0.72–0.76) for the EDACS, and 0.86 (95% CI, 0.85–0.88) for the ICJ (Figure 5). In Addition we calculated the AUC for MACE without index AMI (Supplementary material online, Figure S7).
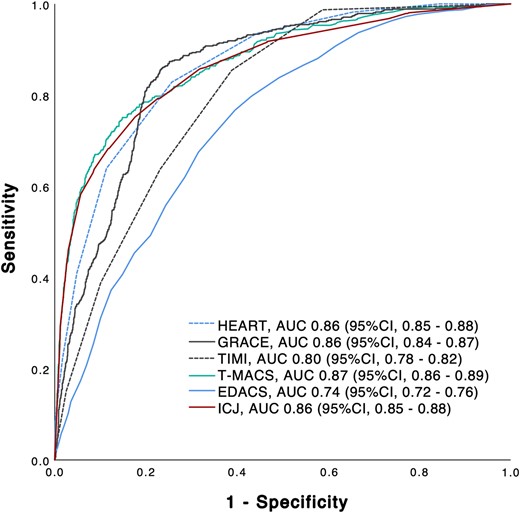
Receiver operating characteristic curve for major adverse cardiac events. Diagnostic accuracy for major adverse cardiac events of clinical risk scores and integrated clinical judgement. T-MACS, Troponin only Manchester Acute Coronary Syndrome Decision Aid; HEART, History, ECG, Age, Risk factors, and Troponin; GRACE, Global Registry of Acute Coronary Events Risk predictor; TIMI, Thrombolysis in Myocardial Infarction; EDACS, Emergency Department Assessment of Chest Pain Score; ICJ, integrated clinical judgement; AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Identification of patients at low risk
The HEART score identified 1281/2849 (45.0%) patients as low risk, 1207/2849 (42.4%) as intermediate risk, and 361/2849 (12.7%) as high risk (see Supplementary material online, Figure S4A). For the rule-out of MACE at 30 days, the NPV was 96.9% (95.8–97.7) and the sensitivity was 93.5% (91.3–95.2). The GRACE score identified 1052/2849 (36.9%) patients as low risk, 741/2849 (26.0%) as intermediate risk, and 1056/2849 (37.1%) as high risk (see Supplementary material online, Figure S4B). For the rule-out of MACE at 30 days, the NPV was 97.3% (96.1–98.1) and the sensitivity was 95.5% (93.5–96.8). The TIMI score identified 1455/2849 (51.1%) patients as low risk and 1394/2849 (48.9%) as high risk (see Supplementary material online, Figure S4C). For the rule-out of MACE at 30 days, the NPV was 94.2% (92.9–95.3) and the sensitivity was 85.4% (82.4–88.0). The T-MACS identified 789/2849 (27.7%) patients as very low risk, 1617/2849 (56.8%) as low risk, 178/2849 (6.2%) as moderate risk, and 265/2849 (9.3%) as high risk (see Supplementary material online, Figure S4D). For the rule-out of MACE at 30 days, the NPV was 97.2% (95.8–98.2) and the sensitivity was 96.4% (94.7–97.6) (see Supplementary material online, Table S5). The EDACS identified 1729/2849 (60.7%) patients as low risk and 1120/2849 (39.3%) as high risk (see Supplementary material online, Figure S4E). For the rule-out of MACE at 30 days, the NPV was 89.2% (87.6–90.7) and the sensitivity was 71.8% (68.2–75.2). The ICJ identified 1202/2849 (42.2%) patients as low risk, 1173/2849 (41.2%) as intermediate risk, and 474/2849 (16.6%) as high risk (see Supplementary material online, Figure S4F). For the rule-out of MACE at 30 days, the NPV was 95.9% (94.7–96.9) and the sensitivity was 92.1% (89.7–94.0). Information about the long term follow-up and the triage according to the formal risk scores as well as according to the ICJ is shown in the Supplemetary material online (see Supplementary material online, Figure S8A–F).
Diagnostic accuracy for index acute myocardial infarction
The adjudicated final diagnosis was AMI in 539/2849 patients (18.9%). The diagnostic accuracy for index AMI was 0.87 (95% CI, 0.86–0.89) for the HEART score, 0.88 (95% CI, 0.87–0.89) for the GRACE score, 0.79 (95% CI, 0.77–0.81) for the TIMI score, 0.91 (95% CI, 0.90–0.93) for the T-MACS, 0.73 (95% CI, 0.71–0.75) for the EDACS, and 0.86 (95% CI, 0.85–0.88) for the ICJ (see Supplementary material online, Figure S5).
Discussion
We performed a large international prospective multicentre study using the central adjudication of 30-day MACE as the primary outcome to directly compare the clinical performance of five well-established clinical risk scores with each other, as well as with the unstructured ICJ of the treating ED physician in patients presenting with acute chest discomfort to the ED. We report six major findings.
First, the prognostic accuracy for 30-day MACE was high and comparable for the HEART score, GRACE score, and T-MACS (AUC 0.85–0.87) while significantly lower and only moderate for the TIMI score and EDACS. Second, accordingly, the safety (NPV) for the identification of low-risk patients eligible for direct discharge from the ED and outpatient management was high if using the HEART score, GRACE score, and T-MACS (sensitivity 93–96%) but significantly lower and only moderate for the TIMI score (sensitivity 85–87%) and EDACS (sensitivity 72%). Third, the simple unstructured ICJ by the treating ED physician, mostly residents, achieved comparable prognostic performance vs. the best formal risk scores for 30-day MACE with and without index AMI and higher AUC and sensitivity vs. the TIMI score and EDACS. Fourth, the clinical risk scores and ICJ also strongly predicted the risk of death within 2 years. Fifth, the prognostic accuracy of ICJ was higher in patients in whom hs-cTn was used clinically vs. patients in whom conventional cTn was used clinically, verifying the hypothesis that the use of hs-cTn increases also the prognostic accuracy of ICJ for 30-day MACE.14,15Sixth, similar findings emerged when assessing the diagnostic accuracy for index AMI.
These findings corroborate and extend previous work on clinical risk scores, e.g. initial observations made in the Netherlands and Singapore that the HEART score had a higher prognostic accuracy for 30-day MACE vs. the TIMI score.26–32 Similarly, our findings regarding the excellent performance of the unstructured clinical impression are supported by pilot data generated in 434 patients presenting to the ED in the USA,33 providing equal performance as compared with the several formal clinical risk scores, and in 255 patients presenting to the ED in the Netherlands, providing equal performance to the HEART score.27
The findings of this multicentre study also corroborate and extend recent insights gained from analyses exploring the incremental value of clinical risk scores to validate rapid hs-cTn-based triage algorithms:18,19 consistently, clinical risk scores did not provide incremental value on top of the ESC hs-cTn-0/1h-algorithm or the high-sensitivity troponin in the evaluation of patients with ACS-0/3h-algorithm in the diagnosis of AMI and only marginally for 30-day MACE. The need to combine one of the formal scores or ICJ with hs-cTn-based rapid algorithms for the early rule-out of Non-ST-segment Elevation Myocardial Infarction (NSTEMI) was also documented by the fact that none of the formal risk scores on its own achieved the sensitivity required by ED physicians for safe discharge.34
It is important to highlight that the use of formal clinical risk scores may also have educational purposes. By forcing the physician to systematically assess established high-risk features, physicians are continuously trained in remembering them. However, as the individual high-risk features selected within any given score are always a suboptimal compromise between trying to reflect all clinically relevant details and still having to limit the number of variables for feasibility, they should never be used as an alternative to the full clinical assessment and the resulting ICJ.35 The ICJ may also have the additional advantage of clinical intuition. Even implicit judgements, such as the physician’s disease severity ratings, have been found to quite accurately predict clinical outcomes including mortality.36 The clinical value of ICJ vs. formal clinical risk scores is further highlighted by the fact that it was obtained from ED residents rather than experienced ED physicians in this study. These data thereby clearly highlight that physicians and institutions may select among several attractive options for the risk stratification of patients presenting with acute chest discomfort to the ED: one of the formal clinical risk scores with high prognostic accuracy including the HEART score, GRACE score, or T-MACS, or unstructured ICJ.
Some limitations need to be considered when interpreting the findings of this study. First, this was a secondary analysis from a large multicentre study. As such, no specific power calculation was performed to justify the sample size for this analysis. However, it is by far the largest study ever performed directly comparing established clinical risk scores, both among each other and with ICJ. It is therefore unlikely that we have missed a clinically relevant difference. Second, this study was conducted in patients presenting with acute chest discomfort to the ED. Further studies are required to directly compare the formal clinical risk scores and ICJ in patients with lower pre-test probability (e.g. in a general practitioner setting). Third, the data presented were obtained from a prospective diagnostic study. Studies applying the ICJ and clinical risk scores prospectively for clinical decision-making are warranted. Fourth, although we used a very stringent methodology to adjudicate MACE including central adjudication by experienced cardiologists, we still may have misclassified a small number of patients. Fifth, we cannot generalize these findings to patients with terminal kidney failure on chronic dialysis since they were excluded from this study.
Conclusions
The HEART score, GRACE score, T-MACS, and unstructured ICJ of the treating physician, not the TIMI score or EDACS, performed well in the prediction of 30-day MACE and may be considered for routine clinical use.
Michael Freese1,2, Paul David Ratmann1,2, Ivo Strebel1,2, Valentina Troester1,2, Benjamin Hafner1,2, Jeanne du Fay de Lavallaz1,2, Petra Hillinger1, Beatriz López2,3, Carolina Fuenzalida2,3, Esther Rodriguez Adrada2,4, Eva Ganovská2,5, Jens Lohrmann1, Michael Christ2,6, Andreas Buser7, Arnold von Eckardstein8, Beata Morawiec2,9, Piotr Muzyk2,9, Franz Bürgler10, Nicolas Geigy10, and Katharina Rentsch11
1Cardiovascular Research Institute Basel (CRIB) and Department of Cardiology, University Hospital Basel, University of Basel, Switzerland; 2GREAT network; 3Emergency Department, Hospital Clinic, Barcelona, Catalonia, Spain; 4Servicio de Urgencias, Hospital Clínico San Carlos, Madrid, Spain; 5Department of Cardiology, University Hospital Brno, Brno, Czech Republic and Medical Faculty, Masaryk University, Brno, Czech Republic; 6Emergency Department, Kantonsspital Luzern, Switzerland; 7Blood Transfusion Centre, Swiss Red Cross, Basel, Switzerland and Department of Hematology, University Hospital Basel, University of Basel, Switzerland; 8Emergency Department of Laboratory Medicine, University Hospital Zurich, Switzerland; 92nd Department of Cardiology, School of Medicine with the Division of Dentistry in Zabrze, Medical University of Katowice, Poland; 10Emergency Department, Kantonsspital Liestal; and 11Laboratory Medicine, University Hospital Basel, Switzerland.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Acknowledgements
We are indebted to the patients who participated in the study and to the emergency department staff as well as the laboratory technicians of all the participating sites for their most valuable efforts. In addition, we wish to thank Esther Garrido, MD, Isabel Campodarve, MD, Joachim Gea, MD (Hospital del Mar, IMIM, Barcelona, Spain), Helena Mañé Cruz (Hospital Clinic, Barcelona, Spain), and Miguel Angel García Briñón (Hospital Clínico San Carlos, Madrid, Spain).
Funding
The study was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the University of Basel, the Universitätsspital Basel, Abbott, Beckman Coulter, Brahms, Idorisa Pharmaceuticals, Novartis, Ortho Clinical Diagnostics, Quidel, Roche, Siemens, and Singulex.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
Author notes
Mario Meier and Jasper Boeddinghaus contributed equally to the study and should be considered first authors.
Conflict of interest: The authors designed the study, gathered and analysed the data, vouch for the data and analysis, wrote the paper, and decided to submit it for publication. J.B., T.N., R.T., L.K., P.B., M.R.G., K.W., and C.M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the manuscript. The sponsors had no role in designing or conducting the study and no role in gathering or analysing the data or writing the manuscript. The manuscript and its content have not been published previously and are not being considered for publication elsewhere in whole or in part in any language, including publicly accessible websites or e-print servers. We disclose that J.B. received research grants from the University of Basel, the University Hospital of Basel and the Division of Internal Medicine, the Swiss Academy of Medical Sciences, and the Gottfried and Julia Bangerter-Rhyner-Foundation and speaker honoraria/consulting honoraria from Siemens, Roche Diagnostics, Ortho Clinical Diagnostics, and Quidel Corporation. T.N. received research support from the Swiss National Science Foundation (P400PM_191037/1), the Prof. Dr. Max Cloëtta Foundation, the Margarete und Walter Lichtenstein-Stiftung (3MS1038), and the University Hospital Basel as well as speaker honoraria/consulting honoraria from B.Braun, Siemens, Beckman Coulter, Bayer, Ortho Clinical Diagnostics, Edwards Lifesciences, and Orion Pharma, outside the submitted work. L.K. received a research grant from the Swiss Heart Foundation, the University of Basel, the Swiss Academy of Medical Sciences, and the Gottfried and Julia Bangerter-Rhyner Foundation, as well as the ‘Freiwillige Akademische Gesellschaft Basel’. J.E.W. received a research grant from the Swiss Academy of Medical Sciences and the Gottfried and Julia Bangerter-Rhyner Foundation as well as research grants from the Swiss Heart Foundation. P.B. received research funding from the ‘Stiftung für Herzschrittmacher und Elektrophysiologie’ and the FAG Basel (‘Freiwillige Akademische Gesellschaft’) and from the University of Basel. K.W. received a research grant from the ‘Freiwillige Akademische Gesellschaft Basel’, the Gottfried and Julia Bangerter-Rhyner Foundation, and the Prince Charles Hospital Foundation and a PhD scholarship from the University of Queensland. C.P. received research support from the Swiss Heart Foundation and Roche Diagnostics, outside the current study. T.Z. received research support from the ‘Freiwillige Akademische Gesellschaft Basel’. M.R.G. received research grants from the Swiss National Science Foundation (P400PM_180828), the Swiss Heart Foundation, and the Women and Heart Foundation and speaker/consulting honoraria from Abbott, Ortho Clinical Diagnostics, Quidel, Roche, and Siemens. D.M.G. reports grants from the Swiss Heart Foundation and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil) and personal fees from Roche, outside the submitted work. R.T. received research support from the Swiss National Science Foundation (P300PB_167803), the Swiss Heart Foundation, the Swiss Society of Cardiology, the University Hospital of Basel, the University of Basel, and the Cardiovascular Research Foundation Basel as well as speaker honoraria/consulting honoraria from Abbott, Amgen, Brahms, Roche, Singulex, and Siemens. C.M. has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the University of Basel, the University Hospital Basel, Abbott, Beckman Coulter, Brahms, Idorsia, Novartis, Ortho Clinical Diagnostics, Quidel, Roche, Siemens, Singulex, and Sphingotec as well as speaker honoraria/consulting honoraria from Amgen, Astra Zeneca, Bayer, Beckman Coulter, Boehringer Ingelheim, BMS, Idorsia, Novartis, Osler, Roche, Sanofi, Siemens, and Singulex. All other authors declare that they have no conflict of interest with this study. The hs-cTn assays investigated were donated by the manufacturers, who had no role in the design of the study, the analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.




Comments