-
PDF
- Split View
-
Views
-
Cite
Cite
Gregory Piazza, Off the beaten path: the need for innovation in medical therapy to improve outcomes in acute pulmonary embolism, European Heart Journal. Acute Cardiovascular Care, Volume 11, Issue 1, January 2022, Pages 10–12, https://doi.org/10.1093/ehjacc/zuab100
Close - Share Icon Share
‘Do not go where the path may lead, go instead where there is no path and leave a trail’.
-Ralph Waldo Emerson
Despite major innovations in anticoagulation and device therapy for acute pulmonary embolism (PE), short- and long-term mortality and morbidity remain high, especially in patients with intermediate–high and high risk.1 While overall case fatality rate has improved over the past two decades in the USA and Europe,2 in-hospital mortality for acute PE still exceeds that of ST-elevation myocardial infarction.3,4 Furthermore, advances in PE therapy, in particular pulmonary artery reperfusion, do not appear to impact long-term complications, including the post-PE syndrome and chronic thromboembolic pulmonary hypertension.5,6
Recently, the majority of PE treatment clinical trials has focused on device therapy for pulmonary artery reperfusion, including catheter-directed fibrinolysis and mechanical thrombectomy. These devices, such as ultrasound-facilitated catheter-directed fibrinolysis and suction-catheter embolectomy, have been evaluated in a series of small randomized and single-arm trials and have demonstrated comparable impact on surrogate imaging endpoints.7 However, very few of these trials have assessed impact on long-term or patient-centred outcomes,8 and accordingly, provide limited data for evidence-based guideline recommendations. Recently, the HI-PEITHO randomized trial of ultrasound-facilitated, catheter-based fibrinolysis vs. anticoagulation alone began enrolling patients with intermediate–high-risk PE in order to fill this critical data void (NCT04790370).
Reliance on imaging surrogates, such as right ventricle (RV)-to-left ventricle (LV) diameter ratio and thrombus volume reduction, fails recognize the multifactorial pathophysiology of PE and may provide an incomplete assessment of treatment benefit.9,10 While baseline RV-to-LV diameter, as measured by computed tomography (CT) or echocardiography, correlates with increased PE-related mortality, its reduction or lack thereof has not been proven to accurately predict clinical benefit post-reperfusion therapy such that it could serve as a reliable surrogate in clinical trials.7 Similarly, proximal thrombus volume reduction as measured by standard contrast-enhanced chest CT does not account for distal pulmonary artery perfusion and the impact of hypoxaemia and circulating pulmonary vasoconstrictors on pulmonary vascular resistance and RV pressure overload. Accordingly, proximal thrombus burden and changes post-intervention has not consistently correlated with PE mortality.11 In contrast, distal pulmonary vascular perfusion may be a more promising surrogate for RV dysfunction and mortality after PE.12,13 Ultrasound-facilitated catheter-based fibrinolysis has been shown to increase distal pulmonary vascular perfusion with potential mechanisms of peripheral fibrinolytic effect and ultrasound-mediated vasodilation.13
In light of these trends and limitations in current PE-related research, there is a critical need for clinical trials that explore therapies beyond proximal thrombus debulking. In addition to interventions that facilitate distal thrombus resolution, medical therapies focused on reversing pulmonary vasoconstriction in acute PE may have an important role in relieving RV pressure overload. While inhaled nitric oxide did not significantly reduce a primary endpoint of normalized troponin and echocardiographic parameters in a trial of intermediate-risk PE, it improved RV function compared with placebo.14
In this issue of the European Heart Journal: Acute Cardiovascular Care, Lim et al.15 explore another potentially important avenue for medical therapy in PE: optimizing RV mechanics and ventricular interdependence. In this randomized controlled trial, the investigators assigned 276 normotensive patients with intermediate-risk PE to a single 80 mg bolus of furosemide or placebo to assess the impact of diuresis on oligo-anuria and simplified PE severity index items. Bolus furosemide improved the primary endpoint, attributable to increased urine output, but did not improve heart rate, systolic blood pressure, or arterial oxygenation. While the trial did not show that RV decompression with bolus diuretic improved haemodynamics in intermediate-risk PE, it did suggest that a wider range of RV loading conditions may be tolerated and a more individualized approach to optimizing RV and LV loading conditions might be beneficial.
The current study by Lim et al. highlights the need to expand beyond anticoagulation, fibrinolysis, and device therapy for acute PE management. Pulmonary embolism comprises a wide spectrum of presentations with varying pathophysiological mechanisms dominating from one patient to the next. While thrombus burden may be an important contributor to RV dysfunction in many patients, reductions in distal pulmonary perfusion, pulmonary artery vasoconstriction, the relationship between RV and LV filling, and the acute inflammatory response to PE may provide additional avenues for therapeutic benefit (Figure 1). Indeed, haemodynamic-based therapies, and possibly anti-inflammatory treatments, may represent what has been missing in our care of patients with intermediate–high- and high-risk PE and much needed tools to reduce mortality and morbidity. For the benefit of the field and, more importantly, our patients, let us hope that investigators such as Lim et al. continue to ‘veer off the beaten path’ and blaze trails towards a greater understanding of safe and effective medical therapies for PE.
Conflict of interest: Dr. Piazza has received research grant support paid to his institution from Boston Scientific Corporation, Bayer, Bristol Myers Squibb/Pfizer Alliance, Alexion, Janssen, and Amgen and consulting fees from Amgen, Pfizer, Boston Scientific Corporation, Syntactx, and Prairie Education and Research Cooperative.
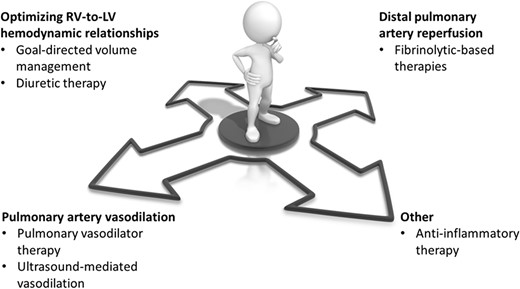
Potential pathways for scientific investigation in medical therapy for acute pulmonary embolism beyond anticoagulation. LV, left ventricle; RV, right ventricle.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal – Acute Cardiovascular Care or of the European Society of Cardiology.




Comments