-
PDF
- Split View
-
Views
-
Cite
Cite
Pascal Lim, Clément Delmas, Olivier Sanchez, Nicolas Meneveau, Roger Rosario, Helene Bouvaist, Anne Bernard, Jacques Mansourati, Francis Couturaud, Mustapha Sebbane, Pierre Coste, Gwenole Rohel, Bernard Tardy, Caroline Biendel, Olivier Lairez, Fabrice Ivanes, Romain Gallet, Jean-Luc Dubois-Rande, Damien Fard, Gilles Chatelier, Tabassome Simon, Muriel Paul, Pierre-André Natella, Richard Layese, Sylvie Bastuji-Garin, Diuretic vs. placebo in intermediate-risk acute pulmonary embolism: a randomized clinical trial, European Heart Journal. Acute Cardiovascular Care, Volume 11, Issue 1, January 2022, Pages 2–9, https://doi.org/10.1093/ehjacc/zuab082
Close - Share Icon Share
Abstract
The role of diuretics in patients with intermediate-risk pulmonary embolism (PE) is controversial. In this multicentre, double-blind trial, we randomly assigned normotensive patients with intermediate-risk PE to receive either a single 80 mg bolus of furosemide or a placebo.
Eligible patients had at least a simplified PE Severity Index (sPESI) ≥1 with right ventricular dysfunction. The primary efficacy endpoint assessed 24 h after randomization included (i) absence of oligo-anuria and (ii) normalization of all sPESI items. Safety outcomes were worsening renal function and major adverse outcomes at 48 hours defined by death, cardiac arrest, mechanical ventilation, or need of catecholamine. A total of 276 patients underwent randomization; 135 were assigned to receive the diuretic, and 141 to receive the placebo. The primary outcome occurred in 68/132 patients (51.5%) in the diuretic and in 49/132 (37.1%) in the placebo group (relative risk = 1.30, 95% confidence interval 1.04–1.61; P = 0.021). Major adverse outcome at 48 h occurred in 1 (0.8%) patients in the diuretic group and 4 patients (2.9%) in the placebo group (P = 0.19). Increase in serum creatinine level was greater in diuretic than placebo group [+4 µM/L (−2; 14) vs. −1 µM/L (−11; 6), P < 0.001].
In normotensive patients with intermediate-risk PE, a single bolus of furosemide improved the primary efficacy outcome at 24 h and maintained stable renal function. In the furosemide group, urine output increased, without a demonstrable improvement in heart rate, systolic blood pressure, or arterial oxygenation.
ClinicalTrials.gov identifier NCT02268903.
Introduction
Acute pulmonary embolism is a common disease, with an early mortality of 7–11%.1–3 The outcome is associated mainly with the patient’s hemodynamic status and occurrence of acute right-ventricular failure4,5 related to the abrupt increase in right ventricular afterload. The initial adaptive mechanism relies on dilatation of the right ventricular chamber to maintain cardiac output,6 and may become deleterious, primarily due to a paradoxical septal motion that may impair left ventricular compliance and filling, thus decreasing cardiac output.7 Thrombolysis in association with unfractionated heparin is indicated in pulmonary embolism-related shock,8 whereas the benefit of thrombolysis is counterbalanced by a risk of bleeding in normotensive patients with intermediate to high-risk pulmonary embolism.9–11 Management of such patients is not clearly established despite their elevated risk of pulmonary embolism-related shock (10%) and in-hospital death (5%).12 Volume expansion may be recommended, but can reduce cardiac output through excessive septal paradoxical motion.13,14 Therapies that reduce right ventricular preload, such as diuretics, may be more appropriate but are commonly viewed as contraindicated because of the fear of depressing right ventricular function by unbalancing the right ventricular preload (Frank–Starling mechanism). The Diuretic Versus Placebo in Pulmonary Embolism (DiPER) trial was designed to investigate the clinical efficacy and safety of a single-bolus injection of the loop-diuretic furosemide in addition to standard anticoagulation therapy in normotensive patients with acute pulmonary embolism and at intermediate risk of an adverse outcome.
Methods
Trial design and oversight
We performed a multicentre, double-blind, placebo-controlled randomized trial in two, parallel groups (ClinicalTrials.gov identifier NCT02268903). The trial was initiated by the investigators and was sponsored by Assistance Publique—Hôpitaux de Paris and Délégation à la Recherche Clinique et à l’Innovation (Clinical Research and Innovation Department). Trial funding was provided by Programme Hospitalier de Recherche Clinique in France (AOM 130519). The protocol was written by the academic principal investigators and approved by the ethics review board (Committee for the Protection of People, Ile de France VII). Trial oversight was provided by an independent data and safety monitoring board whose members periodically reviewed the safety data. A clinical research organization appointed by the sponsor was responsible for data collection and monitoring at the study sites. Data were gathered using electronic case report forms. The trial statistician performed all statistical analyses before the code for concealing the study arms was broken. The principal investigators had unrestricted access to the data after database lock.
Study population
Patients were recruited from 12 hospitals in France. Adults were eligible for inclusion if they met the following criteria: acute pulmonary embolism objectively confirmed by spiral computed tomography scan with the onset of symptoms ≤15 days before randomization; right ventricular dysfunction confirmed by echocardiography, computed tomography, or elevated B-type natriuretic peptide; and one abnormal item on the simplified Pulmonary Embolism Severity Index (sPESI) (i.e.). Elevated B-type natriuretic peptide was defined as >200 pg/mL for B-type natriuretic peptide and >600 pg/mL for N-terminal pro-B-type natriuretic peptide. Exclusion criteria were cardiogenic shock or hypotension, severe left ventricular dysfunction defined by left ventricular ejection fraction <45%, and diuretic treatment (to avoid bias towards a higher response to diuretics). Further information is provided in Supplementary material online, Appendix S1. Written informed consent was provided by all patients.
Study treatment and follow-up
Eligible patients were randomly assigned (1:1) to receive a single intravenous bolus of 80 mg furosemide or a matching placebo. Furosemide dose was set according to the median dose used in the preliminary study. Details on the randomization process are provided in Supplementary material online, Appendix S1. Conventional treatment for pulmonary embolism was left to the investor’s discretion (anticoagulation, supportive care, and reperfusion strategies) and was recorded. Anticoagulants comprised unfractionated heparin, low-molecular-weight heparin, and fondaparinux, according to local practice. Patients were followed for 30 days or to hospital discharge or death, whichever came first.
Study outcomes
The primary outcome, assessed 24 h after randomization, was normalization of hemodynamic and respiratory status, defined as urine output >0.5 mL/kg/min over the previous 24 h (i.e. absence of oligoanuria) and normalization of all sPESI score items (heart rate ≤110 bpm, systolic blood pressure ≥100 mmHg, and arterial oxyhaemoglobin level ≥90% in ambient air) (Supplementary material online, Appendix S1). Secondary efficacy outcomes included the use of fluid expansion, changes in brain natriuretic peptide level (at 24 h), changes in right ventricular/left ventricular diameter ratio (at 24 h), systolic pulmonary artery pressure, right ventricular function measured by tricuspid annular plane systolic excursion (all at or within 24 h after randomization), and duration of hospitalization in the intensive care unit. Echocardiography data were recorded for offline analysis by the echocardiography core laboratory (Imaging Laboratory of Rangueil University, Toulouse, France).
Safety outcomes included worsening renal function (changes in serum creatinine at 24 h) and major adverse events (death, cardiac arrest, mechanical ventilation, or catecholamine use) 48 h after randomization. All patients were followed for 30 ± 60 days (30–90 days) and were evaluated for death, haemodynamic decompensation (or collapse), bleeding, stroke, recurrent pulmonary embolism, and serious adverse events.
Statistical analysis
The planned sample size of 270 patients was estimated to provide 90% power to detect a relative between-group difference of 30% in the risk of hemodynamic stability at 24 h, assuming a two-sided P-value of 0.025 (one interim analysis) and lack of ascertainment of the primary outcome in 5% (further details are provided in the Supplementary material online, Appendix S1).
The main efficacy and safety analyses were based on all events that occurred in the intention-to-treat population, defined as all patients who underwent randomization. Analysis of safety outcomes was performed in the safety population, defined as all patients who received the study drug. We first used a multilevel model (a random-centre effect logistic regression) to account for potential clustering. As neither a centre effect nor an effect modification by centre was observed, clustering was ignored. Therefore, the primary efficacy outcome was analysed by means of a two-sided chi-square test of proportions, and the relative risk (RR) and the absolute risk reduction (ARR) were calculated together with their 95% CIs. For these estimations, to account for the missing assessments, we performed multiple imputations under the assumption that data were missing at random, with the use of ancillary variables (placebo/furosemide group, age, sex, and history of venous thrombosis). The estimations of RR and ARR in multiple imputations framework were performed using log-binomial generalized linear models. A sensitivity analysis was performed in patients whose primary outcome was available (complete case). A random-centre effect multivariable logistic regression model was also fitted to compare the primary outcome between both groups, to take into account centre and potential confounders. Adjusted odds ratios (ORs) were estimated with their 95% CIs. Secondary analyses included the Mann–Whitney U test for continuous variables and the χ2 or Fisher test for proportions. The incidences of major adverse outcomes (defined as death, cardiac arrest, mechanical ventilation, or need for catecholamine) were compared between groups at 3 months. Cumulative clinical event rates at 3 months were calculated according to the Kaplan–Meier method, and the differences between the treatment groups were assessed with the log-rank test. Cox proportional hazards modelling was not performed because of the small number of events. Analyses were performed with Stata software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX, USA: StataCorp LLC). Details of the sample-size estimation and the interim analyses are given in the Supplementary material online, Appendix.
Results
Patients
From April 2015 through December 2019, 280 patients were enrolled and 276 underwent randomization. Of these patients, 135 were randomly assigned to furosemide and 141 to placebo (Figure 1). One patient in the placebo group withdrew consent and was excluded from all analyses. Thus the intention-to-treat population comprised 275 patients, with primary outcome data in 264 (96%) patients. All patients received the assigned study drug except one in the placebo group, who was therefore excluded from the safety analysis. Thus, the safety population comprised 274 patients.
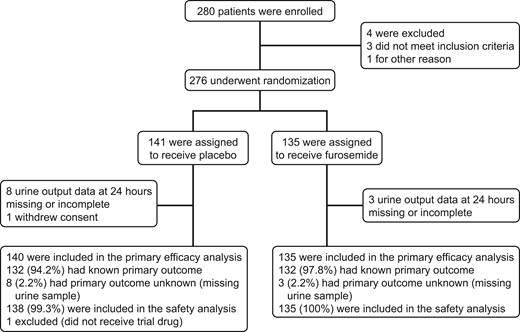
Patient demographic data, clinical status at baseline, and medical history were well matched (Table 1 and Supplementary material online, Table S1 in Appendix S1). Median age was 71 years. Right ventricular dysfunction was diagnosed by echocardiography or computed tomography in 264 patients (95.7%). Natriuretic peptide, assessed in 264 patients, was elevated in 211 patients (79.9%). Low-molecular-weight heparin or fondaparinux was administered before randomization in 121 patients; the remaining patients received unfractionated heparin (n = 118) or a direct oral anticoagulant (n = 22).
| . | Furosemide . | Placebo . |
|---|---|---|
| (n = 135) . | (n = 140) . | |
| Demographics and clinical characteristics | ||
| Age, years (IQR) | 71 (59–76) | 71 (58–80) |
| Male sex, n (%) | 70 (51.9) | 62 (44.3) |
| History of venous thrombosis, n (%) | 38 (28.2) | 37 (26.4) |
| History or active cancer, n (%) | 28 (21.1) | 22 (15.7) |
| Chronic obstructive pulmonary disease, n (%) | 9 (6.7) | 8 (5.7) |
| Chronic renal failure, n (%) | 7 (5.2) | 15 (10.7) |
| Body mass index, kg/m² (IQR) | 28 (25–31) | 30 (26–34) |
| Systolic blood pressure, mmHg (IQR) | 129 (118–145) | 132 (119–145) |
| Systolic blood pressure <100 mmHg, n (%) | 5 (3.7) | 6 (4.3) |
| Heart rate, beats/min (IQR) | 96 (85–112) | 92 (81–107) |
| Heart rate ≥110 beats/min, n (%) | 44 (32.6) | 32 (23.0) |
| Oxygen saturation in ambient air, % (IQR) | 89 (88–93) | 89 (88–93) |
| Oxygen saturation ≤90% in ambient air, n (%) | 86 (64.7) | 84 (61.3) |
| Biological data | ||
| Creatinine, µM/L (IQR) | 78 (64–93) | 83 (68–99) |
| Kalemia, mM/L (IQR) | 4.1 (3.7–4.5) | 4.1 (3.8–4.4) |
| Natremia, mL/L (IQR) | 139 (138–141) | 140 (138–141) |
| B-type natriuretic peptide, pg/mL (IQR) (n = 94; 50/44) | 260 (137–598) | 418 (162–611) |
| N-terminal pro-B-type natriuretic peptide, pg/mL (IQR) (n = 173; 85/88) | 2018 (1115–4884) | 2798 (1214–4774) |
| B-type natriuretic peptide >200 pg/mL or N-terminal pro-B-type natriuretic peptide >600 pg/mL (n = 264; 136/128), n (%) | 101 (78.9) | 110 (80.9) |
| Echocardiography data | ||
| Right ventricle/left ventricle diameter ratio (IQR) (n = 262; 133/129) | 1.2 (1.1–1.4) | 1.2 (1–1.4) |
| Systolic pulmonary artery pressure, mmHg (IQR) (n = 187; 103/84) | 50 (40–62) | 47 (40–60) |
| Tricuspid annulus plane systolic excursion, mm (IQR) (n = 206; 109/97) | 16 (14–20) | 17 (14–20) |
| Left ventricular ejection fraction, % (IQR) (n = 204; 111/93) | 60 (52–65) | 60 (55–65) |
| . | Furosemide . | Placebo . |
|---|---|---|
| (n = 135) . | (n = 140) . | |
| Demographics and clinical characteristics | ||
| Age, years (IQR) | 71 (59–76) | 71 (58–80) |
| Male sex, n (%) | 70 (51.9) | 62 (44.3) |
| History of venous thrombosis, n (%) | 38 (28.2) | 37 (26.4) |
| History or active cancer, n (%) | 28 (21.1) | 22 (15.7) |
| Chronic obstructive pulmonary disease, n (%) | 9 (6.7) | 8 (5.7) |
| Chronic renal failure, n (%) | 7 (5.2) | 15 (10.7) |
| Body mass index, kg/m² (IQR) | 28 (25–31) | 30 (26–34) |
| Systolic blood pressure, mmHg (IQR) | 129 (118–145) | 132 (119–145) |
| Systolic blood pressure <100 mmHg, n (%) | 5 (3.7) | 6 (4.3) |
| Heart rate, beats/min (IQR) | 96 (85–112) | 92 (81–107) |
| Heart rate ≥110 beats/min, n (%) | 44 (32.6) | 32 (23.0) |
| Oxygen saturation in ambient air, % (IQR) | 89 (88–93) | 89 (88–93) |
| Oxygen saturation ≤90% in ambient air, n (%) | 86 (64.7) | 84 (61.3) |
| Biological data | ||
| Creatinine, µM/L (IQR) | 78 (64–93) | 83 (68–99) |
| Kalemia, mM/L (IQR) | 4.1 (3.7–4.5) | 4.1 (3.8–4.4) |
| Natremia, mL/L (IQR) | 139 (138–141) | 140 (138–141) |
| B-type natriuretic peptide, pg/mL (IQR) (n = 94; 50/44) | 260 (137–598) | 418 (162–611) |
| N-terminal pro-B-type natriuretic peptide, pg/mL (IQR) (n = 173; 85/88) | 2018 (1115–4884) | 2798 (1214–4774) |
| B-type natriuretic peptide >200 pg/mL or N-terminal pro-B-type natriuretic peptide >600 pg/mL (n = 264; 136/128), n (%) | 101 (78.9) | 110 (80.9) |
| Echocardiography data | ||
| Right ventricle/left ventricle diameter ratio (IQR) (n = 262; 133/129) | 1.2 (1.1–1.4) | 1.2 (1–1.4) |
| Systolic pulmonary artery pressure, mmHg (IQR) (n = 187; 103/84) | 50 (40–62) | 47 (40–60) |
| Tricuspid annulus plane systolic excursion, mm (IQR) (n = 206; 109/97) | 16 (14–20) | 17 (14–20) |
| Left ventricular ejection fraction, % (IQR) (n = 204; 111/93) | 60 (52–65) | 60 (55–65) |
IQR, interquartile range.
| . | Furosemide . | Placebo . |
|---|---|---|
| (n = 135) . | (n = 140) . | |
| Demographics and clinical characteristics | ||
| Age, years (IQR) | 71 (59–76) | 71 (58–80) |
| Male sex, n (%) | 70 (51.9) | 62 (44.3) |
| History of venous thrombosis, n (%) | 38 (28.2) | 37 (26.4) |
| History or active cancer, n (%) | 28 (21.1) | 22 (15.7) |
| Chronic obstructive pulmonary disease, n (%) | 9 (6.7) | 8 (5.7) |
| Chronic renal failure, n (%) | 7 (5.2) | 15 (10.7) |
| Body mass index, kg/m² (IQR) | 28 (25–31) | 30 (26–34) |
| Systolic blood pressure, mmHg (IQR) | 129 (118–145) | 132 (119–145) |
| Systolic blood pressure <100 mmHg, n (%) | 5 (3.7) | 6 (4.3) |
| Heart rate, beats/min (IQR) | 96 (85–112) | 92 (81–107) |
| Heart rate ≥110 beats/min, n (%) | 44 (32.6) | 32 (23.0) |
| Oxygen saturation in ambient air, % (IQR) | 89 (88–93) | 89 (88–93) |
| Oxygen saturation ≤90% in ambient air, n (%) | 86 (64.7) | 84 (61.3) |
| Biological data | ||
| Creatinine, µM/L (IQR) | 78 (64–93) | 83 (68–99) |
| Kalemia, mM/L (IQR) | 4.1 (3.7–4.5) | 4.1 (3.8–4.4) |
| Natremia, mL/L (IQR) | 139 (138–141) | 140 (138–141) |
| B-type natriuretic peptide, pg/mL (IQR) (n = 94; 50/44) | 260 (137–598) | 418 (162–611) |
| N-terminal pro-B-type natriuretic peptide, pg/mL (IQR) (n = 173; 85/88) | 2018 (1115–4884) | 2798 (1214–4774) |
| B-type natriuretic peptide >200 pg/mL or N-terminal pro-B-type natriuretic peptide >600 pg/mL (n = 264; 136/128), n (%) | 101 (78.9) | 110 (80.9) |
| Echocardiography data | ||
| Right ventricle/left ventricle diameter ratio (IQR) (n = 262; 133/129) | 1.2 (1.1–1.4) | 1.2 (1–1.4) |
| Systolic pulmonary artery pressure, mmHg (IQR) (n = 187; 103/84) | 50 (40–62) | 47 (40–60) |
| Tricuspid annulus plane systolic excursion, mm (IQR) (n = 206; 109/97) | 16 (14–20) | 17 (14–20) |
| Left ventricular ejection fraction, % (IQR) (n = 204; 111/93) | 60 (52–65) | 60 (55–65) |
| . | Furosemide . | Placebo . |
|---|---|---|
| (n = 135) . | (n = 140) . | |
| Demographics and clinical characteristics | ||
| Age, years (IQR) | 71 (59–76) | 71 (58–80) |
| Male sex, n (%) | 70 (51.9) | 62 (44.3) |
| History of venous thrombosis, n (%) | 38 (28.2) | 37 (26.4) |
| History or active cancer, n (%) | 28 (21.1) | 22 (15.7) |
| Chronic obstructive pulmonary disease, n (%) | 9 (6.7) | 8 (5.7) |
| Chronic renal failure, n (%) | 7 (5.2) | 15 (10.7) |
| Body mass index, kg/m² (IQR) | 28 (25–31) | 30 (26–34) |
| Systolic blood pressure, mmHg (IQR) | 129 (118–145) | 132 (119–145) |
| Systolic blood pressure <100 mmHg, n (%) | 5 (3.7) | 6 (4.3) |
| Heart rate, beats/min (IQR) | 96 (85–112) | 92 (81–107) |
| Heart rate ≥110 beats/min, n (%) | 44 (32.6) | 32 (23.0) |
| Oxygen saturation in ambient air, % (IQR) | 89 (88–93) | 89 (88–93) |
| Oxygen saturation ≤90% in ambient air, n (%) | 86 (64.7) | 84 (61.3) |
| Biological data | ||
| Creatinine, µM/L (IQR) | 78 (64–93) | 83 (68–99) |
| Kalemia, mM/L (IQR) | 4.1 (3.7–4.5) | 4.1 (3.8–4.4) |
| Natremia, mL/L (IQR) | 139 (138–141) | 140 (138–141) |
| B-type natriuretic peptide, pg/mL (IQR) (n = 94; 50/44) | 260 (137–598) | 418 (162–611) |
| N-terminal pro-B-type natriuretic peptide, pg/mL (IQR) (n = 173; 85/88) | 2018 (1115–4884) | 2798 (1214–4774) |
| B-type natriuretic peptide >200 pg/mL or N-terminal pro-B-type natriuretic peptide >600 pg/mL (n = 264; 136/128), n (%) | 101 (78.9) | 110 (80.9) |
| Echocardiography data | ||
| Right ventricle/left ventricle diameter ratio (IQR) (n = 262; 133/129) | 1.2 (1.1–1.4) | 1.2 (1–1.4) |
| Systolic pulmonary artery pressure, mmHg (IQR) (n = 187; 103/84) | 50 (40–62) | 47 (40–60) |
| Tricuspid annulus plane systolic excursion, mm (IQR) (n = 206; 109/97) | 16 (14–20) | 17 (14–20) |
| Left ventricular ejection fraction, % (IQR) (n = 204; 111/93) | 60 (52–65) | 60 (55–65) |
IQR, interquartile range.
Primary outcome
Data for the primary outcome were available in 132 patients (97.8%) in the diuretic group and 132 patients (94.3%) in the placebo group. Between randomization and 24 h, the primary efficacy outcome occurred in 68 patients (51.5%) in the diuretic group and in 49 patients (37.1%) in the placebo group (RR 1.30, 95% CI 1.04–1.61; P = 0.021; ARR 14.3%; 95% CI 2.4–26.1, with multiple imputation) (Table 2). Similar results were observed for complete cases (RR 1.30, 95% CI 1.04–1.61; P = 0.019; ARR 14.4%, 95% CI 2.5–26.3) and on multivariable analysis considering potential confounders (Supplementary material online, Table S2.1). Relative risk ratio after adjustment to the level of intermediate risk (low- or high-risk) of pulmonary embolism is displayed in the Supplementary material online, Table S2.2. Oligoanuria 24 h after randomization was reported in 8.7% of patients in the diuretic group and in 41.5% in the placebo group (P < 0.001); heart rate 110 beats/min or higher was reported in 12.6% of patients in the diuretic group and in 3.6% in the placebo group (P = 0.008) (Table 2).
| . | Furosemide . | Placebo . | RR (95% CI) . | P-value . | ARR (95% CI) . |
|---|---|---|---|---|---|
| Primary outcome assessed at 24 h, n (%)a (urine output >0.5 mL/kg/h, and heart rate ≤110 b.p.m. and systolic blood pressure ≥100 mmHg, oxygen saturation ≥90%) | 68/132 (51.5) | 49/132 (37.1) | 1.30 (1.04–1.61) | 0.021 | 14.3 (2.4 to 26.1) |
| Components of the primary outcome, n (%) | |||||
| Urine output >0.5 mL/kg/h | 116/127 (91.3) | 76/130 (58.5) | 1.56 (1.34–1.83) | <0.001 | 0.33 (0.23 to 0.43) |
| Heart rate ≤110 b.p.m. | 118/135 (87.4) | 133/138 (96.4) | 0.91 (0.84–0.97) | 0.008 | −0.09 (−0.15 to −0.03) |
| Systolic blood pressure ≥100 mmHg | 131/135 (97) | 133/139 (95.7) | 1.04 (0.97–1.06) | 0.55 | 0.01 (−0.03 to 0.06) |
| Oxygen saturation ≥90% | 95/134 (71.0) | 96/138 (69.5) | 1.02 (0.87–1.19) | 0.81 | 0.01 (−0.10 to 0.12) |
| . | Furosemide . | Placebo . | RR (95% CI) . | P-value . | ARR (95% CI) . |
|---|---|---|---|---|---|
| Primary outcome assessed at 24 h, n (%)a (urine output >0.5 mL/kg/h, and heart rate ≤110 b.p.m. and systolic blood pressure ≥100 mmHg, oxygen saturation ≥90%) | 68/132 (51.5) | 49/132 (37.1) | 1.30 (1.04–1.61) | 0.021 | 14.3 (2.4 to 26.1) |
| Components of the primary outcome, n (%) | |||||
| Urine output >0.5 mL/kg/h | 116/127 (91.3) | 76/130 (58.5) | 1.56 (1.34–1.83) | <0.001 | 0.33 (0.23 to 0.43) |
| Heart rate ≤110 b.p.m. | 118/135 (87.4) | 133/138 (96.4) | 0.91 (0.84–0.97) | 0.008 | −0.09 (−0.15 to −0.03) |
| Systolic blood pressure ≥100 mmHg | 131/135 (97) | 133/139 (95.7) | 1.04 (0.97–1.06) | 0.55 | 0.01 (−0.03 to 0.06) |
| Oxygen saturation ≥90% | 95/134 (71.0) | 96/138 (69.5) | 1.02 (0.87–1.19) | 0.81 | 0.01 (−0.10 to 0.12) |
ARR, absolute risk reduction; CI, confidence interval; RR, relative risk.
RR and ARR were estimated using log-binomial generalized linear models after multiple imputations. ARR was estimated using this formula: (rate of events in diuretic group—rate of events in placebo group).
| . | Furosemide . | Placebo . | RR (95% CI) . | P-value . | ARR (95% CI) . |
|---|---|---|---|---|---|
| Primary outcome assessed at 24 h, n (%)a (urine output >0.5 mL/kg/h, and heart rate ≤110 b.p.m. and systolic blood pressure ≥100 mmHg, oxygen saturation ≥90%) | 68/132 (51.5) | 49/132 (37.1) | 1.30 (1.04–1.61) | 0.021 | 14.3 (2.4 to 26.1) |
| Components of the primary outcome, n (%) | |||||
| Urine output >0.5 mL/kg/h | 116/127 (91.3) | 76/130 (58.5) | 1.56 (1.34–1.83) | <0.001 | 0.33 (0.23 to 0.43) |
| Heart rate ≤110 b.p.m. | 118/135 (87.4) | 133/138 (96.4) | 0.91 (0.84–0.97) | 0.008 | −0.09 (−0.15 to −0.03) |
| Systolic blood pressure ≥100 mmHg | 131/135 (97) | 133/139 (95.7) | 1.04 (0.97–1.06) | 0.55 | 0.01 (−0.03 to 0.06) |
| Oxygen saturation ≥90% | 95/134 (71.0) | 96/138 (69.5) | 1.02 (0.87–1.19) | 0.81 | 0.01 (−0.10 to 0.12) |
| . | Furosemide . | Placebo . | RR (95% CI) . | P-value . | ARR (95% CI) . |
|---|---|---|---|---|---|
| Primary outcome assessed at 24 h, n (%)a (urine output >0.5 mL/kg/h, and heart rate ≤110 b.p.m. and systolic blood pressure ≥100 mmHg, oxygen saturation ≥90%) | 68/132 (51.5) | 49/132 (37.1) | 1.30 (1.04–1.61) | 0.021 | 14.3 (2.4 to 26.1) |
| Components of the primary outcome, n (%) | |||||
| Urine output >0.5 mL/kg/h | 116/127 (91.3) | 76/130 (58.5) | 1.56 (1.34–1.83) | <0.001 | 0.33 (0.23 to 0.43) |
| Heart rate ≤110 b.p.m. | 118/135 (87.4) | 133/138 (96.4) | 0.91 (0.84–0.97) | 0.008 | −0.09 (−0.15 to −0.03) |
| Systolic blood pressure ≥100 mmHg | 131/135 (97) | 133/139 (95.7) | 1.04 (0.97–1.06) | 0.55 | 0.01 (−0.03 to 0.06) |
| Oxygen saturation ≥90% | 95/134 (71.0) | 96/138 (69.5) | 1.02 (0.87–1.19) | 0.81 | 0.01 (−0.10 to 0.12) |
ARR, absolute risk reduction; CI, confidence interval; RR, relative risk.
RR and ARR were estimated using log-binomial generalized linear models after multiple imputations. ARR was estimated using this formula: (rate of events in diuretic group—rate of events in placebo group).
Safety and secondary outcomes
Other secondary outcomes and safety data are shown in Table 3. At 24 h, hypotension requiring fluid loading was recorded in 16 (11.9%) in the diuretic group and in 19 (13.9%) patients in the placebo group (P = 0.62). Blood samples taken 24 h after randomization showed that plasma creatinine was more elevated in the diuretic group than in the placebo group [+4 µM/L; interquartile range (IQR), −2 to 14 vs. −1 µM/L; IQR −11 to 6; P < 0.001] and plasma potassium level was lower in the furosemide group (P < 0.01). Increase in plasma creatinine level >26 µM/L was reported in 1 patient (0.7%) in the diuretic group and in 11 patients (8.3%) in the placebo group (P = 0.005). The rate of severe hypokalaemia (≤3.0 mM/L) was similar in the diuretic (n = 2) and placebo (n = 2; P = 1.0) groups. The decrease in B-type natriuretic concentration at 24 h was 39% (IQR −59 to 10) in the diuretic group and 24% (IQR −51 to 22) in the placebo group (P = 0.10). Changes in echocardiography data at 24 h are reported in Supplementary material online, Table S3.
| . | Furosemide . | Placebo . | P-value . |
|---|---|---|---|
| (n = 135) . | (n = 140) . | ||
| Components of the primary outcome at 48 h, n (%) | |||
| Urine output >0.5 mL/kg/h, heart rate ≤110 b.p.m., SBP ≥100 mmHg, oxygen saturation ≥90% | 80/122 (65.6) | 74/126 (58.7) | 0.27 |
| Urine output >0.5 mL/kg/h | 118/124 (95.2) | 95/124 (76.6) | <0.001 |
| Heart rate ≤110 beats/min | 127/134 (94.8) | 136/138 (98.6) | 0.10 |
| Systolic blood pressure ≥100 mmHg | 133/135 (98.5) | 134/138 (97.1) | 0.68 |
| Oxygen saturation ≥90% | 116/133 (87.2) | 122/137 (89.1) | 0.64 |
| Other secondary outcomes (change from baseline to 24 h) | |||
| Relative change B-type in natriuretic peptide or N-terminal pro-B-type natriuretic peptide, % (IQR) (n = 234; 122/112) | −39 (−59 to 10) | −24 (−51 to 22) | 0.10 |
| Median absolute change in right ventricular/left ventricular ratio (IQR) (n = 191; 100/91) | −0.2 (−0.3 to 0) | −0.1 (−0.3 to 0) | 0.12 |
| Median absolute change in systolic pulmonary artery pressure (IQR), mmHg (n = 159; 139/135) | −5.0 (−14.0 to 0.0) | 4.5 (−10.0 to 3.0) | 0.29 |
| Median absolute change in tricuspid annulus plane systolic excursion (IQR), mm (n = 182; 95/87) | 1 (−3 to 4) | 1 (0 to 4) | 0.10 |
| Fluid overload, n (%) | |||
| Baseline to 24 h (n = 272; 137/135) | 16 (11.9) | 19 (13.9) | 0.62 |
| Baseline to 48 h (n = 272; 137/135) | 23 (17.0) | 24 (17.9) | 0.89 |
| Median length of intensive care unit stay (IQR), days | 3 (2 to 4) | 2 (2 to 4) | — |
| Safety outcomes (n = 274/275) | |||
| Change in creatinine level at 24 h, µM/L (IQR) (n = 266; 133/133) | 4 (−2 to 14) | −1 (−11 to 6) | <0.001 |
| Increase in creatinine level >26.5 µM/L, n (%) (n = 266; 133/133) | 11 (8.3) | 1 (0.7) | 0.005 |
| Severe hypokalaemia (potassium <3.0 mM/L), n (%) | 2 (1.5) | 1 (0.8) | 1.00 |
| Plasma potassium, mM/L (IQR) | 3.8 (3.5–4.1) | 4.0 (3.8–4.3) | <0.01 |
| Major adverse event from baseline to 48 h, n (%) (n = 262; 135/127) | 1 (0.8) | 4 (2.9) | 0.19 |
| Catecholamine support | 1 (0.8) | 1 (0.7) | 0.99 |
| Mechanical ventilation | 0 | 3 (2.2) | 0.08 |
| Cardiac arrest | 0 | 0 | — |
| Death | 0 | 0 | — |
| Thrombolysisa | 2 (1.5) | 2 (1.5) | 1.00 |
| Major adverse event from baseline to 1 month, n (%) (n = 239; 123/116) | 6 (4.5) | 9 (6.6) | 0.45 |
| Catecholamine support | 2 (1.5) | 1 (0.7) | 0.56 |
| Mechanical ventilation | 1 (0.7) | 3 (2.2) | 0.32 |
| Death | 5 (3.8) | 6 (4.4) | 0.83 |
| Cardiac arrest | 1 (0.7) | 0 | 0.33 |
| Thrombolysisa | 3 (2.5) | 2 (1.6) | 0.68 |
| . | Furosemide . | Placebo . | P-value . |
|---|---|---|---|
| (n = 135) . | (n = 140) . | ||
| Components of the primary outcome at 48 h, n (%) | |||
| Urine output >0.5 mL/kg/h, heart rate ≤110 b.p.m., SBP ≥100 mmHg, oxygen saturation ≥90% | 80/122 (65.6) | 74/126 (58.7) | 0.27 |
| Urine output >0.5 mL/kg/h | 118/124 (95.2) | 95/124 (76.6) | <0.001 |
| Heart rate ≤110 beats/min | 127/134 (94.8) | 136/138 (98.6) | 0.10 |
| Systolic blood pressure ≥100 mmHg | 133/135 (98.5) | 134/138 (97.1) | 0.68 |
| Oxygen saturation ≥90% | 116/133 (87.2) | 122/137 (89.1) | 0.64 |
| Other secondary outcomes (change from baseline to 24 h) | |||
| Relative change B-type in natriuretic peptide or N-terminal pro-B-type natriuretic peptide, % (IQR) (n = 234; 122/112) | −39 (−59 to 10) | −24 (−51 to 22) | 0.10 |
| Median absolute change in right ventricular/left ventricular ratio (IQR) (n = 191; 100/91) | −0.2 (−0.3 to 0) | −0.1 (−0.3 to 0) | 0.12 |
| Median absolute change in systolic pulmonary artery pressure (IQR), mmHg (n = 159; 139/135) | −5.0 (−14.0 to 0.0) | 4.5 (−10.0 to 3.0) | 0.29 |
| Median absolute change in tricuspid annulus plane systolic excursion (IQR), mm (n = 182; 95/87) | 1 (−3 to 4) | 1 (0 to 4) | 0.10 |
| Fluid overload, n (%) | |||
| Baseline to 24 h (n = 272; 137/135) | 16 (11.9) | 19 (13.9) | 0.62 |
| Baseline to 48 h (n = 272; 137/135) | 23 (17.0) | 24 (17.9) | 0.89 |
| Median length of intensive care unit stay (IQR), days | 3 (2 to 4) | 2 (2 to 4) | — |
| Safety outcomes (n = 274/275) | |||
| Change in creatinine level at 24 h, µM/L (IQR) (n = 266; 133/133) | 4 (−2 to 14) | −1 (−11 to 6) | <0.001 |
| Increase in creatinine level >26.5 µM/L, n (%) (n = 266; 133/133) | 11 (8.3) | 1 (0.7) | 0.005 |
| Severe hypokalaemia (potassium <3.0 mM/L), n (%) | 2 (1.5) | 1 (0.8) | 1.00 |
| Plasma potassium, mM/L (IQR) | 3.8 (3.5–4.1) | 4.0 (3.8–4.3) | <0.01 |
| Major adverse event from baseline to 48 h, n (%) (n = 262; 135/127) | 1 (0.8) | 4 (2.9) | 0.19 |
| Catecholamine support | 1 (0.8) | 1 (0.7) | 0.99 |
| Mechanical ventilation | 0 | 3 (2.2) | 0.08 |
| Cardiac arrest | 0 | 0 | — |
| Death | 0 | 0 | — |
| Thrombolysisa | 2 (1.5) | 2 (1.5) | 1.00 |
| Major adverse event from baseline to 1 month, n (%) (n = 239; 123/116) | 6 (4.5) | 9 (6.6) | 0.45 |
| Catecholamine support | 2 (1.5) | 1 (0.7) | 0.56 |
| Mechanical ventilation | 1 (0.7) | 3 (2.2) | 0.32 |
| Death | 5 (3.8) | 6 (4.4) | 0.83 |
| Cardiac arrest | 1 (0.7) | 0 | 0.33 |
| Thrombolysisa | 3 (2.5) | 2 (1.6) | 0.68 |
IQR, interquartile range.
Not included in major adverse events.
| . | Furosemide . | Placebo . | P-value . |
|---|---|---|---|
| (n = 135) . | (n = 140) . | ||
| Components of the primary outcome at 48 h, n (%) | |||
| Urine output >0.5 mL/kg/h, heart rate ≤110 b.p.m., SBP ≥100 mmHg, oxygen saturation ≥90% | 80/122 (65.6) | 74/126 (58.7) | 0.27 |
| Urine output >0.5 mL/kg/h | 118/124 (95.2) | 95/124 (76.6) | <0.001 |
| Heart rate ≤110 beats/min | 127/134 (94.8) | 136/138 (98.6) | 0.10 |
| Systolic blood pressure ≥100 mmHg | 133/135 (98.5) | 134/138 (97.1) | 0.68 |
| Oxygen saturation ≥90% | 116/133 (87.2) | 122/137 (89.1) | 0.64 |
| Other secondary outcomes (change from baseline to 24 h) | |||
| Relative change B-type in natriuretic peptide or N-terminal pro-B-type natriuretic peptide, % (IQR) (n = 234; 122/112) | −39 (−59 to 10) | −24 (−51 to 22) | 0.10 |
| Median absolute change in right ventricular/left ventricular ratio (IQR) (n = 191; 100/91) | −0.2 (−0.3 to 0) | −0.1 (−0.3 to 0) | 0.12 |
| Median absolute change in systolic pulmonary artery pressure (IQR), mmHg (n = 159; 139/135) | −5.0 (−14.0 to 0.0) | 4.5 (−10.0 to 3.0) | 0.29 |
| Median absolute change in tricuspid annulus plane systolic excursion (IQR), mm (n = 182; 95/87) | 1 (−3 to 4) | 1 (0 to 4) | 0.10 |
| Fluid overload, n (%) | |||
| Baseline to 24 h (n = 272; 137/135) | 16 (11.9) | 19 (13.9) | 0.62 |
| Baseline to 48 h (n = 272; 137/135) | 23 (17.0) | 24 (17.9) | 0.89 |
| Median length of intensive care unit stay (IQR), days | 3 (2 to 4) | 2 (2 to 4) | — |
| Safety outcomes (n = 274/275) | |||
| Change in creatinine level at 24 h, µM/L (IQR) (n = 266; 133/133) | 4 (−2 to 14) | −1 (−11 to 6) | <0.001 |
| Increase in creatinine level >26.5 µM/L, n (%) (n = 266; 133/133) | 11 (8.3) | 1 (0.7) | 0.005 |
| Severe hypokalaemia (potassium <3.0 mM/L), n (%) | 2 (1.5) | 1 (0.8) | 1.00 |
| Plasma potassium, mM/L (IQR) | 3.8 (3.5–4.1) | 4.0 (3.8–4.3) | <0.01 |
| Major adverse event from baseline to 48 h, n (%) (n = 262; 135/127) | 1 (0.8) | 4 (2.9) | 0.19 |
| Catecholamine support | 1 (0.8) | 1 (0.7) | 0.99 |
| Mechanical ventilation | 0 | 3 (2.2) | 0.08 |
| Cardiac arrest | 0 | 0 | — |
| Death | 0 | 0 | — |
| Thrombolysisa | 2 (1.5) | 2 (1.5) | 1.00 |
| Major adverse event from baseline to 1 month, n (%) (n = 239; 123/116) | 6 (4.5) | 9 (6.6) | 0.45 |
| Catecholamine support | 2 (1.5) | 1 (0.7) | 0.56 |
| Mechanical ventilation | 1 (0.7) | 3 (2.2) | 0.32 |
| Death | 5 (3.8) | 6 (4.4) | 0.83 |
| Cardiac arrest | 1 (0.7) | 0 | 0.33 |
| Thrombolysisa | 3 (2.5) | 2 (1.6) | 0.68 |
| . | Furosemide . | Placebo . | P-value . |
|---|---|---|---|
| (n = 135) . | (n = 140) . | ||
| Components of the primary outcome at 48 h, n (%) | |||
| Urine output >0.5 mL/kg/h, heart rate ≤110 b.p.m., SBP ≥100 mmHg, oxygen saturation ≥90% | 80/122 (65.6) | 74/126 (58.7) | 0.27 |
| Urine output >0.5 mL/kg/h | 118/124 (95.2) | 95/124 (76.6) | <0.001 |
| Heart rate ≤110 beats/min | 127/134 (94.8) | 136/138 (98.6) | 0.10 |
| Systolic blood pressure ≥100 mmHg | 133/135 (98.5) | 134/138 (97.1) | 0.68 |
| Oxygen saturation ≥90% | 116/133 (87.2) | 122/137 (89.1) | 0.64 |
| Other secondary outcomes (change from baseline to 24 h) | |||
| Relative change B-type in natriuretic peptide or N-terminal pro-B-type natriuretic peptide, % (IQR) (n = 234; 122/112) | −39 (−59 to 10) | −24 (−51 to 22) | 0.10 |
| Median absolute change in right ventricular/left ventricular ratio (IQR) (n = 191; 100/91) | −0.2 (−0.3 to 0) | −0.1 (−0.3 to 0) | 0.12 |
| Median absolute change in systolic pulmonary artery pressure (IQR), mmHg (n = 159; 139/135) | −5.0 (−14.0 to 0.0) | 4.5 (−10.0 to 3.0) | 0.29 |
| Median absolute change in tricuspid annulus plane systolic excursion (IQR), mm (n = 182; 95/87) | 1 (−3 to 4) | 1 (0 to 4) | 0.10 |
| Fluid overload, n (%) | |||
| Baseline to 24 h (n = 272; 137/135) | 16 (11.9) | 19 (13.9) | 0.62 |
| Baseline to 48 h (n = 272; 137/135) | 23 (17.0) | 24 (17.9) | 0.89 |
| Median length of intensive care unit stay (IQR), days | 3 (2 to 4) | 2 (2 to 4) | — |
| Safety outcomes (n = 274/275) | |||
| Change in creatinine level at 24 h, µM/L (IQR) (n = 266; 133/133) | 4 (−2 to 14) | −1 (−11 to 6) | <0.001 |
| Increase in creatinine level >26.5 µM/L, n (%) (n = 266; 133/133) | 11 (8.3) | 1 (0.7) | 0.005 |
| Severe hypokalaemia (potassium <3.0 mM/L), n (%) | 2 (1.5) | 1 (0.8) | 1.00 |
| Plasma potassium, mM/L (IQR) | 3.8 (3.5–4.1) | 4.0 (3.8–4.3) | <0.01 |
| Major adverse event from baseline to 48 h, n (%) (n = 262; 135/127) | 1 (0.8) | 4 (2.9) | 0.19 |
| Catecholamine support | 1 (0.8) | 1 (0.7) | 0.99 |
| Mechanical ventilation | 0 | 3 (2.2) | 0.08 |
| Cardiac arrest | 0 | 0 | — |
| Death | 0 | 0 | — |
| Thrombolysisa | 2 (1.5) | 2 (1.5) | 1.00 |
| Major adverse event from baseline to 1 month, n (%) (n = 239; 123/116) | 6 (4.5) | 9 (6.6) | 0.45 |
| Catecholamine support | 2 (1.5) | 1 (0.7) | 0.56 |
| Mechanical ventilation | 1 (0.7) | 3 (2.2) | 0.32 |
| Death | 5 (3.8) | 6 (4.4) | 0.83 |
| Cardiac arrest | 1 (0.7) | 0 | 0.33 |
| Thrombolysisa | 3 (2.5) | 2 (1.6) | 0.68 |
IQR, interquartile range.
Not included in major adverse events.
Other secondary outcomes and safety data are shown in Table 3. At 48 h, the primary outcome was available in 122 patients in the diuretic group and 126 patients in the placebo group. The primary outcome occurred in 65.6% (n = 80) of patients in the diuretic group and in 58.7% (n = 74) of those in the placebo group (P = 0.27). The cumulative number of patients requiring fluid loading 48 h after randomization was 23 (17.0%) in the diuretic group and 24 (17.9%) in the placebo group (P = 0.89). Major adverse events at 48 h were reported in 1 patient (0.8%) in the diuretic group and in 4 patients (2.9%) in the placebo group (P = 0.19). None of the patients in the diuretic group required mechanical ventilation vs. 3 patients in the placebo group (P = 0.08). Catecholamine infusion was needed in 1 patient in the diuretic group and in 1 patient in the placebo group. Duration of intensive care unit stay was 3 days (95% CI 2–4) in the diuretic group and 2 days (95% CI 2–4) in the placebo group (P = 0.69). At 1 month (±30 days), a major adverse event was reported in 6 patients (4.5%) in the diuretic group and in 9 patients (6.6%) in the placebo group (P = 0.45, Figure 2).
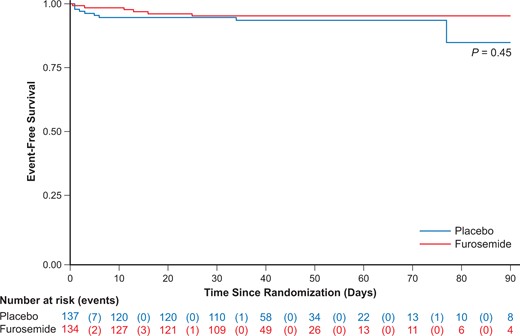
Cumulative major adverse event-free survival. Shown is the cumulative incidence of freedom from a major adverse event (death, cardiac arrest, mechanical ventilation, or catecholamine use) after 30 ± 60 days of follow-up.
Discussion
Among normotensive patients with intermediate-risk pulmonary embolism, a higher proportion of participants who received a single-bolus injection of 80 mg furosemide, as compared with placebo, achieved the primary efficacy outcome within 24 h of randomization. Furosemide was not associated with an increase in adverse events.
Normotensive patients with pulmonary embolism have an increased risk of early death or major complications if they present with right ventricular dysfunction.5,15 The right ventricle plays a key role in maintaining haemodynamic stability during pulmonary embolism.16 The compensatory maintenance of cardiac output is achieved by tachycardia and right ventricular chamber dilatation.6,12 However, an increase in the right ventricular radius aggravates right ventricular wall stress and causes a paradoxical septal wall motion that impairs left ventricular compliance and filling, leading to a decrease in cardiac output.17–19 At this point, right ventricular dilatation becomes deleterious, and a vicious cycle is established between right ventricular enlargements and decreased cardiac output.20 Intravenous thrombolysis accelerates the decrease in right ventricular afterload and may reduce the risk of clinical deterioration, but at a cost of a higher bleeding risk, resulting in a null net clinical benefit, particularly in patients with normotensive pulmonary embolism.21,22 Modest volume expansion is commonly considered acceptable in patients with oligoanuria and haemodynamic instability,23 whereas diuretics are usually forbidden because of the fear of abrupt loss of cardiac output. However, controversial and mostly pre-clinical studies demonstrate that volume expansion may worsen right ventricular function through a mechanical overstretch that increases right ventricular wall stress, and a paradoxical septal wall motion that increases left ventricular filling pressure.24,25 A non-randomized clinical study reported a benefit of diuretics in normotensive patients with an intermediate risk of pulmonary embolism.26,27 Our results indicate that a single bolus of furosemide was safe and appeared to result in a reduction of oligoanuria at 24 h but without sPESI score improvement.
Oligoanuria is a key symptom of low cardiac output which may occur within the first 48 h in intermediate-risk pulmonary embolism because right ventricular dysfunction is not sufficiently compensated by the inotropic and chronotropic adaptive stimulations. This might be attributable to a potentially detrimental combination of increased right ventricular myocardial oxygen demand, decreased right ventricular coronary perfusion gradient, and left ventricular diastolic dysfunction because of ventricular compression.17,20 Together, these elements contribute to right ventricular ischaemia and dysfunction, and may initiate a vicious circle leading to a poor outcome.15 In this setting, similar to heart failure, diuretic treatment probably should reduce the right ventricular congestion (preload) but this was not translated in a significant decreased B-type natriuretic peptide (−39% vs. −24%; P = 0.10) or right ventricular/left ventricular diameter ratio reduction (−0.2 vs. −0.1; P = 0.12). The absence of difference for cardiac arrest, death, thrombolysis, and the need for catecholamine between furosemide and placebo groups may indicate that diuretic effect has no benefit on acute RV dysfunction. Increase in urine output may result from the decongestion effect of diuretic on heart failure related to diastolic or systolic left ventricular dysfunction. Indeed, diastolic dysfunction is probably prevalent in this population because of RV compression and increase in brain natriuretic peptide and response to diuretic could probably be attributed to both left and right ventricular dysfunction. The absence of significant effect on clinical hemodynamic status may be more explained by an underpowered population sample because the expected rate of the primary outcome in the furosemide group was overestimated from the observational study (83%; 95% CI 71–95).26 Future studies should include more patients and target higher-risk population with both elevated B-type natriuretic peptide and troponin to ensure a higher event rate.
Increase in creatinine level and decrease in systolic blood pressure at 24 h in the furosemide group suggested that furosemide dose may be excessive even if this was not translated in a greater need for fluid overload or catecholamine use. The furosemide dose was set according to the median dose reported in the preliminary study. In future studies, this dose should probably be reduced or titrated dose to target a urine output >0.5 mL/kg/L, as suggested elsewhere.27 In addition, assessment of inferior vena cava size and variation may also be helpful to identify best candidate to diuretic treatment. Troponin should also be included as a risk marker to select patient at high risk of clinical event. Finally, despite a randomized and placebo-controlled design, the blinding was challenging because of the need to quantify the urine output.
Conclusions
In normotensive patients with intermediate-risk PE, a single bolus of furosemide improved the primary efficacy outcome at 24 h and maintained stable renal function. In the furosemide group, urine output increased, without a demonstrable improvement in heart rate, systolic blood pressure, or arterial oxygenation.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care.
Funding
The trial was initiated by the investigators and sponsored by Assistance Publique—Hôpitaux de Paris and by delegation Délégation à la Recherche Clinique et à l’Innovation (Clinical Research and Innovation Department).
Acknowledgements
A list of the Study Committees and Investigators is available in the Supplementary material online, Appendix S2.
Data availability
The investigators will consider on a case-by-case basis requests for data sharing.
Conflict of interest: none declared.





Comments