-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher E. Filardi, Catherine E. Smith, Social Selection and Geographic Variation in Two Monarch Flycatchers from the Solomon Islands, The Condor: Ornithological Applications, Volume 110, Issue 1, 1 February 2008, Pages 24–34, https://doi.org/10.1525/cond.2008.110.1.24
Close - Share Icon Share
Abstract
We tested the potential for social selection to act as a mechanism driving rapid plumage divergence in two sympatric monarch flycatchers, the White-capped Monarch (Monarcha richardsii) and the Kolombangara Monarch (M. browni), endemic to a single island group in the Solomon Archipelago. Solomon Island monarchs are famous for dramatic patterns of morphological divergence across very narrow water gaps, little parallel ecological variation, and minimal genetic differentiation among sister taxa inhabiting adjacent island groups. Social selection theory predicts that plumage traits evolved in allopatry may transmit important social information and that responses of dimorphic and monomorphic taxa to territorial intrusions will differ. For the dimorphic M. richardsii, we presented mounted specimens of subadult or female—and adult male—plumaged individuals to territorial birds and quantified their responses to these simulated intrusions. Territorial male M. richardsii generally responded alone, reacting most aggressively to adult male mounts. This response suggests that the bright white cap and occipital and nape patches on male M. richardsii function as social signals. In contrast, in the monomorphic M. browni, both sexes responded aggressively to intrusions of an adult-plumaged conspecific mount. Furthermore, in a variety of Melanesian forest passerines, individuals of dimorphic species generally responded singly to simulated territorial intrusions, whereas individuals of monomorphic taxa usually responded in pairs. Together, these data suggest social selection may be an important mechanism of population divergence driving some of the most extreme patterns of geographic variation among birds.
Resumen
Probamos el potencial de la selección social como mecanismo que originó la rápida divergencia del plumaje entre dos especies simpátricas, Monarcha richardsii y M. browni, endémicas de un único grupo de islas del Archipiélago Solomon. Las especies de Monarcha de las Islas Solomon son famosas por sus dramáticos patrones de divergencia morfológica separados por cortas distancias de agua, por la poca variación ecológica paralela y por la mínima diferenciación genética entre taxones hermanos que habitan grupos de islas adyacentes. La teoría de selección social predice que los caracteres de plumaje que evolucionaron en alopatría pueden transmitir información social importante, y que las respuestas por parte de taxones dimórficos y monomórficos a intrusiones territoriales deberían ser diferentes. Para la especie dimórfica M. richardsii, expusimos especimenes embalsamados subadultos y adultos, con plumaje de hembras o machos, a individuos territoriales, y cuantificamos sus respuestas ante estas intrusiones simuladas. El macho territorial de la especie M. richardsii respondió generalmente solo y reaccionó con mayor agresividad hacia el plumaje de macho adulto. Esta respuesta sugiere que los parches blanco radiantes en la porción occipital de la cabeza y nuca de los machos de M. richardsii funcionan como señal social. De modo contrastante, en la especie monomórfica M. browni, ambos sexos respondieron agresivamente a intrusiones simuladas con individuos embalsamados coespecíficos de plumaje adulto. Además, en una variedad de paserinos de bosque de Melanesia, los individuos de especies dimórficas generalmente respondieron solitariamente a intrusiones territoriales simuladas, mientras que los individuos de taxones monomórficos generalmente respondieron en pareja. En conjunto, estos datos sugieren que la selección social puede ser un mecanismo importante para la divergencia entre poblaciones, originando uno de los patrones de variación geográfica más extremos entre las aves.
Introduction
Island birds have provided great insight into the processes involved in the diversification of life, and have influenced the study of associated subjects ranging from general speciation theory (Darwin 1859, Mayr 1942, Lack 1947, Grant 1965) to specific mechanisms driving adaptive radiation (Grant and Grant 1996). Notably, the well-known geographic variation of many island birds that has attracted so much interest is often confined to plumage traits—namely color—rather than metric traits, such as bill shape or body size. In turn, many complex interisland radiations are accompanied by little or no ecological variation among closely related taxa and can involve increases in conspicuousness on some islands and decreases on others, as well as dramatic shifts in patterns of dichromatism (Omland 1997, Mayr and Diamond 2001). Despite these patterns and the influence of insular avifaunas on modern biology, few studies have tested potential selective forces driving plumage variation among allopatric bird populations across island archipelagos (West-Eberhard 1983, Price 2007).
Flycatchers in the genus Monarcha exhibit some of the most extreme patterns of geographic variation among birds (Mayr and Diamond 2001, Filardi and Moyle 2005, Filardi and Smith 2005). Although the genus is widely distributed in Australasia and Oceania, its center of diversity is found within the many islands of northern Melanesia (Sibley and Monroe 1990, Mayr and Diamond 2001). In particular, the endemic Pied and Chestnut-bellied Monarch Flycatchers of the Solomon archipelago exhibit endemic subspecies or allospecies on nearly all major island groups (Filardi and Smith 2005; Fig. 1). These two monarch lineages have long attracted the attention of biogeographers due to their dramatic patterns of plumage differentiation associated with a striking absence of ecological differentiation across very narrow water gaps (Mayr 1942, 1963, Greenslade 1968, Mayr and Diamond 2001).
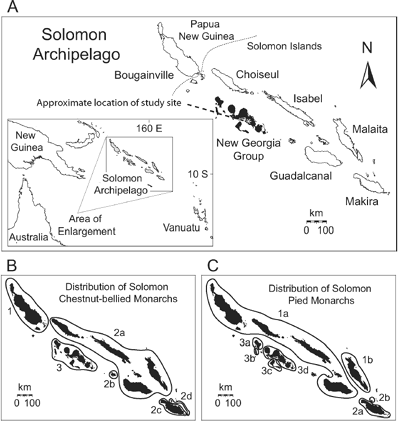
(A) Map showing the geographic location of the Solomon Archipelago and the names of its seven major island groups. The New Georgia group is in black. (B) Distribution of the allospecies and subspecies of chestnut-bellied monarchs across the Solomons: 1 = Monarcha erythrostictus; 2a = M. castaneiventris castaneiventris, 2b = M. c. obscurior, 2c = M. c. megarynchus, 2d = M. c. ugiensis; 3 = M. richardsii. (C) Distribution of the allospecies and subspecies of pied monarchs across the Solomons: 1a = Monarcha barbatus barbatus, 1b = M. b. malaitae; 2a = M. viduus viduus, 2b = M. v. squamulatus; 3a = M. browni nigrotectus, 3b = M. b. ganongae, 3c = M. b. meeki, 3d = M. b. browni.
The lack of both ecological divergence and sympatry between sister taxa in the Solomons has historically led biogeographers to suggest that relative plumage differences are indicative of the gradual effects of physical isolation on the speciation process, as opposed to divergent natural selection (Mayr 1942, 1963, Mayr and Diamond 2001). Yet assumptions of reproductive isolation among early colonizing populations are complicated by the exceedingly narrow water gaps separating island populations that show species-level morphological differentiation. However, if premating barriers to gene flow can arise before postmating genetic incompatibilities have evolved (Grant 2001), such behavioral barriers to reproduction (e.g., distinctive plumage traits that are advantageous in procuring mates or resources) are a likely mechanism leading to early stages of allopatric speciation, particularly if total isolation across short geographic distances is unlikely.
Recent behavioral and comparative studies suggest that in some cases divergent, socially relevant plumage traits may be a powerful primary driver of speciation (Møller and Cuervo 1998, Uy and Borgia 2000, Price 2007). This work corroborates earlier theoretical contributions describing social selection as an extension of sexual selection theory, encompassing all characters conferring an advantage in conspecific competition over access to any resource, including, under sexual selection, mates (West-Eberhard 1979, 1983). A role for social selection should be especially likely in birds with conspicuous plumages that evolve as a result of the accumulation of novel social signals that confer a selective advantage (Rohwer 1982, Mitra et al. 1996, Burley and Symanksi 1998). In this paper, we test whether distinctive plumage characters may function as social signals in isolated populations of two sympatric species of monarch flycatchers, the dichromatic White-capped Monarch, M. richardsii and the monochromatic Kolombangara Monarch, M. browni, both endemic to the New Georgia group of the Solomon Islands (Fig. 1).
Recent studies have revealed two patterns in Solomon monarchs consistent with a role for social selection as a mechanism of morphological differentiation. First, phylogenetic reconstructions of relationships within Solomon Island Monarcha reveal a decoupling of molecular and morphological traits over what appear to be short periods of time, suggesting divergent selection on morphology in some taxa (Filardi and Smith 2005). Specifically, the sexually dimorphic M. richardsii, whose adult males have conspicuous and dramatic white crowns, eyerings, and napes (Filardi 2003), differs minimally in genetic characters from its more widespread and monomorphic allospecies, the Chestnut-bellied Monarch, M. castaneiventris (Filardi and Smith 2005; Fig. 1B). Second, relative degrees of morphological differentiation mirror relative degrees of behavioral or social differentiation. M. richardsii exhibits striking morphological and behavioral differences with adjacent populations of M. castaneiventris. In contrast, across these same geographic gradients, relatively slight patterns of plumage variation among the allospecies of the Solomon Islands Pied Monarchs, M. barbatus and M. browni, reflect general behavioral similarities among allopatric populations (Filardi 2003).
If, as these patterns suggest, social selection has influenced plumage evolution in the Solomon Island monarchs, distinctive plumage traits that evolved in allopatry should function as socially informative signals. Importantly, Solomon monarch species are territorial, and presentation of mounted specimens to territorial species can be effective in probing the social landscapes of wild birds (Rohwer 1978, Pearson and Rohwer 1999, McDonald et al. 2001, VanderWerf and Freed 2003). More specifically, presentation of mounted specimens to free-living individuals provides a means to test the role of competition for resources in the development of plumage characters that may have driven the initial stages of speciation. If plumage traits are transmitting important social information, differences in response to mounts of differing plumage types are expected. Responses of males and females in the dichromatic M. richardsii should differ, reflecting differing sex-specific selective pressures on resource defense. In the case of the monochromatic M. browni, males and females should respond similarly to the threat represented by the mounts, reflecting similar selective pressure driving monochromatism in this taxon.
More generally, under social selection theory, we predict a greater difference in response between the sexes in dichromatic species relative to monochromatic species across a broad range of taxa. In territorial passerines dichromatic species are generally characterized by relatively conspicuous males with duller females, and monochromatic species by conspicuous males and females. We predict singly responding males should be the norm in dichromatic species, with both sexes generally responding to territorial intrusion in monochromatic taxa. Thus, to examine the possibility of a more general influence of social selection on geographic variation in tropical Pacific bird plumages, we opportunistically simulated territorial intrusions for a variety of monochromatic and dichromatic Melanesian birds. Collectively, the data presented here comprise some of the first behavioral tests implicating social selection as a mechanism generating plumage variation in northern Melanesian passerines.
Methods
Study Site
Mount presentations were conducted in February 2000, and March–May 2001 at a single site between the village of Arara and the Sakambare River on the island of New Georgia, Western Province, Solomon Islands (Fig. 1). The study site was primarily low elevation (30–60 m above sea level) swamp forest dominated by Terminalia (Combretaceae) and Eugenia (Myrtaceae) species and numerous palms (Aracacaeae) and pandans (Pandanacaeae). The relatively dense understory contained various woody species (including saplings of overstory species), ground palms and rattans (Aracacaeae), gingers (Zingiberaceae), a variety of forbs, and climbing epiphytes.
Species
M. browni and M. richardsii are broadly sympatric throughout the New Georgia group in most forest habitats below 800 m. Little is known about the natural history of these common forest insectivores, and until recently M. richardsii was thought to be monomorphic like other M. castaneiventris allospecies (Filardi 2003). In fact, it is sexually dimorphic, with the adult male characterized by glossy blue-black upperparts, throat, and breast, a rich chestnut abdomen, and conspicuous snow-white feathering of the occiput, cap and nape. The adult female is duller, with grey upperparts, throat and breast, a variably chestnut abdomen, and small but variable amounts of white around the occiput, supercilliary, and sides of nape. Subadult male plumage is generally similar to that of females (though less variable), and the immature bird of either sex is dull gray with a dull chestnut abdomen and pale chestnut wash on the breast (Filardi 2003). In contrast to M. richardsii, in M. browni, both sexes are similar in appearance. The adult is generally black and white overall, with upperparts, throat, and breast glossy black, and differing amounts of white on the underparts, sides of throat, rump, wings, and tail (dependent on subspecies; Fig. 1). The immature bird is dramatically different, with gray-brown or dark brown above, a blackish tail with white tips, dark gray or blackish throat, and pale rufous underparts.
Like many Monarcha species (Coates 1990, Schodde and Mason 1999), roosting, foraging and vocal behavior suggest that both M. richardsii and M. browni defend territories (CEF, pers. obs.). Although it is not known whether these territories are defended year-round, both species show evidence of breeding throughout the year (CEF, unpubl. data). Preliminary UV reflectance scans of both species confirm the absence of sex-related variation in M. browni and of any visually cryptic signaling in M. richardsii (J. A. Uy, Syracuse University, unpubl. data).
Identification of Territories
Both M. richardsii and M. browni consistently roosted and sang from discreet areas that could be roughly mapped. If birds were encountered within an area three days in succession, the area was considered an active territory. An area approximately 20 m2 was then designated as the territory core based upon the location of encounters with singing or roosting monarchs. Mount presentations were conducted from within this core.
Song Recording and Playback
Behavioral responses of territorial individuals to a trespasser were measured using mounted specimens and playback of locally recorded vocalizations. All recordings were made using a Sennheiser ME66 Short Shotgun directional microphone (with a K6P powering module; Sennheiser Electric Corporation, Old Lyme, Connecticut) attached to a TCM-5000 analog recorder (Sony Corporation of America, New York, New York). For the mount presentations (see below), playbacks were done by connecting a 7.62 cm AA battery-powered external speaker (Radio Shack Corporation, Fort Worth, Texas) to the TCM-5000 via a 20 m cable connector. For the simulated territorial intrusions (see below), songs were recorded using the same equipment as with the mount presentations, but because of the incidental nature of these experiments, playbacks were done using the recorder's built-in speaker as opposed to external speakers.
Mount Presentations
Mounts were prepared in the field from local birds in a posture similar to that observed in a bird singing or calling in the presence of a conspecific individual; wings were slightly drooped, tail slightly fanned, and head up and bill slightly open, as if singing. Using recorded vocalizations in conjunction with mounted specimens is potentially problematic because some of the behavioral responses may be attributed to the recording and not the mount. To control for this effect, in addition to conspecific mounts, we presented a neutral control mount. Cockerell's Fantails (Rhipidura cockerelli) occupy the same midstrata and subcanopy habitat as M. richardsii and M. browni, and neither they nor monarchs are aggressive toward one another (CEF, pers. obs.). We played the same tapes of monarch vocalizations used with the monarch mounts while presenting the fantail mounts. Differences between responses to fantail mounts and monarch mounts can thus be attributed to the effects of the monarch mounts and not the recordings. At each M. richardsii territory, we presented a total of three mounts: an adult male M. richardsii mount, a subadult or female plumaged M. richardsii mount, and a control mount. The plumages of the subadult and female mounts were indistinguishable (Filardi 2003). At each M. browni territory, we presented a total of two mounts: an adult-plumaged M. browni and a control mount.
All mounts (three for M. richardsii and two for M. browni) were presented on one of two similar poles (made from a local tree species in which both species commonly perch) and were situated approximately 2 m above the ground, and within 5 cm of vegetation on which responding birds could alight without being forced to contact the mount.
We presented one mount per territory per day and systematically altered the sequence of presented mounts of differing plumage from one territory to the next. We conducted presentations between the hours of 06:15 and 10:00 and between 15:00 and 17:00. Presentations during the midday hours were avoided because many species regularly become inactive during the midday heat. At each territory, all presentations were conducted at the same place and at the same time of day.
We attracted birds to the mounts by playing a standard sequence of locally recorded conspecific vocalizations with the remote playback speaker placed approximately 1.5 m below the mount. During each mount presentation, the observer was partially concealed 10–15 m from the mount (depending on the density of the understory) and started the playback after a 5 min quiet period. We used a 3 min section of tape to call birds in and then switched to a 10 min tape when a responding bird became visible. Variable plumage characters evident from a distance (e.g., extent of white on the breast or head, plumage wear) were used to verify that the same individuals responded to the different presentations on each territory. Situations in which the identity of the responding individual was uncertain were eliminated from further analyses. Responses to the mounts were characterized for 10 min starting when a responding individual was sighted. All birds responded for the full 10 min period. In total, we presented mounts on 22 M. richardsii territories and 20 M. browni territories.
Quantifying Responses to Intruders
To quantify responses to mount presentations, we identified eight behavioral variables for M. richardsii and M. browni during a set of trial mount presentations (see Appendix for detailed description of variables). Behavioral differences between the two species mandated slightly different sets of variables. Briefly, four variables were identical for both species. Two categories of flight distances were recorded (i.e., “number of flights >5 m” and “number of flights <5 m”), as most birds made either a limited number of flights far from the mount or many flights close to the mount. Also, individuals of both species would sometimes make repeated flights over a mount in a manner that differed in character and flight posture than typical movements within 5 m of the mount. Thus, “number of flights over mount” quantified the relative agitation of a responding bird. Finally, for both species, we recorded “time spent within 1 m of the mount” to measure interest in remaining within close proximity to the mount.
Four additional variables were quantified for M. richardsii. “Time out of view” measured the response of birds that counter-sang from the subcanopy or distant understrata before approaching the mount. “Number of hits to the mount,” “time spent in flutter-fan display,” and “number of nips” (to the vent and flanks) indexed behavior around the mount. Two behaviors identified in the trial presentations (“whistle” and “scold” displays; see Appendix) were discarded, as they appeared to be a reflexive response to playback and thus could not be used to discern differential responses to the mounts. Four additional variables, “number of burry whistles,” “time spent in wing flick display,” “number of times mount was circled,” and “time spent in wing flutter display” were used to further characterize M. browni's response to the mount presentations.
Statistical Analyses
All statistical analyses were completed using the program StatView 5.0 (SAS Institute 1998). For each species, behavioral variables used to quantify a bird's response to the mounts were combined into two behavioral response scores using unrotated principal components extracted from a Spearman correlation matrix (Rohwer 1978, Langston et al. 1990, Pearson and Rohwer 1999). To simplify the component loadings for interpretation and analysis, principal component scores were orthogonally rotated using Varimax criterion (Cooley and Lohnes 1962).
Using the principal component loadings for each species, we first performed repeated measures ANOVA to assess overall differences in response to the presentations of the different mount types. Second, because we presented three mount types (adult, subadult, and control) to M. richardsii, following Pearson and Rohwer (1999), we used a randomized-block ANOVA and Scheffé's multiple comparisons to compare responses among mount types when three mounts were presented on each territory. Additionally, we compared responses of paired and single M. browni using t-tests.
Simulated Territorial Instrusions
From 1999 to 2001, we opportunistically simulated territorial intrusions on a suite of mono- and dichromatic Melanesian species. These intrusions were performed in the forests of Madang, Chimbu, Gulf, Central and West New Britain Provinces of Papua New Guinea and in Malaita, Guadalcanal, Isabel, Choiseul, and Western Provinces of the Solomon Islands. We identified territories (via crepuscular song; see above) of five monochromatic and six dichromatic bird species and used locally recorded song to solicit a response from resident birds.
To simulate territorial intrusions, we played a standardized tape for each species for approximately 3–8 minutes and recorded the number of responding birds. A bird was considered responsive if it approached to within approximately 10 m of the playback speaker and counter-sang or scolded, or moved onto a perch within 5 m of the speaker. Individual birds that simply flew through the area without stopping or that maintained distant, unseen perches and counter-sang were considered unresponsive. In total, we simulated intrusions on 246 territories with one or more responding birds. Because taxa used for simulated intrusions were diverse and encountered opportunistically, we were unable to fully standardize our methods, and statistical analyses are necessarily limited both in scope and implication. To test differences in the responses of monochromatic and dichromatic species, we performed sign tests on the data for each species and then performed a Fisher's Exact Test on the pooled data for species for which results were statistically significant.
Results
Defining Principal Components
Responses to the mount presentations by territorial individuals were varied, with some attacking the mount outright and others failing to approach it at all. Birds that actively responded to the mount differed significantly by species in both the behavioral quality of their responses (e.g., M. browni never struck the mount, but M. richardsii commonly did) and in the quantitative intensity of their responses (Table 1, Appendix). Single adult males responded for all 22 M. richardsii presentations, whereas 13 of 20 presentations on M. browni territories induced responses from two individuals. A response by two individuals complicated quantification of behavior, but in all cases, we were able to follow the actions of the individual that responded most vigorously to the playback and mount. In general, this primary-responding individual tended to dominate interactions with the mount, with the other bird scolding or watching silently from farther away.
Summary of mean ± SE of the behaviors measured in response to the presentation of conspecific adult-plumaged and adult male-plumaged mounts of Monarcha browni and M. richardsii, respectively. Mounts were presented on active territories in lowland forest of New Georgia, Solomon Islands in 2000 and 2001. Behaviors that do not apply to one or the other species are marked N/A (see Appendix for detailed explanations of behaviors).
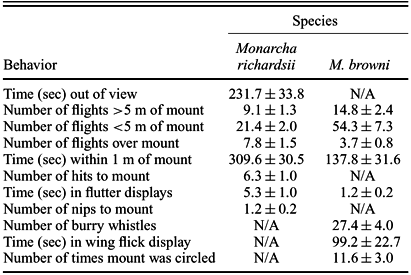
Summary of mean ± SE of the behaviors measured in response to the presentation of conspecific adult-plumaged and adult male-plumaged mounts of Monarcha browni and M. richardsii, respectively. Mounts were presented on active territories in lowland forest of New Georgia, Solomon Islands in 2000 and 2001. Behaviors that do not apply to one or the other species are marked N/A (see Appendix for detailed explanations of behaviors).

Principal component analysis extracted two components that described >70% of the variation in the responses of both M. richardsii and M. browni (Table 2) and thus are considered valid measures of differing behavioral responses to the mount presentations. Two additional factors support the use of the first and second principal components (hereafter PC1 and PC2, respectively) for our analyses. First, for both species, only PC1 and PC2 were associated with eigenvalues greater than one (eigenvalues not presented; Cooley and Lohnes 1962). Second, only components that explain more than 1/n percent of the variance (here n = 8 behavioral variables; i.e., 13%) are generally considered informative. In both species, PC1 and PC2 explain well over that threshold (Table 2), and in each case, the next component (PC3) explained less than 13% of the remaining variance.
Principal component loadings for each of eight behavioral response variables to 10 min mount presentations by (A) Monarcha richardsii, and (B) M. browni in lowland forest of New Georgia, Solomon Islands in 2000 and 2001. The majority of variance in the data is explained by the first two principal components (PC1 and PC2), which measure aggressive responses and nonaggressive solicitation responses, respectively.
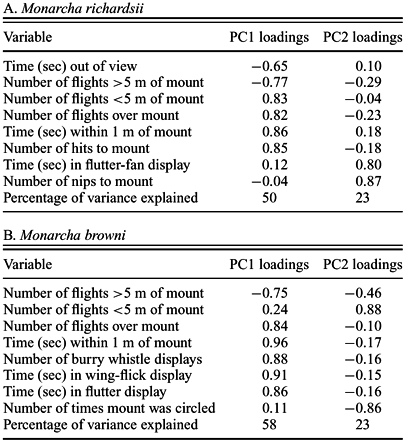
Principal component loadings for each of eight behavioral response variables to 10 min mount presentations by (A) Monarcha richardsii, and (B) M. browni in lowland forest of New Georgia, Solomon Islands in 2000 and 2001. The majority of variance in the data is explained by the first two principal components (PC1 and PC2), which measure aggressive responses and nonaggressive solicitation responses, respectively.

In both M. richardsii and M. browni, PC1 appears to provide a measure of aggression (hereafter “aggression score”). In M. richardsii, number of flights <5 m from the mount, flights over the mount, time within 1 m of the mount, and number of hits to the mount all received strong positive loadings (Table 2A). In contrast, less aggressive behaviors received loadings near zero (flutter-fan and number of nips) or strong negative loadings (time out of view and flights >5 m from the mount). For M. browni, number of flights over the mount, time within 1 m, and the burry whistle, wing flick, and flutter displays all received strong positive loadings (Table 2B). In contrast, number of flights >5 m from the mount received a strong negative loading. The loadings for the remaining variables were weak, but interpretation may be complicated by M. browni's responses being subtler than those of M. richardsii.
In both species, PC2 appears to measure solicitation behavior (hereafter “solicitation score”). In M. richardsii, only flutter-fan display and number of nips received strong positive loadings, whereas the behaviors with strong positive loadings for aggression generally received negative loadings (Table 2A). The weak positive loading for time within 1 m of the mount is again consistent with PC2 measuring a solicitation response. Similarly, for M. browni, PC2 loadings were strongly positive for number of flights within 5 m of the mount and time circling the mount (Table 2B), behaviors consistent with a responding individual nonaggressively soliciting a response from the (static) mount.
Responses of M. Richardsii to the Differing Mounts
Figure 2 compares the aggression and solicitation scores for responses to the two monarch mounts on 22 M. richardsii territories. We were able to present the fantail control mounts on only 17 territories. Because the variance in behavioral responses to the controls was minimal (Fig. 2), we simply compared the responses of individuals to the mounts. Responses to subadult male mounts (n = 16) and to a female mount with similar plumage (n = 6) were not significantly different (t = 0.94, P = 0.38) and were therefore pooled for all subsequent analyses (hereafter referred to collectively as “subadult”).
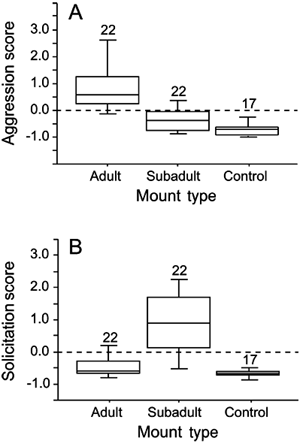
Scores of (A) aggression and (B) solicitation responses to three mount presentation types (adult male, subadult and female, and control) by Monarcha richardsii on 22 territories, New Georgia, Solomon Islands in 2000 and 2001. Controls were presented on 17 territories. Sample sizes are given above each category.
Three key results support plumage traits of M. richardsii functioning as signals in social competition. First, on each of all 22 territories of the dichromatic M. richardsii on which a mount was presented, a single adult male responded. Second, the overall ANOVA comparing aggression scores for M. richardsii males responding to the three mount types was highly significant (F2,16 = 22.9, P < 0.001). Responding males were significantly more aggressive toward the adult male mounts than to the subadult mounts (P < 0.001; Fig. 2A) or to the controls (P < 0.001), with no difference in aggression between controls and subadult mounts (P = 0.27). Third, solicitation scores for subadult mounts were significantly higher than those for adult mounts or controls (P < 0.001; Fig. 2B for both comparisons) and did not differ significantly between males responding to controls and adult mounts (P = 0.59). The overall ANOVA comparing solicitation scores across all three mounts was again highly significant (F2,16 = 26.9, P < 0.001).
Responses of M. Browni: Single Versus Paired Response
We completed mount presentations of adult male M. browni on 20 active territories. On each of 13 of these, two individuals responded to the mount. The remaining seven presentations were on territories either known or suspected to have had one or two formerly resident birds removed (in two of the seven cases, adult females were removed from the territories; the other five cases involved removal of adult males). In all seven of these cases, a single bird responded to the mount and playback, allowing for a contrast between the responses of presumed territorial pairs and either newly resident or newly unpaired birds. Time constraints permitted presentation of the fantail control on only 13 of the 20 M. browni territories.
Figure 3 compares aggression and solicitation responses for single and paired M. browni to the monarch mount and the control. Individuals responding as part of a pair were significantly more aggressive toward the monarch mount than toward the control (t = 16.2, P < 0.001). In contrast, singly responding birds showed similar aggressive response toward the monarch mount and controls (t = −1.0, P = 0.34). The birds responding in a pair were significantly more aggressive than those responding singly (t = 4.1, P < 0.001). For solicitation scores, the reverse was true (Fig. 3B). Singly responding birds showed significantly more solicitation toward the monarch mount than did the primary responders from a pair (t = −2.6, P = 0.02). Solicitation scores for single responders to the monarch mount differed significantly from solicitation scores by these same individuals to controls (t = 11.7, P < 0.001), as did those for paired responders (t = 8.7, P < 0.001).
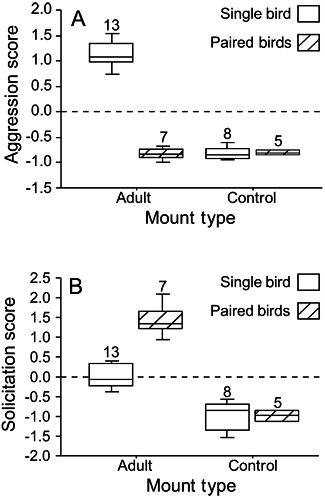
Scores of (A) aggression and (B) solicitation responses by single and paired individuals to adult-plumaged and control mounts by Monarcha browni on 20 territories, New Georgia, Solomon Islands in 2000 and 2001. Controls were presented on 13 territories. Sample sizes are given above each category.
Simulated Territorial Intrusions
Consistent with predictions of social selection theory (West-Eberhard 1983), in our 246 simulated territorial intrusions, we found that monochromatic species generally responded in pairs, and dichromatic species tended to respond singly. In some species (both monochromatic and dichromatic), three or more individuals sometimes responded to the playbacks (Table 3). Because these group responses were difficult to quantify and could be an artifact of the cursory methods used to define territories (i.e., playbacks were conducted on territory boundaries), they were excluded from analyses. Comparing only paired versus single bird responses, seven of the 11 species (three dichromatic and four monochromatic; Table 3) had sample sizes sufficient to be statistically significant. For these seven species, overall differences in the responses of dichromatic and monochromatic species were significant (Fisher's Exact P = 0.03).
Characterization of the responses of five dichromatic and six monochromatic Melanesian passerines to simulated territorial intrusions. Intrusions were conducted opportunistically across Papua New Guinea and the Solomon Islands in 1999–2001, using locally recorded vocalizations to solicit responses from resident birds. Values in parentheses indicate number of intrusions that elicited responses from three or more birds and were excluded from analyses.
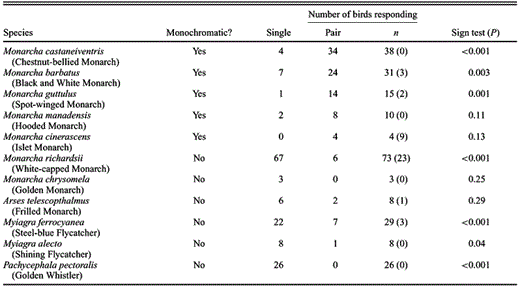
Characterization of the responses of five dichromatic and six monochromatic Melanesian passerines to simulated territorial intrusions. Intrusions were conducted opportunistically across Papua New Guinea and the Solomon Islands in 1999–2001, using locally recorded vocalizations to solicit responses from resident birds. Values in parentheses indicate number of intrusions that elicited responses from three or more birds and were excluded from analyses.

Discussion
Social Signals in M. Richardsii
Consistent with results of studies on other Pacific monarchs (VanderWerf and Freed 2003, Cibois et al. 2004) and more broadly across birds (Price 2007), the differential responses of M. richardsii to adult and subadult mounts suggest that plumage traits function as social signals in this species, implicating social selection in their evolution. In all mount presentations, territorial M. richardsii males responded more aggressively to adult male mounts than to subadult mounts. In contrast, responding males appeared to solicit responses from subadult mounts, possibly to establish whether the intruder represented a threat (i.e., a competitor) or an opportunity (i.e., a possible mate). However, although beyond the scope of this study, determining that social signals function as potential speciating mechanisms—as opposed to simple engines for morphological divergence—requires demonstrating correlated mate preference for the distinctive traits separating allopatric populations.
Differential responses by territorial males are consistent with a number of possible (but nonexclusive) evolutionary mechanisms that relate to honest or deceptive signaling of subordinance, reproductive maturity, or sex (Studd and Robertson 1985, Lyon and Montgomerie 1986, Foster 1987, Kallioinen et al. 1995). For instance, between-class plumage variability may have evolved in response to living at least part of the time in groups, within which social competition results in a hierarchy that determines access to critical resources. In such a case, signaling subordinance or dominance could be advantageous (Rohwer and Ewald 1981, Rohwer et al. 1981, Rohwer 1982, Studd and Robertson 1985, Kallioinen et al. 1995). Incidental observations of what appear to be large, nonbreeding flocks of individuals of varying appearance (and presumably age classes) provide some support for this scenario. Data on the breeding status, sex, and age of individuals in such flocks of monarchs are lacking, but once obtained, will provide important clues about social competition and life history strategies in this and other species of Melaniesian flycatchers.
The adaptive significance of between-class plumage variability in males may also explain differential responses to mounts. Presentations of subadult mounts elicited solicitory responses from responding males and aggressive responses no different than to controls. Adult male mounts, in contrast, were aggressively attacked. The conspicuous white crown, eye ring, and nape of the adult male M. richardsii distinguishes its plumage from all other M. castaneiventris allospecies and is consistent with the idea that arbitrary yet novel plumage characters may evolve as reliable indicators of fighting ability (Rohwer 1982, Butcher and Rohwer 1989, Rohwer and Røskraft 1989, McDonald et al. 2001). Once such a trait becomes fixed in a population (as the narrow plumage variation among adult M. richardsii males suggests; Filardi 2003), it ceases to confer information with respect to fighting ability and becomes a signal of class identity (i.e., adult male). In such a case, unknown adult male–plumaged birds are expected to represent a general threat to defended resources. Subadult or immaturely plumaged males would not present an equivalent threat and would therefore be treated differently. Among M. richardsii, high-quality territories and associated breeding opportunities may be difficult to obtain, and young males may benefit by advertising their subordinance and thus delaying the development of mature adult male plumage, as has been suggested for other Pacific monarchs (VanderWerf and Freed 2003).
Contrasting Dichromatic and Monochromatic Species
M. browni mount presentations provide an important contrast to those of M. richardsii. Sexually monomorphic development of socially selected traits should evolve when both sexes compete with other same-sex conspecifics. Therefore, both males and females of monomorphic bright (following the definition in Butcher and Rohwer 1989) species like M. browni should respond to territorial intrusions with degrees of aggression highest in same-sex interactions. On active territories from which an individual had not previously been removed, two birds responded to the mount presentations. In most cases, differences in behavior between presumed males and females suggested that the two responding individuals were a mated pair and also that the male responded more aggressively (CEF, unpubl. data). On two occasions, after completing the mount presentations, the two responding birds were collected, and the primary responder was identified as a male and the other as a female. This is consistent with the nature of the playbacks (dominated by male song and calls) and mounts (generally adult males) used in our experiments eliciting more aggressive response from the same-sex, or adult male, territory resident.
Further support for paired territorial defense consistent with predictions of social selection is evident in the seven presentations on M. browni territories from which one individual had been previously removed. In all cases, singly responding birds were not aggressive and actively solicited a response from the intruding monarch mount. Our observations that both sexes of this monochromatic and bright-plumaged species may defend territorial resources, and that single birds occupying a territory may test intruders for their potential as mates, supports the operation of social selection in molding monarch plumages.
Simulated territorial intrusions for 11 additional taxa strengthen support for a broad influence of social selection on plumage variability across multiple taxa. Across all 11 taxa, individuals of monochromatic species generally responded in pairs, whereas those of dichromatic species tended to respond singly. Although statistically marginal, the results of these 246 individual simulated territorial intrusions are broadly consistent with the potential for certain patterns of plumage variation in these bird groups having evolved in response to selective pressures on social signaling. Importantly, some of the taxa in these tests (e.g., Pachycephala and Monarcha spp.) exhibit some of the most complex geographic patterns of plumage variation among birds, including dramatic shifts in sexual dichromatism.
Although simulated intrusions with responses from two or more individuals were excluded from analyses, patterns of group responses across species are likely to have biological significance. For example, in the dichromatic species M. richardsii, birds often responded in groups, possibly reflecting the presence of large postbreeding flocks that appear to form at certain times of the year. In the future, more detailed studies within individual populations are needed to clarify the significance of apparent responses from three or more birds to simulated territorial intrusions in both monochromatic and dichromatic Melanesian passerines.
Social Selection and the Geography of Plumage Variation
The results of the studies presented here provide support for the existence of plumage signals in monarchs, but our behavioral tests are limited in their taxonomic scope and compel more detailed study of social signaling across the great diversity of Melanesian birds that exhibit marked geographic variation in plumages. In particular, mount presentations to monochromatic allospecies of Solomon Islands chestnut-bellied monarchs (e.g., M. castaneiventris) will broaden understanding of the relative role of plumage signaling in the dramatic population differentiation exhibited by this endemic species group. Notably, the simulated territorial intrusions did include numerous M. castaneiventris territories, and their responses were consistent with those of other monochromatic species. Presenting mounts of the different chestnut-bellied allospecies to one another (e.g., presentation of M. castaneiventris to M. richardsii) might expose the efficacy of current plumage differences as mechanisms of premating isolation across narrow geographic barriers and between populations with very little genetic differentiation (Filardi and Smith 2005). Testing the specific function of plumage traits in different age classes of M. richardsii will require the manipulation of plumage traits on mounted specimens for presentation and on free-living marked individuals via plumage dyeing (Rohwer 1978, Rohwer and Røskraft 1989), or the use of other types of mount presentation experiments (see VanderWerf and Freed 2003).
Manipulating the plumages of free-living individuals or mounts will also improve understanding of plumage patterns in M. browni, which has four subspecies across the New Georgia group with distinct patterns of white and black on the wings, head, breast, and tail. Additionally, the endemic pied monarchs of the Solomons offer an opportunity to explore the function of distinct juvenile plumages in territorial passerines. Like many of the pied monarch species, M. browni has a juvenile plumage with extensive rufous feathering that differs dramatically from adult plumages. The adaptive significance of distinctive juvenile plumages is poorly understood, but presentations of juvenile and adult mounts across a series of taxa will be informative. Also, mount presentation experiments on monochromatic and dichromatic (e.g., M. trivirgatus) pied monarchs across their range could be compared to similar contrasts among chestnut-bellied species (e.g., M. melanopsis species complex) to yield additional insight into the role social competition plays in driving geographic patterns of dichromatism.
In theory, social selection should operate in all contexts and on any organism in an appropriate social context. However, insular bird populations should be particularly subject to divergence mediated by selection for traits that serve individuals in social competition. Geographic isolation, even if incomplete, in combination with relatively small population sizes, should increase the opportunity for fixation of novel traits (e.g., plumage characters) that are linked to competitive ability. Further applying this adaptationist approach to some of the famously variable bird groups such as the genera Pachycephala and Petroica, and nonpasserines such as variable kingfisher taxa (e.g. Tanysiptera and Halcyon spp.; Price 2007) may add a critical tool to biogeographic studies of island birds. If, as results presented here suggest, social selection is an important speciation mechanism in island radiations, a series of phylogenetically independent contrasts among bird groups with differing patterns of geographic variation may greatly improve understanding of the origins of diversity among island birds.
Acknowledgments
This study was inspired by the work and intellect of S. Rohwer and would not have been possible without his thoughtful support and guidance. S. Birks, P. D. Boersma and S. Edwards provided helpful comments on an earlier draft. S. Pearson gave invaluable statistical assistance and advice about presenting mounts to wild birds. D. Steadman and an anonymous reviewer provided helpful comment, and both D. Dobkin and A. Bontrager provided detailed editorial comment that greatly improved the final product. M. Biliki, J. Horokou, and T. Masolo at the Solomon Islands Ministry of Natural Resources, and D. Malasa at the Ministry of Education facilitated the necessary permitting and assisted with logistics. D. Kwon and others at Eagon Resource Development Company provided access to the study site and generous hospitality. J. Tasker Kikolole was and continues to be a field mentor to CEF in the Solomons; we are indebted to his bushcraft, seamanship, and easy way with the sublime. South Pacific Oil has provided key logistic support in the Solomons. We are also grateful to J. and L. Entrikin and Zipolo Habu, M. Hemmer, and many other Solomon Islanders—without their patient support, guidance, and friendship, our time in the Solomons would have been far less possible, productive, and pleasurable.
Literature Cited
Appendix
Description of variables used to quantify responses of territorial Monarcha richardsii and M. browni to presentations of mounted conspecifics and controls.
For both species:
Number of flights and perch shifts >5 m from the mount. All flights and perch shifts >5 m from the mount.
Number of flights and perch shifts <5 m from the mount. All flights and perch shifts <5 m from the mount that did not involve movement directly over the mount.
Number of flights over the mount. All flights that involved movement directly over and within 2 m of the mount.
Time (sec) spent within 1 m of the mount. Includes perching in vegetation close to the mount, on the mount perch itself, and sequential, short flights over the mount. Often accompanied by vocalization (quantified separately; see below).
For Monarcha browni only:
Number of burry whistles. Number of times a series of 3–12 low burry whistled notes (sometimes uttered singly) was given; the most diagnostic call of this species; usually given in response to playback or presence of other individuals.
Time (sec) spent in wing-flick display. Time spent with tail sharply cocked, neck arched, throat hackles raised, and wings flicked incessantly flashing the large patch of white upper wing coverts; usually accompanied by buzzy, mechanical scold-like notes in sets of three.
Time (sec) spent in wing flutter display. Time spent with head held low, tail not cocked but slightly fanned, and folded wings rapidly fluttered close to the flanks.
Number of times mount was circled. Number of times a responding bird methodically circled the mount, hopping from perch to perch with a posture similar to that described for the wing-flick display; usually silent.
For Monarcha richardsii only:
Time (sec) spent out of sight or greater than 15 m from mount. Measured response of males that counter-sang from the subcanopy or distant understrata but were unwilling to approach the mount. In some habitats, birds were visible at great (>15 m.) distances from the mount, while in others, they were not consistently visible at any distance greater than 8–10 m.
Number of hits. Number of times a bird hit the mount from a nearby perch, in flight, or from the mount perch.
Time (sec) spent in flutter-fan display. Time spent with head lowered, neck outstretched, crown and throat feathers raised, and tail fanned widely while wings were fluttered rapidly; no vocalizations associated with display.
Number of nips and probes to vent and lower flanks. Measured behavior of individuals who cautiously approached the mount, apparently trying to elicit a response.
Time (sec) spent in whistle display (not used in analyses). Time spent emitting loud, sweet, whistled notes with head lowered and neck outstretched, wings sometimes fluttered, tail fanned and the feathers of the crown and throat raised. Birds sometimes hitched their bodies from side to side while displaying. Whistles (1–2 per second) and posture maintained for several seconds to a minute or more before a pause. Displays were timed from initiation to first pause of three or more seconds.
Time (sec) spent in scold display (not used in analyses). Similar to whistle display except the vocalization was a series of scoldy, “check-check” notes and raspy upslurs; head was not lowered.



