-
PDF
- Split View
-
Views
-
Cite
Cite
Juan Ignacio Areta, Behavior and Phylogenetic Position of Premnoplex Barbtails (Furnariidae), The Condor: Ornithological Applications, Volume 109, Issue 2, 1 May 2007, Pages 399–407, https://doi.org/10.1093/condor/109.2.399
Close - Share Icon Share
Abstract
The Margarornis assemblage includes four genera: Margarornis, Premnoplex, Premnornis, and Roraimia, all thought to be closely related. Differences in vocalizations and habitat use between Premnoplex brunnescens (Spotted Barbtail) and P. tatei (White-throated Barbtail) are consistent with their full species status. In light of the weak anatomical (hindlimb musculature) evidence supporting the inclusion of Premnornis in the Margarornis assemblage, the convergence-prone nature of characters associated with climbing habits, and the differences in their nests and foraging behavior, I propose that Premnornis is not a member of the Margarornis assemblage and that Premnoplex is closely related only to Margarornis. These results are supported by recent molecular phylogenetic analyses. Although Roraimia differs in plumage, tail shape, and song from other members of the Margarornis assemblage, it must be provisionally included in the assemblage until evidence clarifies its phylogenetic position.
Resumen
El ensamble Margarornis incluye cuatro géneros: Margarornis, Premnoplex, Premnornis y Roraimia, aparentemente cercanamente emparentados. Las diferencias de hábitat y voces de Premnoplex brunnescens y de P. tatei son consistentes con su estatus específico. A la luz de la débil evidencia anatómica (musculatura apendicular) apoyando la inclusión de Premnornis en el ensamble Margarornis, la naturaleza convergente de los caracteres asociados a los hábitos trepadores y las diferencias en comportamiento y nidificación, propongo que Premnornis no es un miembro del ensamble Margarornis y que Premnoplex estaría solamente cercamente emparentado a Margarornis. Estos resultados son congruentes con recientes estudios moleculares. Aunque Roraimia difiere en plumaje, forma de la cola y canto de otros miembros del ensamble Margarornis, debe ser provisionalmente incluido en él hasta que más evidencia aclare su posición filogenética.
Introduction
There are two species of Premnoplex barbtails, little-known ovenbirds (Furnariidae) that inhabit humid montane forests in South and Central America. P. brunnescens (Spotted Barbtail) comprises five mountain-dwelling subspecies present in Costa Rica, Panama, and from the Coastal Cordillera of Venezuela through the Andes to Bolivia, whereas P. tatei (White-throated Barbtail) is endemic to northeastern Venezuela and consists of two distinctive subspecies: P. t. tatei in the Turimiquire Massif and P. t. pariae in Península de Paria (Phelps and Phelps 1949, 1963, Hilty 2003, Remsen 2003). Evidence for species status of both taxa is considered to be weak (Remsen 2003, Remsen et al. 2006), and some authors (e.g., Hellmayr 1938, Vaurie 1980) have considered them conspecific. Some classifications (e.g., Meyer de Schauensee 1966, Vaurie 1980) merge Premnoplex into Margarornis. Both Premnoplex species are included in the so-called “Margarornis assemblage,” which consists of eight species in the genera Margarornis, Roraimia, Premnornis, and Premnoplex (Vaurie 1980, Rudge and Raikow 1992a, 1992b). This grouping is largely based on structural (size, wing shape, tarsus proportions), anatomical (hindlimb musculature), and plumage pattern and coloration features (Vaurie 1980, Rudge and Raikow 1992a, 1992b). Relationships among members of the assemblage are not clear, and hypotheses derived from structural and anatomical features conflict with those suggested by natural history and molecular data (Rudge and Raikow 1992b, Dobbs et al. 2003, Irestedt et al. 2006).
Natural history data have proven useful for predicting the phylogenetic relationships of furnariids (Kratter 1994, Rodrigues et al. 1994, Whitney and Pacheco 1994, Kratter and Parker 1997), but the ecology and behavior of the Premnoplex barbtails have been poorly described and are almost unknown for the localized P. tatei (Remsen 2003). Herein I document habitat use and analyze the systematic significance of vocalizations, behavior, and morphology of Premnoplex barbtails. I also reanalyze plumage, morphological, and nesting characters of members in the Margarornis assemblage in an attempt to evaluate the validity of the grouping and the position of Premnoplex within it.
Methods
I studied Premnoplex brunnescens (probably subspecies rostratus) for ca. 100 days from March 28 to June 25 2005 in Parque Nacional Yacambú, Estado Lara, Venezuela (9°24′N, 69°30′W, 1200–1800 m asl), during both prebreeding and breeding seasons. Premnoplex tatei pariae was studied for two days during 8 and 9 July 2005 in Parque Nacional Peninsula de Paria, Estado Sucre, Venezuela (10°41′N, 62°36′W, 700–1150 m asl), apparently during the breeding season. I recorded data on habitat, behavior, and vocalizations for both species. I studied nesting only in P. brunnescens. Recordings of P. brunnescens vocalizations were made with a Sennheiser ME-66 directional microphone and a Sony TCM-5000 tape recorder. P. tatei vocalizations were recorded with an Audio-Technica at-835b directional microphone and a Marantz PMD-222 tape recorder.
I examined and measured 189 specimens housed at the Colección Ornitológica Phelps in the four genera comprising the Margarornis assemblage (29 P. brunnescens brunnescens, nine P. b. rostratus, 26 P. tatei tatei, 49 P. t. pariae, 42 Margarornis squamiger perlatus [Pearled Treerunner], 12 Premnornis guttuligera venezuelana [Rusty-winged Barbtail], and 22 Roraimia adusta adusta [Roraiman Barbtail]). Bill length (exposed culmen) was measured to the nearest 0.05 mm with digital calipers, and wing chord and tail length were measured with a metal ruler to the nearest 0.5 mm.
Results
Habitat
Both Premnoplex barbtails frequented cool, epiphyte-laden, dark rainforest. In Parque Nacional Yacambú, Premnoplex brunnescens was always found close to or at ravines or rivers, both during the breeding and nonbreeding seasons. The species is known to favor humid areas in montane evergreen rainforests, particularly dense undergrowth with vines alongside watercourses (Slud 1964, Skutch 1967, Vaurie 1980; JIA, pers. obs.). In 2.7 km of suitable habitat along the Quebrada Negra, I found 22 territories, giving a density of 8.2 pairs per linear km, or one territory every 120 m.
Premnoplex tatei was found in mossy, epiphyte-laden montane forest in Parque Nacional Peninsula de Paria, usually in flat mountaintop areas with large stands of palms and unidentified Heliconia-like plants (see Hilty [1999, 2003] for habitat in Turimiquire). Only once did I find P. tatei in typical P. brunnescens habitat: a pair was located by a creek with extreme humidity, ferns, and decaying wood as outstanding features. In 1 linear km of palm-dominated habitat I found five P. tatei territories, or one territory every 200 m.
Vocalizations
The noisy environment of Premnoplex brunnescens made it extremely difficult to obtain clear recordings of its vocalizations. I was only able to obtain recordings of its most commonly used call: a short, descending, trilled “prriiip” (Fig. 1A). Three other vocalizations were also heard: a sharp and high-pitched “pssiit” and a cricket-like, high-frequency “ti-ti-ti-ti-ti-ti-ti-ti-ti-ti-ti” slowly descending in pitch and tempo. Only once did I hear its complex and long song, which reminded me of the songs of some Cranioleuca spinetails. Slud (1964), Fjeldså and Krabbe (1990), and Hilty (2003) described the same vocalizations, although using a different phonetic notation. The most common call was heard roughly 95% of the time, and the other two vocalizations were heard 5% of the time. I also obtained recordings of the most common call of Premnoplex tatei. This was the only vocalization that I heard, although its repertoire is surely greater. Its call is reminiscent in pattern to that of P. brunnescens, but differs by being lower in pitch and deeper and somewhat bubbled with an underwater quality (Fig. 1B). This vocalization was highly variable in length, intensity, and tempo, varying from two to five notes, from mildly low to strong in volume, and from a leisurely series of notes to a frantic bubbling that was never as fast as that of P. brunnescens. Vocalizations of P. tatei have previously been described as an “almost bubbly ser. of rather low, soft, reedy whistles, we-whúr, we-whúr, we-heét…varied to be-be-búr, be-be-búr… or pi, pr-pr-pr-prip!… in long ser. when excited” (Hilty 2003:490). Both species used their calls routinely while foraging, moving, perching, or when alarmed, which, when added to song structure, strongly suggests homology.
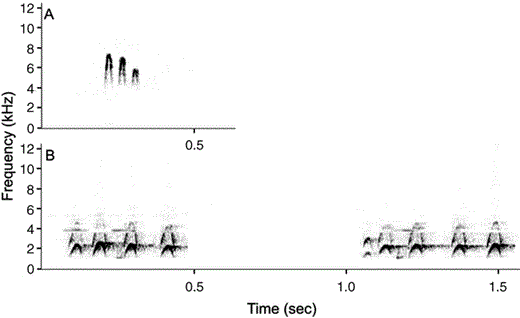
Spectrograms of the most common vocalizations of Premnoplex barbtails. A) P. brunnescens call, recorded at Parque Nacional Yacambú, 11 April 2005. B) P. tatei pariae calls, recorded at Parque Nacional Península de Paria, 9 July 2005. Note the higher pitch and faster delivery rate of each note of P. brunnescens. The interval between successive calls is shorter in P. tatei than in P. brunnescens, thus two consecutive calls are shown for P. tatei. These differences in their calls support their treatment as two separate species.
Behavioral Observations
Wing-flicking and Horizontal Hopping
Two distinctive behaviors of the Premnoplex barbtails that I observed have not previously been noted in the literature: constant high wing-flicking and horizontal hopping. Although some furnariids occasionally flick their wings (Whitney and Pacheco 1994), this behavior is taken to an extreme in Premnoplex. Almost constantly, while foraging, perching, or simply moving, both species flicked their wings high over their backs, in the manner of many Neotropical flycatchers (Tyrannidae). Both wings were flicked at the same time, and this flicking was performed while the body was held in any position, from upright perching to clinging to vegetation while hanging upside down. Horizontal hopping was a behavior that both species displayed when alarmed. Birds would perch in an upright position on a horizontal branch and hop from side to side while facing the intruder. Each hop was accompanied by wing-flicking and the most common call. Individuals usually hopped many times to one side before hopping in the other direction. In the case of Premnoplex brunnescens, this behavior was performed each time I approached a nest with nestlings, and both parents would hop with their beaks full of worms for their nestlings. I did not witness this behavior outside the breeding season, thus I consider this behavior to be an indication of breeding. Therefore, although I did not find a nest of Premnoplex tatei, the horizontal hopping behavior they directed at me suggested that they were nesting (see Breeding).
Foraging
Premnoplex brunnescens foraged mostly by probing and gleaning while climbing small mossy trees, moving along horizontal branches and vines, hanging upside down, or creeping over and under logs. The species only occasionally foraged on the ground (Slud 1964, Wetmore 1972, Remsen 2003; JIA, pers. obs.). While foraging, individuals could spend several minutes carefully searching for arthropods among mosses and epiphytes or in tree bark. All birds were found feeding alone or in pairs, never as part of mixed-species flocks. In contrast to Wetmore (1972), I never saw P. brunnescens searching dead leaves, despite their prevalence in their habitat. As pointed out by Slud (1964) and Hilty (2003), P. brunnescens only rarely used its tail for support while stationary or as a prop while climbing tree trunks. Foraging height ranged from ground level to 5 m, but birds were usually found between 2 and 4 m. In contrast, Premnoplex tatei foraged mostly on the ground and less commonly on tree trunks and branches. While hopping on the ground, its wings usually remained closed, without the characteristic wing-flicking. When foraging in vegetation, it moved much like its congener, but when foraging on the ground, it stuck its head into holes in fallen trunks and rotten logs and frequently tossed leaves aside with its bill to look for arthropods. P. tatei, like P. brunnescens, typically foraged alone or in pairs, although I once observed four P. tatei (a pair and two probable fledglings) foraging in a mixed-species flock composed of Basileuterus tristriatus (Three-striped Warbler) and Myrmotherula schisticolor (Slaty Antwren). Foraging height ranged from ground level to 2 m, but most birds were found on the ground or below 1 m. Hilty (1999) reported a single bird foraging on the ground or on logs (never climbing) at heights from 0.2 to 3 m, loosely associated with a pair of Basileuterus griseiceps (Gray-headed Warbler). A video of a foraging P. tatei on the ground by D. Ascanio (Ascanio Birding Tours) is available at <http://www.hbw.com/ibc/phtml/especie.phtml?idEspecie = 4178>.
Breeding
I found and examined over 35 nests of Premnoplex brunnescens. All were built of moss and were attached to rocks, trees, or roots usually in dark, hidden, and extremely wet areas, but never in what could strictly be considered a cavity. This coincides with previous descriptions (Skutch 1967, Zyskowski and Prum 1999). Nests were usually placed in sites above fast-flowing water, often close to cascades or in areas reached by water spray. The nests were mossy balls with the entrance usually facing downward, and had an inner platform on which the eggs were laid (not visible from the outside). The platforms bent upwards close to the nest entrance, preventing the eggs from falling through the vertical tunnel entrance (see drawing in Skutch [1967]). Nestlings of P. brunnescens showed a complex behavior associated with defecation: both nestlings would turn inside the nest and, while perching on the inner platform, would defecate through the nest entrance; most feces were removed by the water flowing underneath the nest. This behavior frees parents from fecal-sac removal duties and is also shown by Margarornis (Mennill and Doucet 2005). The nest structure and nest-site locations of Premnoplex tatei remain undescribed. Although riverine nesting cannot be excluded, there were no permanent rivers in the habitat where P. tatei was found at Parque Nacional Peninsula de Paria. Available habitat use data suggest that its nest might be built in preexisting clumps of moss, under tree branches, or in crevices in habitat somewhat different from that of P. brunnescens. The finding of apparent fledglings and display of lateral hopping behavior by adults suggests that breeding was going on while fieldwork was carried out.
Comparative Morphology of the Margarornis Assemblage
General Morphology
Three main results emerge when measurements of tail length, wing chord, and exposed culmen of some members of the Margarornis assemblage are compared (Table 1). First, geographic variation in the Premnoplex barbtails is evident when subspecies are analyzed separately (a fact obscured due to extreme species lumping in Vaurie [1980]). Secondly, Premnoplex barbtails have relatively longer bills and shorter tails and wings than the remaining genera, accounting for their wren-like aspect and behavior (Wetmore 1972, Vaurie 1980). Lastly, Premnornis is the only genus in which tail length exceeds wing chord.
Wing chord, tail length, and exposed culmen measurements of some members of the Margarornis assemblage. Values are given in mm as mean ± SD (with number of individuals measured in parentheses). Specimens were housed at the Colección Ornitológica Phelps.
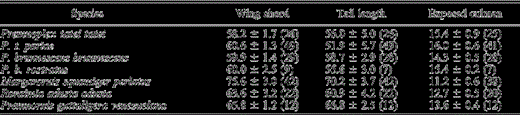
Wing chord, tail length, and exposed culmen measurements of some members of the Margarornis assemblage. Values are given in mm as mean ± SD (with number of individuals measured in parentheses). Specimens were housed at the Colección Ornitológica Phelps.

Tail Shape
Tail shape is extremely variable and plastic in the Furnariidae and can be considered a rough indicator of phylogenetic relationships, with closely related species often having similar tails (Remsen 2003, Fjeldså et al. 2005). Tails of all members of the Margarornis assemblage are composed of 12 rectrices that are more or less graduated (Vaurie 1980). I distinguished three different tail types: the Premnoplex-Margarornis type (Fig. 2A), the Roraimia type (Fig. 2B), and the Premnornis type (Fig. 2C). Tails of Margarornis and Premnoplex are very similar, showing feathers with well-integrated webs ending abruptly by the asymmetrical reduction of vanes, and prolonged in a protruding spine with reduced (and sometimes absent) vanes. The tail of Roraimia has notably less-integrated webs, and feather ends are pointed (“v-shaped tip”) due to the more gradual reduction of the vanes and the lack (or near lack) of spines in tails in good condition. The tail of Premnornis differs strikingly from the others in rectrix shape: the tips are much more rounded, show no asymmetrical vane reduction toward the tip, and lack barbs or spines. Integration of webs is intermediate between that of Roraimia and Premnoplex-Margarornis. Tail shapes change due to abrasion, sometimes so much that they lose diagnostic features (Fig. 2), which must be taken into account when studying tail shape in the group.
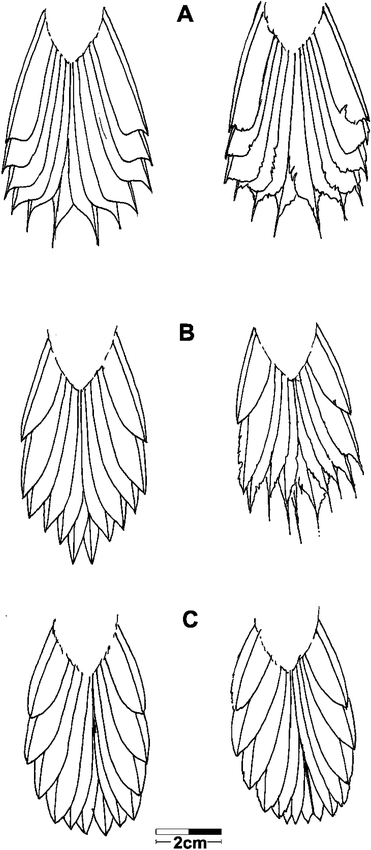
Tail shapes of members of the Margarornis assemblage. A) Premnoplex–Margarornis, B) Roraimia, and C) Premnornis. Tails in good condition are shown on the left side, worn tails on the right side. Note the loss of diagnostic features in the worn tails of Premnoplex–Margarornis and Roraimia, which could suggest a false similitude among their tails. Sketches were based on the examination of individuals housed at the Colección Ornitológica Phelps.
Plumage
An exhaustive plumage analysis of the Margarornis assemblage is beyond the scope of this paper, but some features and ideas are worth noting (see Zimmer [1934], Wetmore [1972], Vaurie [1980], and Remsen [2003] for detailed plumage descriptions). Margarornis and Premnoplex share a common ventral pattern, with the feather edges enclosing a drop-shaped paler center. This pattern is well developed in Margarornis bellulus (Beautiful Treerunner), Margarornis squamiger, Premnoplex tatei tatei, and all subspecies of Premnoplex brunnescens, but less developed in Margarornis stellatus (Fulvous-dotted Treerunner) and Margarornis rubiginosus (Ruddy Treerunner). Notably, Premnoplex tatei pariae shows a different pattern: the dark margin of each breast feather is limited to the sides, hence there is no drop-shaped center, but a light, broad, central stripe, bordered by a narrow dark line to each side. The ventral pattern of Roraimia is similar to that of P. tatei pariae but the central, light part of each feather is wider, thus giving it a strongly flammulated appearance. The facial pattern of Roraimia adusta is strikingly similar to that of Synallaxis [Poecilurus] candei (White-whiskered Spinetail), and Synallaxis [Poecilurus] kollari (Hoary-throated Spinetail): all have a boldly patterned throat, dark facial stripe and auriculars, and a broad reddish-orangish eyebrow that extends downward passing behind the facial stripe, which differs notably from other members of the Margarornis assemblage. Moreover, when the throat feathers of Roraimia are closely examined, black is obvious at their bases exactly where the black gular spot of both previously mentioned Synallaxis (Poecilurus) spp. appears, suggesting a close relationship among these forms. Premnornis differs from the other three genera in that the color of the tail strongly contrasts with the color of the back, but otherwise it is similar in plumage to the Premnoplex-Margarornis barbtails. Without a formal phylogenetic analysis, it is not clear whether the plumage similarities reflect a close relationship or homoplasy.
Discussion
Premnoplex tatei was initially described as a new species by Chapman (1925), but was subsequently considered a subspecies of Premnoplex brunnescens by Hellmayr (1938) and Vaurie (1980). Meyer de Schauensee (1966) and Remsen (2003) treated them as separate species, but published evidence for either treatment is weak (Remsen et al. 2006). Differences presented here regarding vocalizations, foraging mode, and habitat use support the validity of P. brunnescens and P. tatei as separate species.
In furnariids, morphological features associated with foraging are far more plastic than nest-building behaviors (including nest shape, nest placement, and construction materials), as shown by Limnoctites-Cranioleuca and Limnornis-Phleocryptes (Olson et al. 2005), Geositta (Cheviron et al. 2005), and Anumbius-Coryphistera (Zyskowski and Prum 1999, Fjeldså et al. 2005). Thus, a weighting of the characters to be used for phylogenetic reconstruction becomes justified and necessary: more weight should be given to the characters that convey more accurate phylogenetic information (e.g., nesting characters) and less weight given to plastic or convergent-prone characters (e.g., feeding adaptations).
Morphological convergence seems a pervasive phenomenon among trunk-climbers, and the role of morphology in elucidating phylogenetic relationships is usually limited, because convergent adaptations can mask actual phylogenies (Feduccia 1973, Irestedt et al. 2004). Rudge and Raikow (1992b) nonetheless based their phylogenetic analysis of the Margarornis assemblage on myiological hindlimb characters that could represent convergent adaptations for foraging (McCracken et al. 1999, Irestedt et al. 2004).
Rudge and Raikow (1992b) proposed a closer relationship between Premnoplex and Premnornis within the Margarornis assemblage. However, Dobbs et al. (2003) described the nest of Premnornis guttuligera as a cup made of tree-fern petiole scales inside a cavity, contrasting with the mossy ball-shaped nest attached to trees or banks built by Premnoplex and Margarornis (Feduccia 1970, Fjeldså and Krabbe 1990, Skutch 1996, Remsen 2003, Mennill and Doucet 2005). This marked contrast in nest structure prompted Dobbs et al. (2003) to suggest that Premnornis was misplaced within the Margarornis assemblage. Some furnariid species that usually have domed nests have been reported to build cups when nesting in cavities (Johnson 1967, Zyskowski and Prum 1999), but to date no nest of Premnoplex or Margarornis has been found totally within a cavity (contra Irestedt et al. 2006; JIA, pers. obs.), a fact that strengthens the use of a cup-shaped cavity nest in Premnornis as evidence for its exclusion from the Margarornis group (Dobbs et al. 2003). Moreover, the peculiar behavior of defecating through the nest entrance shown by nestlings of Premnoplex and Margarornis would be precluded if nests were placed wholly in a cavity.
A reanalysis of the two anatomical synapomorphies in Rudge and Raikow (1992b) that support the Premnoplex-Premnornis clade reveal their weaknesses as phylogenetic indicators. First, the origin of Tendon M could be a symplesiomorphy or convergence of Premnoplex and Premnornis. Rudge and Raikow (1992b:765) stated that in Premnoplex (brunnescens) and Premnornis, “Tendon M originates entirely from the tibial lobe of M. flexor cruris lateralis pars pelvica,” but in the next sentence write, “A similar modification has also been found among the dendrocolaptines.” Assuming, rather than testing, monophyly of the clade, they go on to conclude that “given the monophyly of the treerunners, [the character] is derived for the clade consisting of Premnoplex brunnescens and Premnornis guttuligera.” In short, Rudge and Raikow (1992b) seem to disregard information suggesting that this character is not necessarily a synapomorphy, but that it can also be interpreted as a symplesiomorphy or as a convergent character. Second, the absence of the third belly of the M. lumbricalis could be an artifact. Rudge and Raikow (1992b) stated that the presence of a third belly in the M. lumbricalis is a synapomorphy of Premnoplex (brunnescens) and Premnornis. However, they noted that the character is not present in every examined individual. The lack of the third belly in some individuals is accounted for by suggesting that it could be due to a preparation artifact because the structure is so small. An alternative explanation seems equally likely: the lack of the third belly in the other species could also be a preparation artifact, not being preserved in any of the few studied individuals in other genera. Additionally, reduced taxon sampling in Rudge and Raikow (1992b) renders their work unsuitable to test for monophyly of the Margarornis assemblage.
Premnornis is not scansorial and almost half of its foraging attacks were directed toward prey in or on suspended dead leaves (Dobbs et al. 2003), unlike Premnoplex and Margarornis, which are scansorial and seldom forage in dead leaves (Fjeldså and Krabbe 1990, Sillett 1994; JIA, pers. obs.). Finally, the reversed tail to wing chord ratio of Premnornis, and its mildly acuminate tail shape with no spines, differs strikingly from Premnoplex. In the light of the convergence-prone nature of characters associated with climbing habits that were suggested as supporting the inclusion of Premnornis in the Margarornis assemblage, and the differences in tail shape, external morphology, nests, and foraging behavior, I propose that Premnornis is not a member of the Margarornis assemblage.
Similarities in vocalizations (high-frequency trilled songs; Moore and Lysinger 1997, Mayer 2000), nests (mossy balls with a lateral or underside entrance; Skutch 1967, Feduccia 1970, Fjeldså and Krabbe 1990, Mennill and Doucet 2005), foraging behavior (scansorial habits and epiphyte-searching; Ridgely and Tudor 1994, Sillett 1994, Remsen 2003), nestling behavior (autonomous defecation; Mennill and Doucet 2005), and tail shape (feathers with barbed tips and asymmetrically reduced vanes; Wetmore 1972, Vaurie 1980) point to a close relationship between Margarornis and Premnoplex (Remsen 2003). Data on osteology and pterylosis are compatible with this view (Wetmore 1972, Clench 1995). No other furnariid genera show such a combination of features; thus, Premnoplex and Margarornis are probably sister taxa, and the core of the Margarornis assemblage.
The phylogenetic relationships of Premnoplex, Margarornis, and Premnornis suggested by natural history, external measurements, and tail shape agree with phylogenetic hypotheses based on DNA sequencing data, which found Premnornis to form a clade with Pseudocolaptes, and Premnoplex to group with Margarornis in a distant clade (Irestedt et al. 2006). These independently derived and coincident hypotheses support each other and disagree with the sister relationship of Premnoplex and Premnornis proposed by Rudge and Raikow (1992b) on anatomical grounds, suggesting that the guttate ventral pattern has evolved many times in the furnariids (Irestedt et al. 2006).
The relationships of Roraimia adusta are enigmatic. Originally described as a Synallaxis spinetail, a monotypic genus was later created for it, despite the lack of any diagnostic character, based on number of rectrices (12 in Roraimia, 10 in Synallaxis), tail shape (pointed in Roraimia, usually rounded in Synallaxis), and hind-toe claw size (long in Roraimia, Margarornis, and Premnoplex, short in Synallaxis; Chapman 1929). Roraimia has a composite plumage pattern, with facial features of some Synallaxis (Poecilurus) spp., a ventral pattern reminiscent of Premnoplex tatei pariae but even more flammulated, and tail shape intermediate between that of Premnoplex-Margarornis and Premnornis. The song of Roraimia differs in pattern, tempo, and harmonic structure from songs of Premnoplex and Margarornis (D. Ascanio and JIA, unpubl. data). The situation of Roraimia is analogous to that of Gyalophylax, which is deemed to be intermediate between Asthenes and Synallaxis (Whitney and Pacheco 1994). The placement of Roraimia in the Margarornis assemblage should be considered provisional until data on genetics, natural history, vocalizations, and nesting are published.
Acknowledgments
I wish to thank the many Venezuelan partners who stimulated my work with the Premnoplex barbtails: Violeta Gómez Serrano was a wonderful field partner both in Yacambú and at Las Melenas, and David Ascanio provided information on the places were P. tatei could be found and shared his recordings of Roraimia. Laura Hernández contributed some papers when the study was beginning. Miguel Lentino and Margarita Hernández at the Colección Ornitológica Phelps kindly guided me through their library and granted permission to study the collection and consult the useful notes of the Phelps. The drawings were skillfully prepared by Robin Restall, who also provided crucial help, becoming an important contributor to this publication despite the geographical distance. The comments of Jorge Pérez and James Van Remsen greatly improved the structure and content of this paper, which also benefited from suggestions made by Kristof Zyskowski and an anonymous reviewer. This study was made possible in part by support from National Science Foundation grants DEB-9981527 and DEB-0543178 to T. E. Martin. Finally, very special thanks to Josecho, Javi, Joaco, and my parents for continuous support and encouragement throughout my life.
Literature Cited
Author notes
E-mail: [email protected]



