-
PDF
- Split View
-
Views
-
Cite
Cite
Susannah C. Buhrman-Deever, Amy R. Rappaport, Jack W. Bradbury, Geographic Variation in Contact Calls of Feral North American Populations of the Monk Parakeet, The Condor: Ornithological Applications, Volume 109, Issue 2, 1 May 2007, Pages 389–398, https://doi.org/10.1093/condor/109.2.389
Close - Share Icon Share
Abstract
Introduced feral populations offer a unique opportunity to study the effects of social interaction and founder effects on the development of geographic variation in learned vocalizations. Introduced populations of Monk Parakeets (Myiopsitta monachus) have been growing in number since the 1970s, with a mixture of isolated and potentially interacting populations. We surveyed diversity in contact calls of Monk Parakeet populations in Connecticut, Texas, Florida, and Louisiana. Contact call structure differed significantly among the isolated populations in each state. Contact call structure also differed significantly among potentially interacting nest colonies in coastal Connecticut, and these differences did not follow a geographic gradient. Limited dispersal distances, founder effects, and social learning preferences may play a role in call structure differences.
Resumen
Las poblaciones introducidas que se han naturalizado ofrecen una oportunidad única para estudiar la influencia de las interacciones sociales y del efecto fundador sobre el desarrollo de la variación geográfica en las vocalizaciones aprendidas. Las poblaciones introducidas de Myiopsitta monachus han aumentado desde los años setenta, y actualmente existe una mezcla de poblaciones aisladas y poblaciones que podrían estar interactuando. En este estudio, examinamos la diversidad de las llamadas de contacto de poblaciones ubicadas en Connecticut, Texas, Florida y Louisiana. La estructura de las llamadas de contacto difirió significativamente entre las poblaciones aisladas en cada estado. La estructura de dichas llamadas también difirió significativamente entre colonias de nidificación que potencialmente podrían interactuar en la costa de Connecticut, y las diferencias observadas no siguieron un gradiente geográfico. Las distancias de dispersión reducidas, los efectos de fundador y las preferencias sociales del aprendimiento podrían jugar un papel en generar las diferencias en la estructura de las llamadas.
Introduction
Geographic variation in vocalizations has been documented in many species with vocal learning. Most research has focused on passerines (Baptista 1977, Payne 1978, Kroodsma et al. 1999), but similar variation is known for a variety of nonpasserines (Peake and McGregor 1999), rodents (Eiler and Banack 2004), primates (Mitani et al. 1992), pinnipeds (Van Parijs et al. 1999), and cetaceans (Helweg et al. 1998, Rendell and Whitehead 2005). In addition, geographic variation has been found in several species of parrots (Wright 1996, Baker 2000, 2003, Bradbury et al. 2001, Bond and Diamond 2005, Kleeman and Gilardi 2005), and there is evidence that parrots, like songbirds, attend to these differences in the wild (Wright and Dorin 2001, Vehrencamp et al. 2003).
Many hypotheses have been proposed to explain the development and maintenance of geographic variation in vocalizations of species with vocal learning. Call structure may be adapted for transmission through the local environment (Hansen 1979). Founder effects and cultural drift may cause elements to be lost through sampling error or accident; these may be especially important factors in establishing differences among small or isolated populations (Catchpole and Slater 1995, Lynch 1996). Local dialects may be important in mate choice; females may prefer to mate with males from their natal area (Nottebohm 1969) to maintain locally adaptive alleles in their offspring. Social interactions may also be a strong selective force for local geographic variation. In many passerines, song-type matching is a key signal in male–male agonistic interactions (Vehrencamp 2001, Wilson and Vehrencamp 2001). Group-specific call types may be necessary in a variety of taxa for coordinating group activities or identifying group members and discriminating between different groups (Nowicki 1989, Boughman and Wilkinson 1998, Wilkinson and Boughman 1998).
Social factors may be particularly important in the development of local homogeneity in parrot vocalizations. Laboratory studies of Budgerigars (Melopsitta undulatus) have shown that individuals converge on group or pair contact call types after prolonged social interaction (Farabaugh et al. 1994, Bartlett and Slater 1999, Hile et al. 2000), but it remains unclear whether this also occurs in the wild. Some species (e.g., Yellow-naped Amazons [Amazona auropalliata]) have sharp dialect boundaries (Wright 1996), while others have graded variation on a scale commensurate with measured home-range size (e.g., Orange-fronted Conures [Aratinga canicularis]; Bradbury et al. 2001). Differing patterns of geographic variation in call structure may be the result of different social pressures (Bradbury et al. 2001).
Feral parrot populations offer an interesting opportunity to study the effects of group dynamics and social interactions on the development of geographic variation in vocalizations. Geographically disparate introduction events have created isolated populations, some of which have expanded and now form contiguous, interacting populations. Comparing the vocalizations of these isolated and interacting populations could help shed light on the roles of social interaction and cultural drift on the evolution of geographic variation.
Monk Parakeets (Myiopsitta monachus) are a good candidate species for such a study. Native to South America (Bolivia and Southern Brazil south to central Argentina), they were first introduced to the U.S. in the late 1960s and 1970s, and populations have been expanding exponentially ever since (Van Bael and Pruett-Jones 1996, Spreyer and Bucher 1998). Monk Parakeets have established breeding populations in at least 11 states (Spreyer and Bucher 1998) and, nationwide, the population now numbers in the thousands (Spreyer and Bucher 1998, National Audubon Society 2002). Christmas Bird Counts in Connecticut alone counted over 1800 individuals (National Audubon Society 2002).
The Monk Parakeet is the only parrot that builds its own stick nests (Forshaw 1989, Spreyer and Bucher 1998). Nests can be over a meter in diameter (Spreyer and Bucher 1998). Many have multiple chambers, and thus can house several pairs. A large percentage of Monk Parakeet social interactions occur at nests, where they rest during the day and sleep at night (Spreyer and Bucher 1998). Although we have no data for U.S. populations, approximately 50% of adult birds changed nests between years in Argentina, with a median distance moved of approximately 500 m (Martin and Bucher 1993). Similarly, little is known about home ranges in the U.S., however data from Argentina indicate that daily home range can be up to 10 km, although ranges are more usually 3–5 km in diameter (Spreyer and Bucher 1998). Natal dispersal in Argentina is most often restricted to within 2 km of the natal territory (Martin and Bucher 1993).
In this study, we investigated geographic variation of loud contact calls of feral Monk Parakeets in the U.S. The loud contact call is the most common call of the Monk Parakeet (Martella and Bucher 1990), and is given by both sexes in many contexts, including foraging, flying, and approaching the nest. It is a short (approx. 160 msec) and rapidly modulated call, with most of the energy focused between 1 and 3 kHz (Martella and Bucher 1990). We recorded and compared contact calls from isolated feral populations of Monk Parakeets in New Orleans, Louisiana; Austin, Texas; Miami, Florida; and coastal Connecticut. Monk parakeets have been present in Miami since 1969, in Connecticut since the 1970s, and in New Orleans and Austin since at least the 1980s (Spreyer and Bucher 1998, National Audubon Society 2002). We also compared contact calls recorded at six colonies in coastal Connecticut, to examine patterns of fine-scale geographic variation in potentially interacting populations.
Methods
We recorded feral Monk Parakeets either at or within a few city blocks of nesting colonies from March to April 2004 in Connecticut, New Orleans, Miami, and Austin. We recorded calls from three additional colonies in Connecticut in July 2004. All recordings were made with a Sennheiser ME67K6 shotgun microphone and a Marantz PMD 670 or PMD 690 hard drive recorder, digitized at 16 bits and 48 000 Hz. All calls were high-pass filtered at 600 Hz to remove low-frequency noise. We selected clear contact call recordings, free of background interference, for analysis.
It was often difficult to pick out a single parakeet in a large, mobile flock, but including repeat recordings of the same individual could overestimate local homogeneity. Thus, we used the following two techniques to ensure analyzed calls were from different individuals: 1) we only selected calls recorded from birds either as they left or joined groups, thereby avoiding repeated recordings from group members, 2) because preliminary analysis indicated that individuals may use a consistent call type (SCB-D, unpubl. data), we culled repeat exemplars from the analysis. Removing what appeared to be the same call type reduced the risks of inflating our samples with repeat calls from the same bird; thus, by excluding these calls, we reduced within-site relative to among-site homogeneity. This should decrease the likelihood of finding differences among the populations. Therefore, removing very similar call types from our samples is a conservative safeguard (Bradbury et al. 2001, Cortopassi and Bradbury 2006).
Statistical Analyses
To compare spectral differences among populations, we performed spectrographic cross-correlation and principal coordinates analyses (Legendre and Legendre 1998) using a specially designed Matlab (Mathworks 2006) routine (K. Cortopassi, Cornell University, unpubl. data; see Vehrencamp et al. [2003] for further details). We first generated spectrograms of the selected calls for each region (512-point FFT, 90% overlap, Hanning window). The spectrograms were then cross-correlated with all other calls in the sample, generating a symmetrical matrix of similarity values for all calls, on a scale of 0 (complete mismatch) to 1 (exact match). The similarity matrix was run through a principal coordinates analysis in the Matlab routine. To test for differences among populations, we performed a one-way multivariate analysis of variance (MANOVA; Tabachnick and Fidell 2001) in JMP® IN 5.1 (SAS Institute 2003) on the principal coordinate values for each call. For both the within-Connecticut and among-regions MANOVA tests, we used the first five principal coordinates, which explained 87% of the call variation in each case. Because call duration can affect cross-correlation similarity values, we also did a regression of principal coordinates values on call duration, and recalculated the MANOVA using the regression residuals (Bradbury et al. 2001). Both the original and the duration-corrected results are reported. All data were transformed and outliers excluded when necessary to conform to the assumptions of normality for the tests.
The spectrographic cross-correlation and principal coordinates technique has been shown to be very sensitive in finding subtle variation in the frequency and temporal structure of parrot calls (Cortopassi and Bradbury 2000). Such subtle variation is often difficult to detect using specific call measurements. However, it is also useful, if possible, to attempt to find measures that vary systematically, to understand which aspects of calls influence geographic differences. Thus, in addition to the above analyses, we also measured call length and several frequency contour characteristics using the frequency measurement contour plugin (Cortopassi 2006) of the XBAT 0.3 measurement routine (Figueroa 2006) in Matlab. This routine determines the harmonic frequency contour in a user-specified range using autoregression to predict the signal frequency over short periods, followed by a Viterbi-based tracking method to determine the best track through those estimated frequencies (Cortopassi 2006). We extracted the following measurements from this contour: duration, start, end, minimum, mean, and peak frequency, and the number of inflections (number of changes of slope). We found that our analysis settings (data length = 512 points, overlap = 90%, number of poles = 90, frequency range = 2–6 kHz) successfully traced the second harmonic of the contact call. We conducted a principal components analysis (PCA) on the frequency and duration measures, then used analysis of variance (ANOVA) to compare the different populations across the principal components in JMP® IN 5.1. We only compared the principal components that were strongly correlated with at least one measure (a factor loading of at least 50%). We thus compared the first three principal components for both the among-regions and within-Connecticut analyses.
To determine if there was a geographic gradient of call similarity for the Connecticut colonies, we performed a Ward's hierarchical clustering analysis on the distance between each colony's calls in JMP® IN 5.1. We calculated the acoustic difference between each colony's calls by computing the Euclidean distance between the centroid of each colony's calls in the five-dimensional principal coordinate space generated from the spectrographic cross-correlation and principal coordinates analysis (Cortopassi and Bradbury 2006).
Results
For the among-regions comparisons, we selected 20 calls from three nesting colonies in Austin (two, one, and 17 calls per colony), eight calls from three nesting colonies in Miami (five, one, and two calls per colony), 16 calls from six nesting colonies in New Orleans (four, two, five, two, one, and two calls per colony), and 37 calls from three nesting colonies in Connecticut (15, 13, and nine calls per colony). The nesting colonies in Austin, Miami, New Orleans, and Connecticut were all separated by equivalent distances (approximately 2–7 km). We used 65 calls from six different nesting colonies in Connecticut (15, eight, 13, nine, 12, and eight calls per colony) for the comparisons between potentially interacting populations (Fig. 1).
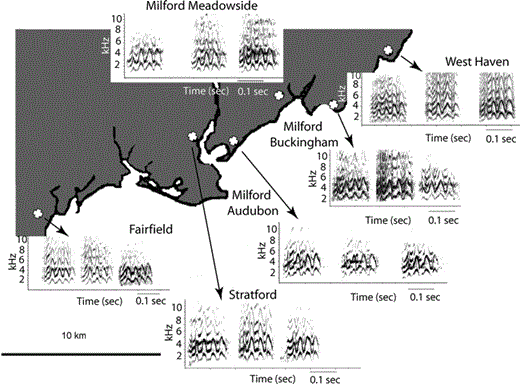
Map of the locations of six Connecticut recording sites used to survey feral Monk Parakeet call diversity. Sample spectrograms of three different Monk Parakeet contact calls from each nesting colony are linked to their respective recording locations with arrows and labels. All spectrograms are on the same scale, and were generated with the same parameters (256 FFT size, 90% overlap, Hanning window). The spacing between the calls is not meaningful.
Among Regions Comparison
Examples of contact calls from Austin, Miami, and New Orleans can be seen in (Fig. 2). Comparisons of the principal coordinates extracted from contact calls of the isolated populations revealed significant differences among regions (Wilk's λ = 0.26, η2 = 74%, P < 0.001), and this result held when we corrected for call length (Wilk's λ = 0.33, η2 = 67%, P < 0.001). The principal coordinates-based canonical plot revealed that Austin and Connecticut calls occupied separate regions of principal coordinates space from the other localities (Fig. 3). Only Miami and New Orleans overlapped; post-hoc contrasts revealed no significant difference between the Miami and New Orleans populations (F5,72 = 2.0, P = 0.08). However, the PCA and ANOVA analyses revealed no significant differences among the populations in any of the principal components generated from the call frequency and duration measures (ANOVA, PC1: F3,70 = 0.7, P = 0.58; PC2: F3,70 = 2.3, P = 0.08; PC3: F3,70 = 0.9, P = 0.44; factor loadings for each principal component are shown in Table 1). Therefore, although there were significant differences in overall call structure, there were no consistent differences in duration or any of the frequency measures among the populations.
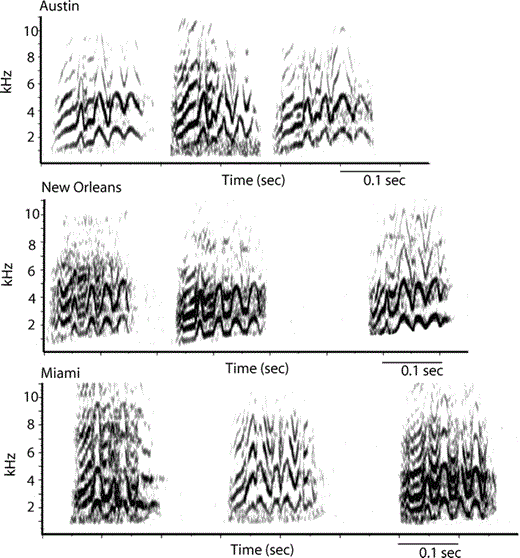
Spectrograms of three representative Monk Parakeet contact calls from Austin, Miami, and New Orleans. All spectrograms are on the same scale, and were generated with the same parameters (256 FFT size, 90% overlap, Hanning window). The spacing between the calls is not meaningful.
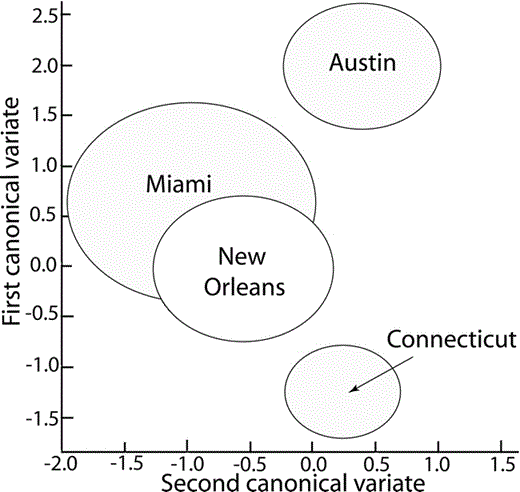
Location of the Monk Parakeet contact calls from each state on the first two canonical axes generated in the spectrographic cross-correlation–principal coordinates–MANOVA analysis. The canonical axes are a linear combination of the principal coordinates that maximally separate the call regions. The first canonical variate produces the best separation; the second canonical variate the next-best separation after the first variate's effects have been removed. The size of the circle represents the 95% confidence interval for the estimate of the mean centroid location.
Results of a principal components analysis on duration and frequency contour measures comparing Monk Parakeet contact calls from Austin, Texas; Miami, Florida; New Orleans, Louisiana; and coastal Connecticut. The factor loading for each call measure is reported for each component. All variables with loadings less than 50% (meaning less than 25% of the variance is accounted for by that variable) for a particular principal component are indicated with a dash. Only the first three principal components had at least one variable with a factor loading of at least 50%.
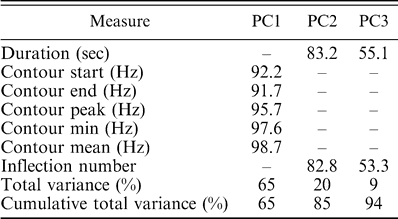
Results of a principal components analysis on duration and frequency contour measures comparing Monk Parakeet contact calls from Austin, Texas; Miami, Florida; New Orleans, Louisiana; and coastal Connecticut. The factor loading for each call measure is reported for each component. All variables with loadings less than 50% (meaning less than 25% of the variance is accounted for by that variable) for a particular principal component are indicated with a dash. Only the first three principal components had at least one variable with a factor loading of at least 50%.

Within-connecticut Comparison
Comparisons of the extracted principal coordinates from the contact calls of the six different nesting colonies revealed significant differences (MANOVA, Wilk's λ = 0.18, η2 = 82%, P < 0.001; corrected Wilk's λ = 0.15, η2 = 85%, P < 0.001; Fig. 4). Post-hoc contrasts revealed significant differences between the three southernmost and three northernmost sites (F5,55 = 0.5, P < 0.001), between Fairfield and Stratford, the two most southern sites (F5,55 = 0.4, P < 0.002), and between Milford Buckingham and Milford Meadowside (F5,55 = 0.4, P < 0.004).
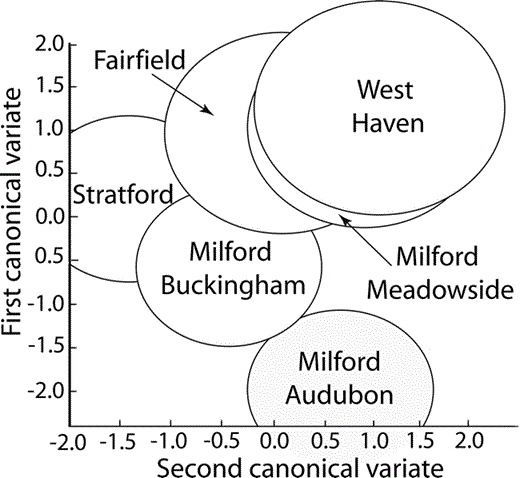
Location of the centroids of the Monk Parakeet contact calls from each nesting colony in Connecticut on the first two canonical axes generated in the spectrographic cross-correlation–principal coordinates–MANOVA analysis. The canonical axes are a linear combination of the principal coordinates that maximally separate the call regions. The first canonical variate produces the best separation; the second canonical variate the next-best separation after the first variate's effects have been removed. The size of the circle represents the 95% confidence interval for the estimate of the mean centroid location.
The results of the principal components analysis are listed in Table 2. There was no significant difference among the populations in PC1 (ANOVA, F5,54 = 0.5, P = 0.78) or PC3 (ANOVA, F5,54 = 2.1, P = 0.08), but there were significant differences in PC2 among sites (ANOVA, F5,54 = 3.4, P = 0.009), with both Stratford and Milford Buckingham significantly different from both Milford Meadowside and Fairfield (Tukey-Kramer HSD, P < 0.05). Thus, in addition to the overall structural differences found in the spectrographic cross-correlation and the principal coordinates analysis, Connecticut populations can be separated in terms of the mean frequency, initial frequency, and minimum frequency of their calls.
Results of a principal components analysis on duration and frequency contour measures comparing Monk Parakeet contact calls from six Connecticut nesting colonies (Fairfield, Stratford, Milford Audubon, Milford Meadowside, Milford Buckingham, and West Haven). The factor loading for each call measure is reported for each component. All variables with loadings less than 50% (meaning less than 25% of the variance is accounted for by that variable) for a particular principal component are indicated with a dash. Only the first three principal components had at least one variable with a factor loading of at least 50%.
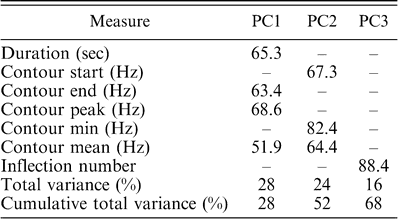
Results of a principal components analysis on duration and frequency contour measures comparing Monk Parakeet contact calls from six Connecticut nesting colonies (Fairfield, Stratford, Milford Audubon, Milford Meadowside, Milford Buckingham, and West Haven). The factor loading for each call measure is reported for each component. All variables with loadings less than 50% (meaning less than 25% of the variance is accounted for by that variable) for a particular principal component are indicated with a dash. Only the first three principal components had at least one variable with a factor loading of at least 50%.

Geographic Distance and Call Similarity
The cluster analysis revealed a lack of geographic gradient in vocal diversity for the Connecticut populations (Fig. 5). This result is not surprising given the results of the MANOVA analysis (Fig. 4). For example, the Milford Meadowside and West Haven colonies were closest in vocal similarity, even though they were separated by 7.5 km, whereas the Milford Audubon and Stratford colonies were separated by only 2.6 km, but were quite different vocally.
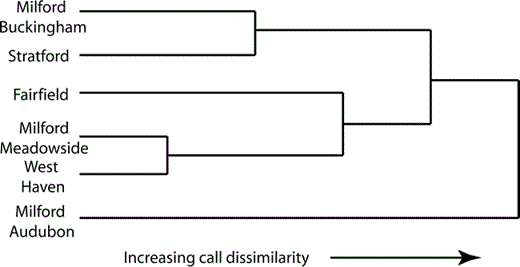
Results of Ward's clustering analysis for Monk Parakeet contact calls for Connecticut nesting colonies. The distances between the calls from each nesting colony were generated using the Euclidean distance between the centroids in the five-dimensional principal coordinates space.
Discussion
We found significant differences in the structure of feral Monk Parakeet contact calls among both isolated and potentially interacting populations. We cannot yet say what factors have led to the observed contact call variation. In the absence of genetic data, few alternative hypotheses can be ruled out. We do believe that the differences are, at least in part, due to founder effects. Our results could be explained by founder populations of each colony coming from different introduction events, or even different native geographic regions; current vocal diversity could reflect initial variation. This interpretation can be tested by examining genetic differences among isolated populations in the different states to see whether the New Orleans and Miami populations have similar origins and the Connecticut and Austin populations have different origins. At the local scale, we would also want to examine the population structure of the Connecticut colonies to determine if vocally similar colonies (i.e., West Haven and Milford Meadowside) are similar genetically.
Unlike other species such as Orange-fronted Conures (Bradbury et al. 2001), Monk Parakeet populations in Connecticut did not exhibit a graded variation in call structure. Rather, several nesting colonies in close proximity (e.g., Milford Audubon and Milford Meadowside, separated by 4.4 km) had quite different vocalization structure, whereas other colonies that were more distant were more similar (e.g., Milford Buckingham and Stratford, separated by 9.2 km). If simple diffusion and drift were the main contributors to variation in vocalization structure, we would expect a graded structure of vocal diversity along overlapping home ranges. Instead, in the Connecticut populations, adjacent colonies had distinct differences in their contact calls. It seems likely, therefore, that factors other than proximity are major determinants of this variation.
While it is possible that each Connecticut colony in this study was founded by a separate introduction event, it seems more likely, given the amount of time that has passed since the first introduction in the 1970s and recent population growth (National Audubon Society 2002), that the current population has spread from only a few initial introduction sites. If so, the observed vocal heterogeneity might have arisen because dispersing founders “leap-frogged” potential settlement sites, perhaps due to the unnatural urban context, or because social factors caused adjacent colonies to diverge more rapidly than more distantly spaced colonies. These two alternatives are not mutually exclusive. In fact, a plausible scenario is one in which a reliance on social learning of native nest colony vocalizations, short dispersal distances in feral populations similar to those of South American populations (Martin and Bucher 1993), and resistance of neighboring colonies to admitting new members who do not use their vocal variants combine to force dispersers to pass up adjacent colonies and instead colonize more distant and uninhabited zones. This would result in geographical patterns like those seen in our Connecticut data.
The comparisons among the frequency contour measures further suggest that the contact call variation in Connecticut is not solely the result of founder effects. The first three principal components of the national analysis accounted for a larger proportion of the variance than the first three components of the Connecticut analysis. However, there were no significant differences among locations in the national analysis, but there were in the Connecticut analysis. This suggests that there may be differences in the factors influencing vocal variation at the micro- and macrogeographic level. Because founder effects are most likely the dominant factors at the nationwide level, other factors are probably dominant at the local level. The lack of a relationship between geography and call structure suggests that simple cultural diffusion is not the dominant local-level effect. Thus, differences in call structure are presumably driven by interactions between birds from adjacent populations.
All available data indicate that parrots continue to learn new vocalizations throughout life (Farabaugh and Dooling 1996, Bradbury 2003), and may alter them to match social partners (Farabaugh et al. 1994, Bartlett and Slater 1999, Hile and Streidter 2000, Hile et al. 2000), although it is not yet clear what benefits vocal conformity may confer. Converging on a common call type may be a criterion for admission to parrot social groups. Thus, in theory, a determined disperser should be able to learn local contact calls sufficiently to join an adjacent colony. In support of this inference, studies examining genetic differences among dialect regions in another parrot, the Yellow-naped Amazon, found no genetic differences between populations with different dialects, indicating that dialects in this species do not limit dispersal (Wright and Wilkinson 2001, Wright et al. 2005).
On the other hand, Monk Parakeets may be sufficiently different from other species that such learning is not sufficient. Existing studies suggest there may be an association between the degree of site residency in parrots and the nature of geographical variation in contact calls. At one extreme are Orange-fronted Conures, which move night roosts daily, live in a fluid fission-fusion society with high turnover group composition, and show gradual and continuous geographical variation in vocalizations (Bradbury et al. 2001). In contrast, the sympatric Yellow-naped Amazon relies on a few traditional communal night roots and shows discrete dialect structure (Wright 1996). However, there are multiple communal night roosts within each dialect region, and while some roost-specific vocal differentiation occurs (Wright 1996), adult birds can relocate among night roosts. Monk Parakeets appear to constitute an extreme on this continuum in their use of a single communal nest as their nightly refuge throughout the year. At least in Argentina, individuals are likely to belong to the same colony for years (Martin and Bucher 1993). The marked heterogeneity in contact calls among communal nests may thus be a consequence of much higher compositional stability of colonies when compared to other species. Unfortunately, we do not know whether such heterogeneity among communal nests is also a characteristic of natural populations of Monk Parakeets in Argentina. This would be worth examining.
The likely continued colonization of regions of North America by this resilient parakeet provides an unusual opportunity to document the generation of geographical variation in vocalizations of wild birds. This study provides timely baseline data on vocal variation in contact calls at a very early stage in this colonization. All of the recordings and associated metadata on which this study was based are being archived in The Macaulay Library (<http://www.birds.cornell.edu/macaulaylibrary/>) and will be available for later comparisons. When combined with relevant genetic data being collected by colleagues, it should be possible to develop a useful history of how genetic and vocal changes interacted during colonization events by a species relying on vocal learning.
Acknowledgments
We thank M. Avery, B. Merchant, J. B. Sevenair, S. Hanks, and J. Eberhard for their hospitality and assistance in locating and recording Monk Parakeets. Comments from two anonymous reviewers greatly improved the manuscript. Our work was supported by a National Science Foundation (NSF) Graduate Research Fellowship to SCB-D, a Howard Hughes Foundation undergraduate research grant to ARR, and NSF Grant IBN 02-29271 to JWB.
Literature Cited
Author notes
Present address: Macaulay Library, Laboratory of Ornithology, 159 Sapsucker Woods Road, Ithaca, NY 14850. E-mail: [email protected]



