-
PDF
- Split View
-
Views
-
Cite
Cite
Sylvain Lehmann, Susanna Schraen-Maschke, Jean-Sébastien Vidal, Constance Delaby, Frédéric Blanc, Claire Paquet, Bernadette Allinquant, Stéphanie Bombois, Audrey Gabelle, Olivier Hanon, the BALTAZAR study group , Head-to-Head Comparison of Two Plasma Phospho-tau Assays in Predicting Conversion of Mild Cognitive Impairment to Dementia, Clinical Chemistry, Volume 69, Issue 9, September 2023, Pages 1072–1083, https://doi.org/10.1093/clinchem/hvad103
Close - Share Icon Share
Abstract
Among blood biomarkers, phospho-tau181 (pTau181) is one of the most efficient in detecting Alzheimer disease across its continuum. However, transition from research to routine clinical use will require confirmation of clinical performance in prospective cohorts and evaluation of cofounding factors.
Here we tested the Lumipulse assay for plasma pTau181 in mild cognitive impairment (MCI) participants from the Baltazar prospective cohort. We compared the performance of this assay to the corresponding Simoa assay for the prediction of conversion to dementia. We also evaluated the association with various routine blood parameters indicative of comorbidities.
Lumipulse and Simoa gave similar results overall, with hazard ratios for conversion to dementia of 3.48 (95% CI, 2.23–5.45) and 3.70 (95%CI, 2.39–5.87), respectively. However, the 2 tests differ somewhat in terms of the patients identified, suggesting that their use may be complementary. When combined with age, sex, and apolipoprotein E (APOE)ε4 status, areas under the curves for conversion detection were 0.736 (95% CI, 0.682–0.791) for Lumipulse and 0.733 (95% CI, 0.679–0.788) for Simoa. Plasma pTau181 was independently associated with renal dysfunction (assessed by creatinine and glomerular filtration) for both assays. Cardiovascular factors (adiponectin and cholesterol), nutritional, and inflammatory markers (total protein content, C-reactive protein) also impacted plasma pTau181 concentration, although more so with the Simoa than with the Lumipulse assay.
Plasma pTau181 measured using the fully automated Lumipulse assay performs as well as the Simoa assay for detecting conversion to dementia of MCI patients within 3 years and Lumipulse is less affected by comorbidities. This study suggests a pathway to routine noninvasive in vitro diagnosis-approved testing to contribute to the management of Alzheimer disease.
NCT01315639
Introduction
Alzheimer disease is among the most common pathologies of old age and a major societal burden; however, pharmacological treatments remain limited (1). Along with several other neurodegenerative diseases like Creutzfeldt-Jakob disease, Parkinson disease, Huntington disease, and amyotrophic lateral sclerosis, Alzheimer disease is considered to be a result of protein aggregation (e.g., tau, amyloid) in the brain (2). Alzheimer disease can be detected at an early stage, or even before symptoms appear, by monitoring pathogenic concentrations of tau and amyloid in cerebrospinal fluid (3). Therefore, there has been active investigation of blood-based biomarkers in an effort to identify a noninvasive test that may eventually avoid or even replace the need for lumbar puncture. One of the most promising blood-based biomarkers is the protein tau, phosphorylated on residue 181 (pTau181) (4–6). Plasma pTau181 is a sensitive marker for Alzheimer disease, which may distinguish different stages of the disease as well as predict conversion to dementia of mild cognitive impairment (MCI) patients (5, 7–10). Plasma pTau181 is also a reliable biomarker of amyloid deposition in the brain (6, 11). Most of the aforementioned studies used the Quanterix Simoa assay. Alternatives to that assay include Fujirebio's Lumipulse G pTau181 Plasma assay. This specific plasma assay was recently compared as 1 of 10 different assays to measure plasma pTau species and could robustly differentiate Alzheimer disease from control participant plasma (12, 13).
Here we compare the Lumipulse and Simoa plasma pTau181 assays in the Biomarker of AmyLoid pepTide and AlZheimer diseAse Risk (BALTAZAR) cohort, which is a unique study combining follow-up of various markers for dementia with biological parameters (14). Previously in this cohort, we demonstrated that the plasma Aβ1-42/Aβ1-40 ratio predicts conversion to dementia of MCI participants (15). The BALTAZAR cohort has an advantage over many cohorts in that we include biological measures such as metabolic markers associated with comorbidities e.g., diabetes, kidney dysfunction, and cardiovascular disease. This is particularly relevant since a history of comorbidities was previously found to correlate with plasma pTau181 concentrations in a large Alzheimer disease cohort (16). Here we test the Simoa and Lumipulse plasma pTau181 assays side-by-side on 360 MCI individuals of the BALTAZAR cohort, including 120 who converted to dementia over the course of 3 years. The rationale for our study was to compare the most frequently used research-grade assay for pTau181 (Simoa) with a new assay running on a certified “in vitro diagnostic” platform (Lumipulse). Our objective was to provide data that would support the use of these tests by clinical laboratories subject to ISO15189 based accreditation.
Materials and Methods
Study Population
The study population corresponds to 360 MCI participants of the BALTAZAR multicenter prospective cohort (ClinicalTrials.gov Identifier #NCT01315639) (14). Written informed consent to participate in the study was provided by all participants. The BALTAZAR study has approval from by the Paris ethics committee (CPP Ile de France IV Saint-Louis Hospital). All participants had clinical, neuropsychological, brain magnetic resonance imaging, and biological assessments (see later discussion). Right and left hippocampal volume was obtained for each participant using automatic segmentation of the hippocampus. APOE was genotyped in a single centralized laboratory. MCI subjects were selected according to the Petersen criteria (17). Participants had visits every 6 months for 3 years where they were reassessed each time for conversion to dementia (14). The progression from MCI to dementia was defined by evaluation of the following parameters: (a) decline in cognitive function [measured by changes from the baseline in scores of the mini-mental state examination (MMSE)], (b) disability in activities of daily living (activities of daily living > 1), and (c) clinical dementia rating sum of boxes (> 1). Conversions from MCI to dementia were reviewed by an adjudication committee, and 95% were conversions to Alzheimer disease dementia. Blood samples were taken at the first visit and, to minimize preanalytical and analytical problems, identical plasma collection tubes were used across centers (EDTA BD Vacutainer K2E, Becton Dickinson). Plasma aliquots were stored at −80°C in low-binding Eppendorf® LoBind microtubes (Eppendorf) until testing.
Plasma pTau181 Measurement
Plasma pTau181 was determined using 2 different pTau181 assay methods. The first was the Quanterix method based on ultrasensitive Simoa technology (18) on an HD-X analytical platform. All samples were diluted 4-fold with the provided dilution buffer, to minimize matrix effects. After dilution, the lowest limit of detection was 0.019 pg/mL and the limit of quantification was 0.085 pg/mL. Quality controls, with low (QC 1 with mean concentration of 3.82 pg/mL) or high (QC 2–52.4 pg/mL) assigned pTau181 concentration, were provided in assay kits. Interassay coefficients of variation for QC 1 and QC 2 were 7% and 5%, respectively. We also added 2 internal QCs (high and low) with pooled serum (mean pTau181 of 4.47 pg/mL and 2.81 pg/mL) to be analyzed at the start and end of each sample plate. These had interassay variation coefficients of 3% and 6%, respectively.
Plasma pTau181 was also measured by a commercial optimized Lumipulse G assay based on an antibody combination targeting tau epitopes proximal to Thr181. Monoclonal antibodies used were for capture AT270 specific for phospho-Thr181 and for detection HT7 and BT2, which recognized 2 different nonphosphorylated epitopes localized in the central core of the protein. Lumipulse G is a 2-step chemiluminescent enzyme immunoassay method in which the luminescent signal reflects the amount of pTau181 present in the sample. Plasma samples were thawed and centrifuged for 5 min at 10 000g before being loaded on the fully automated LUMIPULSE G1200 instrument. The measurement range is between 0.05 and 60 pg/mL, and 100% of plasma samples fell within the quantifiable range. The intrarun assay variation computed with QCs (mean pTau181 of 5.3 pg/mL and 47.7 pg/mL) was 4.2%, and the interrun variation was 6.9%.
Biological Biomarker Measurements
Blood samples, taken at baseline, were used for determination of routine parameters in ISO15189 certified laboratories: fasting glycemia, triglycerides, cholesterol (total, high-density lipoproteins, low-density lipoproteins), creatinine, prealbumin, albumin, total protein, C-reactive protein (CRP), hemoglobin, vitamin B12, TSH, folate, and red-cell folate (14). Estimated glomerular filtration rate (eGFR) based on creatinine, age, and sex was calculated using the CKD Epidemiology Collaboration equation, revised in 2021 without inclusion of race (19). High molecular weight adiponectin was measured on stored samples using the LUMIPULSE G platform. The limit of detection of the Lumipulse G high molecular weight Adiponectin assay was 0.09 µg/mL, and the measurement range was between 0.20 and 15 µg/mL with a coefficient of variation ≤4%.
Statistical Analyses
General characteristics were analyzed in the MCI sample overall and in converter and nonconverter MCI subsets. Categorical variables were analyzed as percentage and counts [% (N)], continuous variables as mean and standard deviation [M (SD)], or median [25–75 percentile interquartile range (IQR)] and comparisons were made by χ2 test, t-test, Mann-Whitney U-test, or ANOVA (Kruskal-Wallis test). Cox proportional hazards regression models for conversion, with time to dementia as a dependent variable, were computed, with adjustment for age at blood draw, sex, and APOEε4 allele carrier status. We additionally plotted Kaplan-Meier curves for the different pTau181 tertiles, and differences between tertiles were calculated by Log rank test. For all analyses, a 2-sided α-level of 0.05 was used for significance testing. Receiving operator characteristic curves, using conversion as a dependent variable, were also used. The corresponding areas under the curve (AUCs) were compared using the Delong method (20). All analyses were performed using MedCalc (20·118) and R [R Core Team (2019)] software.
Results
Baseline MCI Participant Characteristics and Comparison between Converters and Nonconverters
A subset of MCI 360 patients from the BALTAZAR cohort (14) was studied. Mean age at enrollment was 77.9 (SD 5.5) years. One-third of the subjects (120/360) converted to dementia during the 3-year period. The subjects converted to clinically probable Alzheimer disease in 95% of the cases (15). Subjects who converted to dementia (MCI converters) were slightly older than nonconverters [78.7 (SD 5.7) vs 77.6 (SD 5.4) years, P = 0.0105) but did not differ regarding their sex distribution, body mass index, or educational levels (Table 1). The average MMSE score was 26.5 (SD 2.6), and 39.8% (n = 185) of the MCI participants were APOE ε4 carriers. MCI converters had lower MMSE at baseline and a much higher MMSE decline per year, at −3.51 (SD 3.86) on average vs −0.37 (SD 1.99) for the nonconverter population. Hippocampal volume (R + L) (cm3) was also lower in converters than in nonconverters. All these differences remained significant after adjustment for age, sex, educational level, and the APOE ε4 status (Table 1).
| . | n MCI . | Value [mean(SD)] or n . | MCI Nonconverter . | MCI Converter . | Converter vs nonconverter (P or Xi2) . | Adjusted (age, sex, ApoE, education) . | ||
|---|---|---|---|---|---|---|---|---|
| n . | Value [mean (SD)] . | n . | Value [mean (SD)] . | |||||
| Age (years) | 360 | 77.9 (5.5) | 240 | 77.6 (5.4) | 120 | 78.7 (5.7) | 0.0792 | 0.0105 |
| Men/Women | 360 | 139/221 | 240 | 93/147 | 120 | 46/74 | 0.9389 | 0.6727 |
| BMI (kg/m2) | 354 | 25.1 (3.9) | 238 | 25.3 (4) | 116 | 24.7 (3.7) | 0.1780 | 0.7226 |
| MMSE (/30) | 354 | 26.5 (2.6) | 235 | 26.8 (2.5) | 119 | 25.7 (2.5) | 0.0001 | 0.0009 |
| MMSE/year | 327 | −1.51 (3.19) | 209 | −0.37 (1.99) | 118 | −3.52 (3.86) | <0.0001 | <0.0001 |
| 1 or 2 APOE4 alleles | 358 | 231/127 | 238 | 175/63 | 120 | 56/64 | <0.0001 | <0.0001 |
| Hippocampal volume (R + L) (cm3) | 277 | 4.62 (1.08) | 182 | 4.92 (0.94) | 95 | 4.04 (1.11) | <0.0001 | <0.0001 |
| Educational level (years) | 359 | 5.28 (1.58) | 239 | 5.3 (1.6) | 120 | 5.25 (1.52) | 0.7680 | 0.8534 |
| Lumipulse pTau181 (pg/mL) | 360 | 4.34 (1.92) | 240 | 3.91 (1.55) | 120 | 5.21 (2.28) | <0.0001 | <0.0001 |
| Simoa pTau181 (pg/mL) | 360 | 3.21 (1.52) | 240 | 2.89 (1.41) | 120 | 3.85 (1.54) | <0.0001 | <0.0001 |
| . | n MCI . | Value [mean(SD)] or n . | MCI Nonconverter . | MCI Converter . | Converter vs nonconverter (P or Xi2) . | Adjusted (age, sex, ApoE, education) . | ||
|---|---|---|---|---|---|---|---|---|
| n . | Value [mean (SD)] . | n . | Value [mean (SD)] . | |||||
| Age (years) | 360 | 77.9 (5.5) | 240 | 77.6 (5.4) | 120 | 78.7 (5.7) | 0.0792 | 0.0105 |
| Men/Women | 360 | 139/221 | 240 | 93/147 | 120 | 46/74 | 0.9389 | 0.6727 |
| BMI (kg/m2) | 354 | 25.1 (3.9) | 238 | 25.3 (4) | 116 | 24.7 (3.7) | 0.1780 | 0.7226 |
| MMSE (/30) | 354 | 26.5 (2.6) | 235 | 26.8 (2.5) | 119 | 25.7 (2.5) | 0.0001 | 0.0009 |
| MMSE/year | 327 | −1.51 (3.19) | 209 | −0.37 (1.99) | 118 | −3.52 (3.86) | <0.0001 | <0.0001 |
| 1 or 2 APOE4 alleles | 358 | 231/127 | 238 | 175/63 | 120 | 56/64 | <0.0001 | <0.0001 |
| Hippocampal volume (R + L) (cm3) | 277 | 4.62 (1.08) | 182 | 4.92 (0.94) | 95 | 4.04 (1.11) | <0.0001 | <0.0001 |
| Educational level (years) | 359 | 5.28 (1.58) | 239 | 5.3 (1.6) | 120 | 5.25 (1.52) | 0.7680 | 0.8534 |
| Lumipulse pTau181 (pg/mL) | 360 | 4.34 (1.92) | 240 | 3.91 (1.55) | 120 | 5.21 (2.28) | <0.0001 | <0.0001 |
| Simoa pTau181 (pg/mL) | 360 | 3.21 (1.52) | 240 | 2.89 (1.41) | 120 | 3.85 (1.54) | <0.0001 | <0.0001 |
Values in the number (n) of MCI participants for which different data types were available and comparison between nonconverter and converters, with t-student or χ2 and linear regression adjusted for age, sex, and the presence of the APOE ε4 allele; numbers were used to describe categorical variables. Mean ± SD for continuous variables.
Abbreviations: MCI, mild cognitive impairment; BMI, body mass index.
Statistically significant P values are shown in bold.
| . | n MCI . | Value [mean(SD)] or n . | MCI Nonconverter . | MCI Converter . | Converter vs nonconverter (P or Xi2) . | Adjusted (age, sex, ApoE, education) . | ||
|---|---|---|---|---|---|---|---|---|
| n . | Value [mean (SD)] . | n . | Value [mean (SD)] . | |||||
| Age (years) | 360 | 77.9 (5.5) | 240 | 77.6 (5.4) | 120 | 78.7 (5.7) | 0.0792 | 0.0105 |
| Men/Women | 360 | 139/221 | 240 | 93/147 | 120 | 46/74 | 0.9389 | 0.6727 |
| BMI (kg/m2) | 354 | 25.1 (3.9) | 238 | 25.3 (4) | 116 | 24.7 (3.7) | 0.1780 | 0.7226 |
| MMSE (/30) | 354 | 26.5 (2.6) | 235 | 26.8 (2.5) | 119 | 25.7 (2.5) | 0.0001 | 0.0009 |
| MMSE/year | 327 | −1.51 (3.19) | 209 | −0.37 (1.99) | 118 | −3.52 (3.86) | <0.0001 | <0.0001 |
| 1 or 2 APOE4 alleles | 358 | 231/127 | 238 | 175/63 | 120 | 56/64 | <0.0001 | <0.0001 |
| Hippocampal volume (R + L) (cm3) | 277 | 4.62 (1.08) | 182 | 4.92 (0.94) | 95 | 4.04 (1.11) | <0.0001 | <0.0001 |
| Educational level (years) | 359 | 5.28 (1.58) | 239 | 5.3 (1.6) | 120 | 5.25 (1.52) | 0.7680 | 0.8534 |
| Lumipulse pTau181 (pg/mL) | 360 | 4.34 (1.92) | 240 | 3.91 (1.55) | 120 | 5.21 (2.28) | <0.0001 | <0.0001 |
| Simoa pTau181 (pg/mL) | 360 | 3.21 (1.52) | 240 | 2.89 (1.41) | 120 | 3.85 (1.54) | <0.0001 | <0.0001 |
| . | n MCI . | Value [mean(SD)] or n . | MCI Nonconverter . | MCI Converter . | Converter vs nonconverter (P or Xi2) . | Adjusted (age, sex, ApoE, education) . | ||
|---|---|---|---|---|---|---|---|---|
| n . | Value [mean (SD)] . | n . | Value [mean (SD)] . | |||||
| Age (years) | 360 | 77.9 (5.5) | 240 | 77.6 (5.4) | 120 | 78.7 (5.7) | 0.0792 | 0.0105 |
| Men/Women | 360 | 139/221 | 240 | 93/147 | 120 | 46/74 | 0.9389 | 0.6727 |
| BMI (kg/m2) | 354 | 25.1 (3.9) | 238 | 25.3 (4) | 116 | 24.7 (3.7) | 0.1780 | 0.7226 |
| MMSE (/30) | 354 | 26.5 (2.6) | 235 | 26.8 (2.5) | 119 | 25.7 (2.5) | 0.0001 | 0.0009 |
| MMSE/year | 327 | −1.51 (3.19) | 209 | −0.37 (1.99) | 118 | −3.52 (3.86) | <0.0001 | <0.0001 |
| 1 or 2 APOE4 alleles | 358 | 231/127 | 238 | 175/63 | 120 | 56/64 | <0.0001 | <0.0001 |
| Hippocampal volume (R + L) (cm3) | 277 | 4.62 (1.08) | 182 | 4.92 (0.94) | 95 | 4.04 (1.11) | <0.0001 | <0.0001 |
| Educational level (years) | 359 | 5.28 (1.58) | 239 | 5.3 (1.6) | 120 | 5.25 (1.52) | 0.7680 | 0.8534 |
| Lumipulse pTau181 (pg/mL) | 360 | 4.34 (1.92) | 240 | 3.91 (1.55) | 120 | 5.21 (2.28) | <0.0001 | <0.0001 |
| Simoa pTau181 (pg/mL) | 360 | 3.21 (1.52) | 240 | 2.89 (1.41) | 120 | 3.85 (1.54) | <0.0001 | <0.0001 |
Values in the number (n) of MCI participants for which different data types were available and comparison between nonconverter and converters, with t-student or χ2 and linear regression adjusted for age, sex, and the presence of the APOE ε4 allele; numbers were used to describe categorical variables. Mean ± SD for continuous variables.
Abbreviations: MCI, mild cognitive impairment; BMI, body mass index.
Statistically significant P values are shown in bold.
Plasma pTau181 and Comorbidity Biomarkers in MCI Participants
Mean plasma pTau181 levels in the total MCI population were measured as being higher with Lumipulse than when measured with Simoa [4.34 (SD 1.92) vs 3.21 (SD 1.52)] pg/mL, paired t-test P < 0.0001) (Table 1). The 2 sets of values were, however, correlated (Pearson correlation coefficient r = 0.5149, significance level P < 0.0001) with a regression equation as follows: [Simoa] = 2.249 + 0.652[Lumipulse] (Supplemental Fig. 1). Importantly, plasma pTau181 measured with both methods increased significantly, on average by 25%, between MCI nonconverters and converters (P < 0.0001) (Table 1). AUCs of the receiving operator characteristic curves for conversion were similar (P = 0.8669) at 0.685 (95% CI, 0.625–0.744) and 0.690 (95% CI, 0.631–0.748) for Lumipulse and Simoa, respectively (Table 2). AUCs were similar in APOEε4 negative and positive populations. pTau181 AUCs for both methods were also improved in a logistic regression model with age, sex, and APOEε4 status (Table 2). No difference was observed between MCI converters and nonconverters for the following metabolic, cardiovascular, inflammatory, or renal function blood biomarkers: adiponectin, fasting glycemia, triglycerides, cholesterol (total, high-density lipoprotein, low-density lipoprotein), creatinine, eGFR, prealbumin, albumin, total protein, CRP, hemoglobin, vitamin B12, TSH, folate, and red-cell folate (Supplemental Table 1).
| ROC analysis for conversion to dementia . | AUC in total population (95% CI) . | AUC in APOE4 negative population (95% CI) . | AUC in APOE4 negative population (95% CI) . |
|---|---|---|---|
| Lumipulse pTau181 | 0.685 (0.625–0.744) | 0.672 (0.607–0.732) | 0.673 (0.584–0.753) |
| Simoa pTau181 | 0.690 (0.631–0.748) | 0.661 (0.596–0.722) | 0.670 (0.581–0.751) |
| Lumipulse pTau181 adjusted age, sex | 0.694 (0.643–0.741) | 0.708 (0.645–0.766) | 0.706 (0.619–0.783) |
| Simoa pTau181 adjusted age, sex | 0.696 (0.646–0.743) | 0.702 (0.639–0.761) | 0.672 (0.583–0.752) |
| Lumipulse pTau181 adjusted age, sex, ApoE | 0.736 (0.682–0.791) | / | / |
| Simoa pTau181 adjusted age, sex, ApoE | 0.733 (0.679–0.788) | / | / |
| Comparison of AUCs | P (DeLong) | P (DeLong) | P (DeLong) |
| pTau181 Lumipulse vs Simoa | 0.8669 | 0.8270 | 0.9523 |
| pTau181 adjusted age, sex, ApoE Lumipulse vs Simoa | 0.8669 | / | / |
| Lumipulse pTau181 adjusted age, sex, ApoE vs nonadjusted | 0.01776 | / | / |
| Simoa pTau181 adjusted age, sex, ApoE vs nonadjusted | 0.0377 | / | / |
| ROC analysis for conversion to dementia . | AUC in total population (95% CI) . | AUC in APOE4 negative population (95% CI) . | AUC in APOE4 negative population (95% CI) . |
|---|---|---|---|
| Lumipulse pTau181 | 0.685 (0.625–0.744) | 0.672 (0.607–0.732) | 0.673 (0.584–0.753) |
| Simoa pTau181 | 0.690 (0.631–0.748) | 0.661 (0.596–0.722) | 0.670 (0.581–0.751) |
| Lumipulse pTau181 adjusted age, sex | 0.694 (0.643–0.741) | 0.708 (0.645–0.766) | 0.706 (0.619–0.783) |
| Simoa pTau181 adjusted age, sex | 0.696 (0.646–0.743) | 0.702 (0.639–0.761) | 0.672 (0.583–0.752) |
| Lumipulse pTau181 adjusted age, sex, ApoE | 0.736 (0.682–0.791) | / | / |
| Simoa pTau181 adjusted age, sex, ApoE | 0.733 (0.679–0.788) | / | / |
| Comparison of AUCs | P (DeLong) | P (DeLong) | P (DeLong) |
| pTau181 Lumipulse vs Simoa | 0.8669 | 0.8270 | 0.9523 |
| pTau181 adjusted age, sex, ApoE Lumipulse vs Simoa | 0.8669 | / | / |
| Lumipulse pTau181 adjusted age, sex, ApoE vs nonadjusted | 0.01776 | / | / |
| Simoa pTau181 adjusted age, sex, ApoE vs nonadjusted | 0.0377 | / | / |
Abbreviations: APOE4 positive, 1 or 2 APOE4 alleles; APOE4 negative, 0 APOE4 allele; ROC, receiving operator characteristic.
Statistically significant P values are shown in bold.
| ROC analysis for conversion to dementia . | AUC in total population (95% CI) . | AUC in APOE4 negative population (95% CI) . | AUC in APOE4 negative population (95% CI) . |
|---|---|---|---|
| Lumipulse pTau181 | 0.685 (0.625–0.744) | 0.672 (0.607–0.732) | 0.673 (0.584–0.753) |
| Simoa pTau181 | 0.690 (0.631–0.748) | 0.661 (0.596–0.722) | 0.670 (0.581–0.751) |
| Lumipulse pTau181 adjusted age, sex | 0.694 (0.643–0.741) | 0.708 (0.645–0.766) | 0.706 (0.619–0.783) |
| Simoa pTau181 adjusted age, sex | 0.696 (0.646–0.743) | 0.702 (0.639–0.761) | 0.672 (0.583–0.752) |
| Lumipulse pTau181 adjusted age, sex, ApoE | 0.736 (0.682–0.791) | / | / |
| Simoa pTau181 adjusted age, sex, ApoE | 0.733 (0.679–0.788) | / | / |
| Comparison of AUCs | P (DeLong) | P (DeLong) | P (DeLong) |
| pTau181 Lumipulse vs Simoa | 0.8669 | 0.8270 | 0.9523 |
| pTau181 adjusted age, sex, ApoE Lumipulse vs Simoa | 0.8669 | / | / |
| Lumipulse pTau181 adjusted age, sex, ApoE vs nonadjusted | 0.01776 | / | / |
| Simoa pTau181 adjusted age, sex, ApoE vs nonadjusted | 0.0377 | / | / |
| ROC analysis for conversion to dementia . | AUC in total population (95% CI) . | AUC in APOE4 negative population (95% CI) . | AUC in APOE4 negative population (95% CI) . |
|---|---|---|---|
| Lumipulse pTau181 | 0.685 (0.625–0.744) | 0.672 (0.607–0.732) | 0.673 (0.584–0.753) |
| Simoa pTau181 | 0.690 (0.631–0.748) | 0.661 (0.596–0.722) | 0.670 (0.581–0.751) |
| Lumipulse pTau181 adjusted age, sex | 0.694 (0.643–0.741) | 0.708 (0.645–0.766) | 0.706 (0.619–0.783) |
| Simoa pTau181 adjusted age, sex | 0.696 (0.646–0.743) | 0.702 (0.639–0.761) | 0.672 (0.583–0.752) |
| Lumipulse pTau181 adjusted age, sex, ApoE | 0.736 (0.682–0.791) | / | / |
| Simoa pTau181 adjusted age, sex, ApoE | 0.733 (0.679–0.788) | / | / |
| Comparison of AUCs | P (DeLong) | P (DeLong) | P (DeLong) |
| pTau181 Lumipulse vs Simoa | 0.8669 | 0.8270 | 0.9523 |
| pTau181 adjusted age, sex, ApoE Lumipulse vs Simoa | 0.8669 | / | / |
| Lumipulse pTau181 adjusted age, sex, ApoE vs nonadjusted | 0.01776 | / | / |
| Simoa pTau181 adjusted age, sex, ApoE vs nonadjusted | 0.0377 | / | / |
Abbreviations: APOE4 positive, 1 or 2 APOE4 alleles; APOE4 negative, 0 APOE4 allele; ROC, receiving operator characteristic.
Statistically significant P values are shown in bold.
Risk Factors Associated with Conversion to Dementia
In a Cox proportional hazard model, conversion to dementia during our 3-year follow-up after adjustment for age, sex, educational level, and APOE ε4 status showed a significant risk for age, MMSE, APOE ε4, hippocampal volume, and pTau181 (Table 3). None of the comorbidity biomarkers were associated with an increased risk of conversion (Supplemental Table 2). The risk of conversion to dementia linked to high plasma pTau181 is illustrated by Kaplan-Meier curves of pTau181 tertiles (Fig. 1). The hazard ratios between the first and the third tertile were 3.48 (95% CI, 2.23–5.45) and 3.70 (95% CI, 2.39–5.87), for the Lumipulse and the Simoa assays, respectively (log rank P values <0.0001).
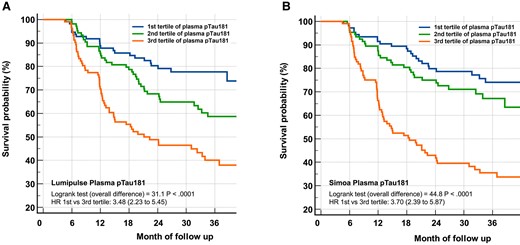
Kaplan-Meier curves of conversion to dementia according to Lumipulse or Simoa pTau181 tertiles. A very significant overall difference in pTau181 was observed (Log rank P < 0.0001) for both Lumipulse (A) and Simoa (B) tertiles. The hazard ratios between the first and the third tertile was 3.48 (95% CI, 2.23–5.45) and 3.70 (95% CI, 2.39–5.87) for the Lumipulse and the Simoa assay, respectively.
| . | n . | HR Conversion (95% CI) . | P . | P adjusted (age, sex, ApoE, education) . |
|---|---|---|---|---|
| Age | 360 | 1.04 (1.01–1.08) | 0.0207 | 0.0020 |
| Sex | 360 | 0.90 (0.63–1.31) | 0.5915 | 0.2169 |
| BMI | 354 | 0.99 (0.94–1.04) | 0.6517 | 0.6490 |
| MMSE | 354 | 0.85 (0.80–0.90) | <0.0001 | <0.0001 |
| APOE4 | 358 | 2.57 (1.79–3.68) | <0.0001 | <0.0001 |
| Hippocampal volume | 277 | 0.50 (0.42–0.60) | <0.0001 | <0.0001 |
| Educational level | 359 | 0.96 (0.86–1.08) | 0.4817 | 0.6675 |
| Lumipulse pTau181 | 360 | 1.30 (1.21–1.40) | <0.0001 | <0.0001 |
| Simoa pTau181 | 360 | 1.39 (1.26–1.54) | <0.0001 | <0.0001 |
| . | n . | HR Conversion (95% CI) . | P . | P adjusted (age, sex, ApoE, education) . |
|---|---|---|---|---|
| Age | 360 | 1.04 (1.01–1.08) | 0.0207 | 0.0020 |
| Sex | 360 | 0.90 (0.63–1.31) | 0.5915 | 0.2169 |
| BMI | 354 | 0.99 (0.94–1.04) | 0.6517 | 0.6490 |
| MMSE | 354 | 0.85 (0.80–0.90) | <0.0001 | <0.0001 |
| APOE4 | 358 | 2.57 (1.79–3.68) | <0.0001 | <0.0001 |
| Hippocampal volume | 277 | 0.50 (0.42–0.60) | <0.0001 | <0.0001 |
| Educational level | 359 | 0.96 (0.86–1.08) | 0.4817 | 0.6675 |
| Lumipulse pTau181 | 360 | 1.30 (1.21–1.40) | <0.0001 | <0.0001 |
| Simoa pTau181 | 360 | 1.39 (1.26–1.54) | <0.0001 | <0.0001 |
Cox proportional hazard model of conversion to dementia in follow-up before and after adjustment for age, sex, educational level, and the APOEε4 status.
Abbreviations: HR, hazard ratio for conversion; BMI, body mass index.
Statistically significant P values are shown in bold.
| . | n . | HR Conversion (95% CI) . | P . | P adjusted (age, sex, ApoE, education) . |
|---|---|---|---|---|
| Age | 360 | 1.04 (1.01–1.08) | 0.0207 | 0.0020 |
| Sex | 360 | 0.90 (0.63–1.31) | 0.5915 | 0.2169 |
| BMI | 354 | 0.99 (0.94–1.04) | 0.6517 | 0.6490 |
| MMSE | 354 | 0.85 (0.80–0.90) | <0.0001 | <0.0001 |
| APOE4 | 358 | 2.57 (1.79–3.68) | <0.0001 | <0.0001 |
| Hippocampal volume | 277 | 0.50 (0.42–0.60) | <0.0001 | <0.0001 |
| Educational level | 359 | 0.96 (0.86–1.08) | 0.4817 | 0.6675 |
| Lumipulse pTau181 | 360 | 1.30 (1.21–1.40) | <0.0001 | <0.0001 |
| Simoa pTau181 | 360 | 1.39 (1.26–1.54) | <0.0001 | <0.0001 |
| . | n . | HR Conversion (95% CI) . | P . | P adjusted (age, sex, ApoE, education) . |
|---|---|---|---|---|
| Age | 360 | 1.04 (1.01–1.08) | 0.0207 | 0.0020 |
| Sex | 360 | 0.90 (0.63–1.31) | 0.5915 | 0.2169 |
| BMI | 354 | 0.99 (0.94–1.04) | 0.6517 | 0.6490 |
| MMSE | 354 | 0.85 (0.80–0.90) | <0.0001 | <0.0001 |
| APOE4 | 358 | 2.57 (1.79–3.68) | <0.0001 | <0.0001 |
| Hippocampal volume | 277 | 0.50 (0.42–0.60) | <0.0001 | <0.0001 |
| Educational level | 359 | 0.96 (0.86–1.08) | 0.4817 | 0.6675 |
| Lumipulse pTau181 | 360 | 1.30 (1.21–1.40) | <0.0001 | <0.0001 |
| Simoa pTau181 | 360 | 1.39 (1.26–1.54) | <0.0001 | <0.0001 |
Cox proportional hazard model of conversion to dementia in follow-up before and after adjustment for age, sex, educational level, and the APOEε4 status.
Abbreviations: HR, hazard ratio for conversion; BMI, body mass index.
Statistically significant P values are shown in bold.
Association of Lumipulse and Simoa Plasma pTau181 Levels with Different Biomarkers and Cohort Characteristics
The relationship between plasma pTau181 concentration and demographic or biological factors, collected at baseline in the BALTAZAR cohort, was investigated using a linear regression approach. Plasma pTau181 was clearly associated with age and APOE status (Fig. 2A). Plasma pTau181 weakly correlated with age in the whole population for both Lumipulse and Simoa assays [Pearson correlation coefficients of r = 0.17 (P = 0.0015) and 0.13 (P = 0.0175), respectively]. The presence of APOE ε4 was associated with significantly higher pTau181 values (t-test between APOE ε4 negative and positive population: P = 0.019 and P = 0.001 for Lumipulse and Simoa, respectively). pTau181 levels were strongly linked to renal function parameters, creatinine, and eGFR with both techniques. Adiponectin, total cholesterol, total protein, and CRP were also significantly associated with pTau181 levels but only when measured with Simoa (Fig. 2B). These associations are illustrated by a plot of the correlation between pTau181 and eGFR or total protein (Fig. 3). Finally, we observed that creatinine and eGFR remained associated with pTau181 independently of APOE status (Supplemental Table 3). The situation is different for the association between pTau181 and CRP or total protein, which is affected differently depending on the type of assay performed and APOE status.
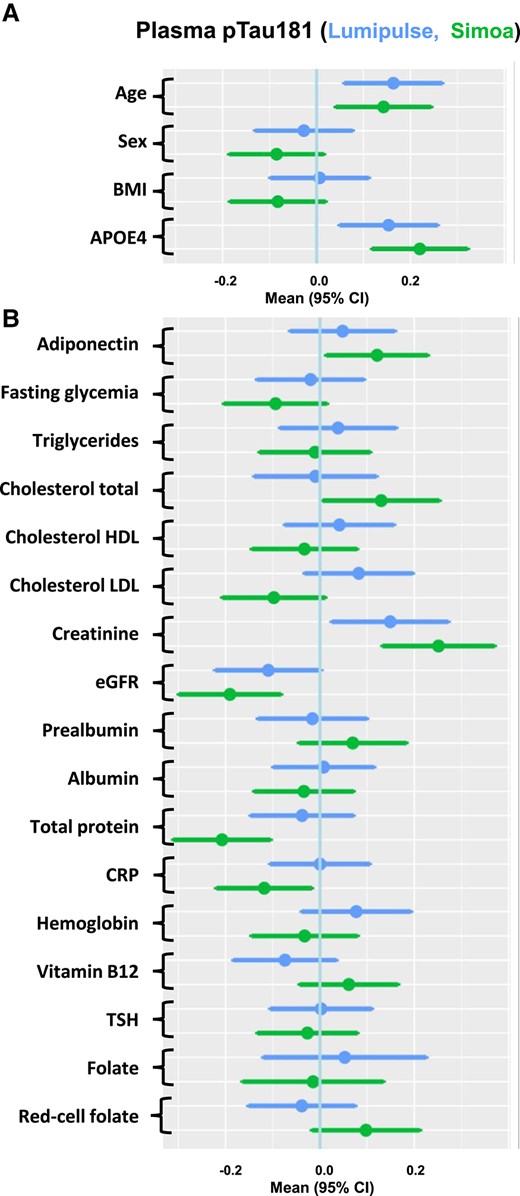
Associations between multiple factors and plasma pTau181 concentrations. Forest plots of associations between demographic (A) and comorbidity (B) biomarkers and Lumipulse (upper bar) or Simoa (lower bar) plasma pTau181, using linear regression of z-scores. Means and 95% CIs are provided. Abbreviations: BMI, body mass index.
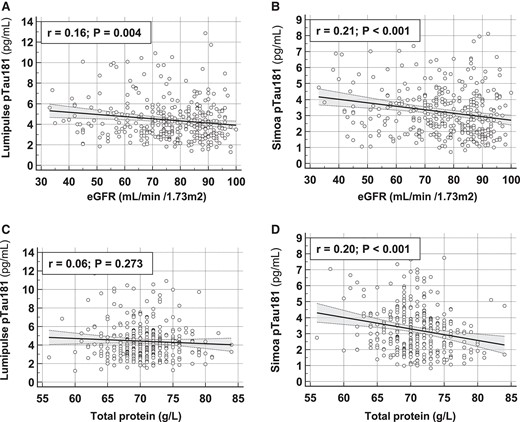
Correlation between pTau181 and eGFR or total protein. Correlation between Lumipulse (A, C) and Simoa (B, D) pTau181 values and eGFR (A, B) or total protein (C, D). Pearson correlation coefficient (r) and significance level (P) are indicated. Abbreviations: eGFR, estimated glomerular filtration rate.
Discussion
Among the blood-based biomarkers for Alzheimer disease, pTau181 is the most powerful in detecting brain amyloidopathy and for detecting Alzheimer disease across its clinical continuum (6–11, 21). Plasma pTau181 thus potentially fulfills an important medical need: predicting conversion from MCI to dementia (22). This predictive information is invaluable for adapting patient care and selecting those who may benefit most from prevention strategies (1). Here we evaluated the performance of plasma pTau181 in the BALTAZAR large-scale multicenter prospective longitudinal cohort. These clinically defined MCI participants were referred to one of the participating memory centers and were followed up for 3 years (14). Historically, many studies of blood protein biomarkers have relied on the Simoa assay, but a new fully automated Lumipulse assay has recently become commercially available (12). Here we compared these 2 technologies and found that their clinical performances are statistically equivalent. Both assays gave >25% higher pTau181 values in MCI participants who converted to dementia and around 3.5 times increased risk for conversion to dementia associated with the highest tertile values of pTau181, at optimal cutpoints of >4.1 pg/mL for Lumipulse and >3.2 pg/mL for Simoa, respectively.
We observed that age and ApoE4 status were also predictive of MCI conversion. When age, ApoE4 status, and sex were included in a logistic regression model, pTau181 predicted conversion, with AUCs close to 0.73 for both techniques.
This AUC value is lower than observed when using pTau181 to identify brain amyloidopathy or when comparing patients with or without Alzheimer disease (4, 13, 16, 23, 24). If we compare our results with the recent evaluation of the Lumipulse pTau181 assay by Wilson et al. (13), performed on a large cohort of patients with Alzheimer disease, we can identify several differences and reasons for the lower AUC observed in our study. First, in our study, some of the subjects (n = 6) converted to other forms of dementia than Alzheimer disease, with more than half being below optimal cutpoints (Supplemental Fig. 2). Second, the average age of our population is higher (78 vs 70 years), implying more advanced clinical stages and presence of a higher rate of comorbidities, both of which may impact biomarker performance. In addition to assessing the clinical performance of the Lumipulse assay, our study adds to the study of Wilson a comparison with the Simoa assay. Interestingly, a previously published comparison of these 2 methods concluded that the Lumipulse performed less well (12), which is in contradiction with our study. However, this result was obtained with a more limited number of patients, which reduces the power of the study, and the authors used an in-house Simoa test rather than the commercial version we used. Our study has also a strong added value in relation to evaluation of the impact of comorbidities on plasma pTau181.
We observed, first, that none of these biological factors were statistically different between MCI converters and nonconverters. Then, when using a linear regression method, we showed that factors linked to renal function (creatinine and eGFR) are strongly associated with plasma pTau181 measured with either Lumipulse or Simoa. This confounding effect has been previously reported (16, 25) and has to be taken into account to avoid false-positive detection in patients with altered kidney dysfunction. Other blood biomarkers, like neurofilament light chain (26), are also influenced by renal function. Additional work is needed to fully understand how renal function impacts blood-based biomarker levels independent of neurodegenerative processes.
In terms of differences between the 2 techniques tested, we observed that factors associated with cardiovascular diseases, like cholesterol, red-cell folate, or adiponectin, were associated with higher pTau181 levels but only when measured by Simoa. The association of cardiovascular diseases with Alzheimer disease is known (27), and, for example, adiponectin has been previously identified as a predictor of cognitive decline (28). It is therefore possible that Alzheimer pathology, and consequently pTau181, is modified by cardiovascular risk or disease. We also observed that CRP, a marker of inflammation and cardiovascular risk, is associated with lower pTau181 levels, as is also the case for high blood total protein (Fig. 3). Again, these associations were present only when pTau181 was measured with Simoa and not with Lumipulse.
These puzzling differences between the 2 immunoassays must also be interpreted bearing in mind their relatively low correlation (Supplemental Fig. 1). This could be related to the use of different combinations of antibodies recognizing different subpopulations/fragments of blood pTau181 with different concentrations or properties in terms of clearance or binding to blood proteins. High protein content or presence of inflammatory proteins could therefore impact the 2 assays in different ways. Another possibility is that Simoa, which relies on a nanobead labelling approach with digital detection (29), might be differently impacted by interfering factors compared with the Lumipulse assay, which is a more classical electrochemiluminescence detection method. Further studies will be needed to investigate this. Finally, another element that illustrates the differences between the assays is represented by the distribution of MCI conversion program participants according to assay results (Supplemental Fig. 2). A significant number of converters were positive for one assay but not for the other. The combined use of the 2 tests could thus be of interest in clinical practice to detect a higher percentage of MCI converters.
Conclusions
From our evaluation of the ability of plasma pTau181 to detect MCI conversion to dementia, we can conclude that the clinical performances of the Simoa and Lumipulse assays are overall similar. However, the 2 tests differ somewhat in terms of the patients identified, suggesting that they are complementary. In addition, there are differences that need to be confirmed regarding confounding factors associated with comorbidities. Thus, the 2 assays have different properties that need to be considered in relation to eventual use in the clinic. Importantly, unlike the Simoa analyzer, LUMIPULSE G is an IVD-approved system that ensures a full traceability of the samples, random access, full automation, and connection to laboratory information systems. These elements will likely lead to easier ISO15189 certification.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Nonstandard Abbreviations
APOE, apolipoprotein E; AUC, area under the receiving operator characteristic curve; BALTAZAR, Biomarker of AmyLoid pepTide and AlZheimer diseAse Risk; eGFR, estimated glomerular filtration rate; MCI, mild cognitive impairment; MMSE, Mini–Mental State Examination
Author Contributions
The corresponding author takes full responsibility that all authors on this publication have met the following required criteria of eligibility for authorship: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. Nobody who qualifies for authorship has been omitted from the list.
Sylvain Lehmann (Conceptualization-Lead, Funding acquisition-Equal, Methodology-Equal, Writing—original draft-Lead, Writing—review & editing-Lead), Susanna Schraen-Maschke (Conceptualization-Equal, Funding acquisition-Equal, Methodology-Lead, Writing—review & editing-Equal), Jean-Sébastien Vidal (Data curation-Lead, Formal analysis-Lead, Methodology-Equal, Writing—review & editing-Equal), Constance Delaby (Data curation-Supporting, Formal analysis-Supporting, Validation-Supporting, Writing—review & editing-Equal), Frédéric Blanc (Data curation-Supporting, Investigation-Equal, Project administration-Supporting, Writing—review & editing-Equal), Claire Paquet (Data curation-Equal, Formal analysis-Equal, Investigation-Equal, Methodology-Equal, Project administration-Equal, Writing—review & editing-Equal), Bernadette Allinquant (Data curation-Supporting, Formal analysis-Equal, Methodology-Supporting, Writing—review & editing-Equal), Stéphanie Bombois (Data curation-Equal, Investigation-Equal, Methodology-Equal, Project administration-Supporting, Writing—review & editing-Equal), Audrey Gabelle (Conceptualization-Equal, Data curation-Equal, Investigation-Equal, Methodology-Equal, Project administration-Equal, Writing—review & editing-Equal), and Olivier Hanon (Conceptualization-Lead, Investigation-Lead, Methodology-Lead, Project administration-Lead, Writing—review & editing-Equal)
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
C. Paquet, Advisory Board for Roche, Biogen, Lilly, and EISAI.
Stock Ownership
None declared.
Honoraria
J.-S. Vidal, honoraria for lectures paid by Bayer to institution; C. Paquet, honorarium for AAIC 2020 presentation supported by Roche; O. Hanon, honoraria from Biogene for presentations; S. Bombois, honoraria for a lecture for the personnel of EISAI (October 20, 2020) and for a presentation at the symposium organized by Biogen on “Digital Tools: Their Place in the Alzheimer Disease Care Pathway” (September 2021).
Research Funding
The French Ministry of Health (Programme Hospitalier de Recherche Clinique), Grant/Award Numbers:PHRC2009/01-04, PHRC-13-0404; The Foundation Plan Alzheimer; Fondation pour la Recherche Médicale; The Gerontopôle d’Ile de France.
Expert Testimony
None declared.
Patents
None declared.
Other Remuneration
C. Paquet, CTAD Congress 2019 travel and facilities support by Biogen.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Data Availability
Data and informed consent form are available upon request after publication (APHP, Paris). Requests will be considered by each study investigator based on the information provided by the requester regarding the study and analysis plan. If the use is appropriate, a data sharing agreement will be put in place before distributing a fully deidentified version of the dataset, including the data dictionary used for analysis with individual participant data.
The BALTAZAR Study Group Collaborators
Olivier Hanon, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Frédéric Blanc, CHRU de Strasbourg, Memory Resource and Research Centre of Strasbourg/Colmar, French National Centre for Scientific Research, ICube Laboratory and Fédération de Médecine Translationnelle de Strasbourg, Team Imagerie Multimodale Intégrative en Santé /Neurocrypto, Université de Strasbourg, F-67000 Strasbourg, France.
Yasmina Boudali, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Audrey Gabelle, Memory Research and Resources Center, Department of Neurology, Inserm INM NeuroPEPs Team, Université de Montpellier, F-34000 Montpellier, France.
Jacques Touchon, Memory Research and Resources Center, Department of Neurology, Inserm INM NeuroPEPs Team, Université de Montpellier, F-34000 Montpellier, France.
Marie-Laure Seux, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Hermine Lenoir, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Catherine Bayle, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
StéphanieBombois, Inserm, CHU Lille, UMR-SU1172, LiCEND, Lille Neuroscience & Cognition, LabEx DISTALZ, University of Lille, F-59000 Lille, France.
Christine Delmaire, Inserm, CHU Lille, UMR-SU1172, LiCEND, Lille Neuroscience & Cognition, LabEx DISTALZ, University of Lille, F-59000 Lille, France.
Xavier Delbeuck, Univ. Lille, Inserm U1171 Degenerative and Vascular Cognitive Disorders, F-59000 Lille, France.
Florence Moulin, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Emmanuelle Duron, Université Paris-Saclay, APHP, Hôpital Paul Brousse, département de gériatrie, Équipe MOODS, Inserm 1178, F-94800 Villejuif, France.
Florence Latour, Centre Hospitalier de la Côte Basque, Department of Gerontology, F-64100 Bayonne, France.
Matthieu Plichart, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Sophie Pichierri, Université de Nantes, EA 4334 Movement-Interactions-Performance, CHU Nantes, Memory Research Resource Center of Nantes, Department of clinical gerontology, F-44000 Nantes, France.
Galdric Orvoën, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Evelyne Galbrun, Sorbonne Université, APHP, Centre Hospitalier Dupuytren, Department of Gérontology 2, F-91210 Draveil, France.
Giovanni Castelnovo, CHU de Nimes, Hôpital Caremeau, Neurology Department, F- 30029 Nimes, France.
Lisette Volpe-Gillot, Hôpital Léopold Bellan, Service de Neuro-Psycho-Gériatrie, Memory Clinic, F-75014 Paris, France.
Florien Labourée, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Pascaline Cassagnaud, Univ. Lille, CHU de Lille, Memory Resource and Research Centre of Lille, Department of Neurology, F-59000 Lille, France.
Claire Paquet, GHU APHP Nord Lariboisière Fernand Widal, Centre de Neurologie Cognitive, Université Paris Cité, F-75010 Paris, France.
Françoise Lala, Université de Toulouse III, CHU La Grave-Casselardit, Memory Resource and Research Centre of Midi-Pyrénées, F-31300 Toulouse, France.
Bruno Vellas, Université de Toulouse III, CHU La Grave-Casselardit, Memory Resource and Research Centre of Midi-Pyrénées, F-31300 Toulouse, France.
Julien Dumurgier, GHU APHP Nord Lariboisière Fernand Widal, Centre de Neurologie Cognitive, Université Paris Cité, F-75010 Paris, France.
Anne-Sophie Rigaud, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Christine Perret-Guillaume, Université de Lorraine, CHRUdeNancy, Memory Resource and Research Centre of Lorraine, F-54500 Vandoeuvre-lès-Nancy, France.
Eliana Alonso, Université de Paris, APHP, Hôpital européen Georges Pompidou, Service de Gériatrie, F-75015, Paris, France.
Foucaud du Boisgueheneuc, CHU de Poitiers, Memory Resource and Research Centre of Poitiers, F-86000 Poitiers, France.
Laurence Hugonot-Diener, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Adeline Rollin-Sillaire, Univ. Lille, CHU de Lille, Memory Resource and Research Centre of Lille, Department of Neurology, F-59000 Lille, France.
Olivier Martinaud, CHU Charles Nicolle, Memory Resource and Research Centre of Haute Normandie, F-76000 Rouen, France.
Clémence Boully, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Yann Spivac, APHP, Centre Hospitalier Émile-Roux, Department of Gérontology 1, F-94450 Limeil-Brévannes, France.
Agnès Devendeville, CHU d'Amiens-Picardie, Memory Resource and Research Centre of Amiens Picardie, F-80000 Amiens, France.
Joël Belmin, Sorbonne Université, APHP, Hôpitaux Universitaires Pitie- Salpêtrière-Charles-Foix, Service de Gériatrie Ambulatoire, F-75013 Paris, France.
Philippe Robert, Université Côte d'Azur, CHU de Nice, Memory Research Resource Center of Nice, CoBTek lab, F-06100 Nice, France.
Thierry Dantoine, CHU de Limoges, Memory Research Resource Center of Limoges, F-87000 Limoges, France.
Laure Caillard, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
David Wallon, Normandie Univ, UNIROUEN, Inserm U1245, CHU de Rouen, Department of Neurology and CNR-MAJ, Normandy Center for Genomic and Personalized Medicine, CIC-CRB1404, F-76000, Rouen, France.
Didier Hannequin, CHU Charles Nicolle, Memory Resource and Research Centre of Haute Normandie, F-76000 Rouen, France.
Nathalie Sastre, Université de Toulouse III, CHU La Grave-Casselardit, Memory Resource and Research Centre of Midi-Pyrénées, F-31300 Toulouse, France.
Sophie Haffen, CHU de Besançon, Memory Resource and Research Centre of Besançon Franche-Comté, F-25000 Besançon, France.
Anna Kearney-Schwartz, Université de Lorraine, CHRUdeNancy, Memory Resource and Research Centre of Lorraine, F-54500 Vandoeuvre-lès-Nancy, France.
Jean-Luc Novella, Université de Reims Champagne-Ardenne, EA 3797, CHU de Reims, Memory Resource and Research Centre of Champagne- Ardenne, F-51100 Reims, France.
Vincent Deramecourt, Univ. Lille, CHU de Lille, Memory Resource and Research Centre of Lille, Department of Neurology, F-59000 Lille, France.
Valérie Chauvire, CHU d'Angers, Memory Resource and Research Centre of Angers, F-49000 Angers, France.
Gabiel Abitbol, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Nathalie Schwald, APHP, Centre Hospitalier Émile-Roux, Department of Gérontology 1, F-94450 Limeil-Brévannes, France.
Caroline Hommet, CHRU de Tours, Memory Resource and Research Centre of Tours, F-37000 Tours, France.
François Sellal, Université de Strasbourg, CHRU de Strasbourg, Memory Resource and Research Centre of Strasbourg/Colmar, Inserm U-118, F-67000 Strasbourg, France.
Marie-Ange Cariot, Université de Paris, APHP, Hôpital européen Georges Pompidou, Service de Gériatrie, F-75015, Paris, France.
Mohamed Abdellaoui, Univ Paris Est Creteil, EA 4391 Excitabilité Nerveuse et Thérapeutique, CHU Henri Mondor, Department of Neurology, F- 94000 Créteil, France.
Sarah Benisty, Hôpital Fondation Rothschild, Department of Neurology, F- 75019 Paris, France.
Salim Gherabli, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Pierre Anthony, Université de Strasbourg, CHRU de Strasbourg, Memory Resource and Research Centre of Strasbourg/Colmar, Inserm U-118, F-67000 Strasbourg, France.
Frédéric Bloch, CHU d'Amiens-Picardie, Department of Gerontology, F-80000 Amiens, France.
Nathalie Charasz, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Sophie Chauvelier, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Jean-Yves Gaubert, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Guillaume Sacco, Université Côte d'Azur, CHU de Nice, Memory Research Resource Center of Nice, CoBTek lab, F-06100 Nice, France.
Olivier Guerin, Université Côte d'Azur, CHU de Nice, Memory Research Resource Center of Nice, CoBTek lab, F-06100 Nice, France.
Jacques Boddaert, Sorbonne Université, APHP, Hôpitaux Universitaires Pitie- Salpêtrière-Charles Foix, Memory Resource and Research Centre, Centre des Maladies Cognitives et Comportementales IM2A, Inserm UMR 8256, F-75013 Paris, France.
Marc Paccalin, CHU de Poitiers, Memory Resource and Research Centre of Poitiers, F-86000 Poitiers, France.
Marie-Anne Mackowiak, Univ. Lille, CHU de Lille, Memory Resource and Research Centre of Lille, Department of Neurology, F-59000 Lille, France.
Marie-Thérèse Rabus, Sorbonne Université, APHP, Centre Hospitalier Dupuytren, Department of Gérontology 2, F-91210 Draveil, France.
Valérie Gissot, Université François-Rabelais de Tours, CHRU de Tours, MemoryResource andResearchCentre of Tours, Inserm CIC 1415, F-37000 Tours, France.
Athanase Benetos, Université de Lorraine, CHRUdeNancy, Memory Resource and Research Centre of Lorraine, F-54500 Vandoeuvre-lès-Nancy, France.
Candice Picard, CHU d'Amiens-Picardie, Memory Resource and Research Centre of Amiens Picardie, F-80000 Amiens, France.
Céline Guillemaud, Sorbonne Université, APHP, Hôpitaux Universitaires Pitie- Salpêtrière-Charles Foix, Memory Resource and Research Centre, Centre des Maladies Cognitives et Comportementales IM2A, F-75013 Paris, France.
Gilles Berrut, Université de Nantes, EA 4334 Movement-Interactions-Performance, CHU Nantes, Memory Research Resource Center of Nantes, Department of clinical gerontology, F-44000 Nantes, France.
Claire Gervais, Université Côte d'Azur, CHU de Nice, Memory Research Resource Center of Nice, CoBTek lab, F-06100 Nice, France.
Jacques Hugon, GHU APHP Nord Lariboisière Fernand Widal, Centre de Neurologie Cognitive, Université Paris Cité, F-75010 Paris, France.
Jean-Marc Michel, Université de Strasbourg, CHRU de Strasbourg, Memory Resource and Research Centre of Strasbourg/Colmar, Inserm U-118, F-67000 Strasbourg, France.
Jean-Philippe David, APHP, Centre Hospitalier Émile-Roux, Department of Gérontology 1, F-94450 Limeil-Brévannes, France.
Marion Paulin, Univ. Lille, CHU de Lille, Memory Resource and Research Centre of Lille, Department of Neurology, F-59000 Lille, France.
Pierre-Jean Ousset, Université de Toulouse III, CHU La Grave-Casselardit, Memory Resource and Research Centre of Midi-Pyrénées, F-31300 Toulouse, France.
Pierre Vandel, Université Bourgogne Franche-Comté, Laboratoire de Recherches Intégratives en Neurosciences et Psychologie Cognitive, CHU de Besançon, Memory Resource and Research Centre of Besançon Franche-Comté, F-25000 Besançon, France.
Sylvie Pariel, Sorbonne Université, APHP, Hôpitaux Universitaires Pitie- Salpêtrière-Charles-Foix, Service de Gériatrie Ambulatoire, F-75013 Paris, France.
Vincent Camus, Université François-Rabelais de Tours, CHRU de Tours, UMR Inserm U1253, F-37000 Tours, France.
Anne Chawakilian, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Léna Kermanac'h, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Anne-Cécile Troussiere, Univ. Lille, CHU de Lille, Memory Resource and Research Centre of Lille, Department of Neurology, F-59000 Lille, France.
Cécile Adam, CHU de Limoges, Memory Research Resource Center of Limoges, F-87000 Limoges, France.
Diane Dupuy, CHU d'Amiens-Picardie, Memory Resource and Research Centre of Amiens Picardie, F-80000 Amiens, France.
Elena Paillaud, Université de Paris, APHP, Hôpital européen Georges Pompidou, Service de Gériatrie, F-75015, Paris, France.
Hélène Briault, Sorbonne Université, APHP, Centre Hospitalier Dupuytren, Department of Gérontology 2, F-91210 Draveil, France.
Isabelle Saulnier, Université de Limoges, EA 6310 HAVAE, CHU de Limoges, Memory Research Resource Center of Limoges, F-87000 Limoges, France.
Karl Mondon, Université François-Rabelais de Tours, CHRU de Tours, UMR Inserm U1253, F-37000 Tours, France.
Marie-Agnès Picat, CHU de Limoges, Memory Research Resource Center of Limoges, F-87000 Limoges, France.
Marie Laurent, Université de Paris, APHP, Hôpital européen Georges Pompidou, Service de Gériatrie, F-75015, Paris, France.
Olivier Godefroy, CHU d'Amiens-Picardie, Memory Resource and Research Centre of Amiens Picardie, F-80000 Amiens, France.
Rezki Daheb, Université de Paris, APHP, Hôpital européen Georges Pompidou, Service de Gériatrie, F-75015, Paris, France.
Stéphanie Libercier, Université de Strasbourg, CHRU de Strasbourg, Memory Resource and Research Centre of Strasbourg/Colmar, Inserm U-118, F-67000 Strasbourg, France.
Djamila Krabchi, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Marie Chupin, Université Paris-Saclay, Neurospin, CEA, CNRS, catineuroimaging.com, CATI Multicenter Neuroimaging Platform, F-91190 Gif-sur-Yvette, France.
Jean Sébastien Vidal, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Edouard Chaussade, EA 4468, APHP, Hospital Broca, Memory Resource and Research Centre of de Paris-Broca-Ile de France, Université Paris Cité, F-75013 Paris, France.
Christiane Baret-Rose, Université de Paris, Institute of Psychiatric and Neurosciences, Inserm UMR-S 1266, F-75014 Paris, France.
Sylvain Lehmann, Univ Montpellier, IRMB CHU de Montpellier, INM INSERM, Montpellier, France.
Bernadette Allinquant, Université de Paris, Institute of Psychiatric and Neurosciences, Inserm UMR-S 1266, F-75014 Paris, France.
Susanna Schraen-Maschke, Inserm, CHU Lille, UMR-SU1172, LiCEND, Lille Neuroscience & Cognition, LabEx DISTALZ, University of Lille, F-59000 Lille, France.



