-
PDF
- Split View
-
Views
-
Cite
Cite
Ioannis Mykoniatis, Pantelis Sarafidis, Dimitrios Memmos, Anastasios Anastasiadis, Georgios Dimitriadis, Dimitrios Hatzichristou, Are endourological procedures for nephrolithiasis treatment associated with renal injury? A review of potential mechanisms and novel diagnostic indexes, Clinical Kidney Journal, Volume 13, Issue 4, August 2020, Pages 531–541, https://doi.org/10.1093/ckj/sfaa020
Close - Share Icon Share
Abstract
Nephrolithiasis is one of the most common urological conditions with a huge socio-economic impact. About 50% of recurrent stone-formers have just one lifetime recurrence and >10% of patients present with a high recurrent disease requiring subsequent and sometimes multiple surgical interventions. The advent of new technology has made endourological procedures the pinnacle of stone treatment, including procedures like percutaneous nephrolithotomy (PCNL), retrograde intrarenal surgery and miniaturized PCNL procedures. Researchers have primarily focused on comparisons with respect to stone-free rates, procedure parameters and post-operative complications. However, the effect of these three procedures on renal function or indexes of renal injury has not been sufficiently examined. This was only reported in a few studies as a secondary objective with the use of common and not the appropriate and detailed renal parameters. This review presents current literature regarding the use of novel and highly predictive biomarkers for diagnosing acute kidney injury, discusses potential mechanisms through which endourological procedures for renal stone treatment may affect renal function and proposes areas with open questions where future research efforts in the field should focus.
INTRODUCTION
Nephrolithiasis is one of the most common urological conditions with a huge socio-economic impact. Its prevalence in the US population is estimated at 10.6% for men and 7.1% for women [1]. Among patients with nephrolithiasis forming recurrent stones, ~50% have just one lifetime recurrence while highly recurrent disease is observed in slightly >10% of patients [2]. Surgical treatment of renal calculi has evolved rapidly in recent years following continuous technological advancements. During the past decade, endourological procedures, including retrograde intrarenal surgery (RIRS), percutaneous nephrolithotomy (PCNL) and miniaturized PCNL (mini-PCNL and micro-PCNL) have become very popular for the management of nephrolithiasis (Figure 1). The reason is the high efficacy of these techniques in combination with low complication rates and decreased hospitalization time due to their minimal invasive character [2]. During RIRS, a flexible ureteroscope is advanced in the caliceal system and the stones are fragmented with the use of laser technology. In PCNL, access to the pyelocaliceal system is achieved by a percutaneous puncture and subsequent dilatation of the track in order to place a percutaneous sheath. The nephroscope insertion and stone removal are performed through the sheath. Fragmentation of the stones can be performed using laser, ultrasound or mechanical techniques. Depending on the outer diameter of the sheath used, PCNL procedures can be categorized as standard PCNL (22–30 Fr), mini-PCNL (14–20 Fr), ultramini-PCNL (11–13 Fr) and micro-PCNL (<11 Fr) (Figure 2).
![Common endourological procedures for the treatment of urinary calculi. (A) ESWL. A correlation between ESWL and AKI has been suggested but more evidence is needed [7]. (B) URS. A semi-rigid ureteroscope is used to treat only ureteral calculi. There is evidence that URS may cause AKI [3] but further research is needed. (C) PCNL. The kidney is punctured and the track is dilated in order to place a sheath for nephroscope insertion and stone fragment removal. PCNL has been associated with AKI but further research is needed [4, 5]. (D) RIRS. A flexible ureteroscope is advanced to the kidney through the ureter in order to perform laser lithotripsy. During RIRS, increased intrarenal pressures are observed, hypothesizing that AKI can manifest following the obstructive uropathy model [6]. Definitive proof of AKI in humans is still lacking.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ckj/13/4/10.1093_ckj_sfaa020/2/m_sfaa020f1.jpeg?Expires=1750196458&Signature=hITXDxMX0l6tWNUxZ9le17qQ4uzkiRfX1L0SAL5Fa9-jF3FB9fVeD~UKYqX~sVIw7CaPkaR1o3VzhCU47tuceJMoLAYrwXuUzes11Ck3k0ORSt6lFzBmoy95D7WtEu0MqLf-060lNR-LeMnqnu6-bHJniJXVhSr49dW-2zikELtWo~VmSezu8h8UIDl1FIqu0kt5RKzvHJ1bPpCxPA4A6VSF42Xb95t5JgfTyGmngce0ZlFt-6RqJU3ID3vQTuWxppbNhh7IRlHLYt2ZHnX6MWUx-rMar4rSsyVBEQ5EIjqIMHOO6zBdhuqayIAdyvNDb2Td5SYLnIaCQnfcvQtbPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Common endourological procedures for the treatment of urinary calculi. (A) ESWL. A correlation between ESWL and AKI has been suggested but more evidence is needed [7]. (B) URS. A semi-rigid ureteroscope is used to treat only ureteral calculi. There is evidence that URS may cause AKI [3] but further research is needed. (C) PCNL. The kidney is punctured and the track is dilated in order to place a sheath for nephroscope insertion and stone fragment removal. PCNL has been associated with AKI but further research is needed [4, 5]. (D) RIRS. A flexible ureteroscope is advanced to the kidney through the ureter in order to perform laser lithotripsy. During RIRS, increased intrarenal pressures are observed, hypothesizing that AKI can manifest following the obstructive uropathy model [6]. Definitive proof of AKI in humans is still lacking.
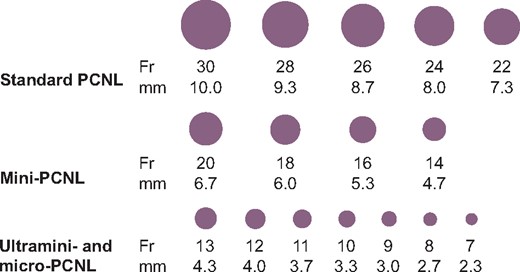
Sheath sizes for different PCNL types. The different types of PCNL differ in the size of the sheath used to access the kidney. An illustration of the different sizes in each type can be seen for comparison. The most common sizes used are 30 Fr for standard PCNL and 16 Fr for mini-PCNL (ultramini- and micro-PCNL are rare and there is no consensus regarding common practice).
Recent systematic reviews and meta-analyses on the efficacy and safety of the aforementioned endourological techniques have focused primarily on comparisons with respect to stone-free rates and post-operative complications such as bleeding and infection [8–10]. Other important parameters regarding the endourological procedures were examined, such as fluoroscopy time, duration of surgery, hospitalization time and reoperation rate for each technique [11, 12]. However, the effect of the three surgical procedures on renal function or indexes of renal injury has not been sufficiently examined. This was only reported in a few studies as a secondary objective using common and not appropriate or detailed renal parameters [i.e. using only indexes of glomerular function such as serum creatinine (sCr) and estimated glomerular filtration rate (eGFR) instead of specific indexes of tubular function and injury] [13]. This review presents current literature regarding the use of novel and highly predictive biomarkers for diagnosing acute kidney injury (AKI) and discusses potential mechanisms through which endourological procedures for renal stone treatment may affect renal function.
FROM Acute Renal Failure (ARF) TO AKI
ARF is a general term describing an acute reversible or non-reversible deterioration of renal function. The term ARF could reflect a decrease in renal function of variable severity, ranging from an asymptomatic increase in common renal function markers to anuria and dialysis requirement. The absence of a commonly accepted definition of ARF [14, 15] was an important reason to fully describe its epidemiology, aetiology and prognostic implications. In 2004, the Acute Dialysis Quality Initiative group proposed the RIFLE criteria to define the syndrome; RIFLE is the acronym for each of the five categories proposed (Risk, Injury, Failure, Loss and End-stage renal disease), each of which was defined with specific criteria, based on changes in sCr, GFR and total diuresis [16]. Following this effort, two classification systems were proposed by the AKI Network and the Kidney Disease: Improving Global Outcomes (KDIGO) organization. Both of them used largely similar criteria to describe the syndrome that covers a wide range of cases, from patients with subclinical mild renal impairment to end-stage patients in need of dialysis [17, 18]. In 2013, the UK National Institute for Health and Care Excellence compared the three main proposed definitions, considering them almost similar, but eventually suggested use of KDIGO categorization as more complete and more recent (Table 1) [19]. AKI has now superseded the term ARF, establishing solid bases for numerous epidemiological studies that have redefined the incidence and severity of acute renal damage in various clinical conditions. Moreover, AKI has been proposed recently as a risk factor for extrarenal complications and mortality in various clinical settings [20–22]. The association of endourological surgeries for lithiasis and AKI has not been adequately studied to date. To our knowledge, the incidence of properly defined AKI after endourological procedures has been examined in only one study, which is discussed below [4].
Classification of AKI according to the KDIGO clinical practice guideline for AKI [18]
| Stage . | sCr . | Diuresis . |
|---|---|---|
| 1 | ↑ of sCr ≥26.5 μmol/L (0.3 mg/dL) within 48 h or 1.5–1.9 × baseline within 7 days | <0.5 mL/kg/h for 6–12 h |
| 2 | ↑ of sCr 2–2.9 × baseline | <0.5 mL/kg/h for ≥12 h |
| 3 | ↑ of sCr 3 × baseline or ≥354 μmol/L (4 mg/dL) or initiation of renal replacement therapy | <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h |
| Stage . | sCr . | Diuresis . |
|---|---|---|
| 1 | ↑ of sCr ≥26.5 μmol/L (0.3 mg/dL) within 48 h or 1.5–1.9 × baseline within 7 days | <0.5 mL/kg/h for 6–12 h |
| 2 | ↑ of sCr 2–2.9 × baseline | <0.5 mL/kg/h for ≥12 h |
| 3 | ↑ of sCr 3 × baseline or ≥354 μmol/L (4 mg/dL) or initiation of renal replacement therapy | <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h |
Classification of AKI according to the KDIGO clinical practice guideline for AKI [18]
| Stage . | sCr . | Diuresis . |
|---|---|---|
| 1 | ↑ of sCr ≥26.5 μmol/L (0.3 mg/dL) within 48 h or 1.5–1.9 × baseline within 7 days | <0.5 mL/kg/h for 6–12 h |
| 2 | ↑ of sCr 2–2.9 × baseline | <0.5 mL/kg/h for ≥12 h |
| 3 | ↑ of sCr 3 × baseline or ≥354 μmol/L (4 mg/dL) or initiation of renal replacement therapy | <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h |
| Stage . | sCr . | Diuresis . |
|---|---|---|
| 1 | ↑ of sCr ≥26.5 μmol/L (0.3 mg/dL) within 48 h or 1.5–1.9 × baseline within 7 days | <0.5 mL/kg/h for 6–12 h |
| 2 | ↑ of sCr 2–2.9 × baseline | <0.5 mL/kg/h for ≥12 h |
| 3 | ↑ of sCr 3 × baseline or ≥354 μmol/L (4 mg/dL) or initiation of renal replacement therapy | <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h |
| Biomarker . | Type . | Source . | Place of express . | Potential uses . |
|---|---|---|---|---|
| NGAL | Iron transporter protein of the lipocalin family | Urine, serum | Proximal tubule (and distal tubule) | Identification of tubular damage, differentiation between prerenal azotaemia and acute tubular necrosis, early diagnosis of AKI |
| KIM-1 | Cell membrane glycoprotein with an extracellular, a transmembrane and an intracellular portion | Urine | Proximal tubule | Identification of tubular damage, early diagnosis, assessment of severity and prediction of mortality of AKI |
| NAG | Lysosomal enzyme | Urine | In lysosomes of proximal tubule | Identification of tubular damage, assessment of severity and prediction of mortality of AKI |
| IL-18 | Pro-inflammatory cytokine | Urine, serum | Proximal tubule, macrophages | Identification of tubular damage, early diagnosis and prediction of mortality of AKI (mediator of inflammation and immune response) |
| Cystatin C | Non-glycosylated protein belonging to cystatin protease inhibitors | Urine, serum | Low molecular weight protein expressed by all nucleated cells and freely filtered in the glomeruli | Urine cystatin C: identification of tubular damage, early diagnosis of AKI |
| Serum cystatin C: used to estimate GFR, increases when GFR is reduced | ||||
| L-FABP | Fatty acid–binding protein | Urine | Expressed in the tubules | Identification of tubular damage, early diagnosis of AKI |
| MIOX | Non-heme di-iron enzyme | Serum | Proximal tubule | Identification of tubular damage, early diagnosis of AKI |
| Biomarker . | Type . | Source . | Place of express . | Potential uses . |
|---|---|---|---|---|
| NGAL | Iron transporter protein of the lipocalin family | Urine, serum | Proximal tubule (and distal tubule) | Identification of tubular damage, differentiation between prerenal azotaemia and acute tubular necrosis, early diagnosis of AKI |
| KIM-1 | Cell membrane glycoprotein with an extracellular, a transmembrane and an intracellular portion | Urine | Proximal tubule | Identification of tubular damage, early diagnosis, assessment of severity and prediction of mortality of AKI |
| NAG | Lysosomal enzyme | Urine | In lysosomes of proximal tubule | Identification of tubular damage, assessment of severity and prediction of mortality of AKI |
| IL-18 | Pro-inflammatory cytokine | Urine, serum | Proximal tubule, macrophages | Identification of tubular damage, early diagnosis and prediction of mortality of AKI (mediator of inflammation and immune response) |
| Cystatin C | Non-glycosylated protein belonging to cystatin protease inhibitors | Urine, serum | Low molecular weight protein expressed by all nucleated cells and freely filtered in the glomeruli | Urine cystatin C: identification of tubular damage, early diagnosis of AKI |
| Serum cystatin C: used to estimate GFR, increases when GFR is reduced | ||||
| L-FABP | Fatty acid–binding protein | Urine | Expressed in the tubules | Identification of tubular damage, early diagnosis of AKI |
| MIOX | Non-heme di-iron enzyme | Serum | Proximal tubule | Identification of tubular damage, early diagnosis of AKI |
| Biomarker . | Type . | Source . | Place of express . | Potential uses . |
|---|---|---|---|---|
| NGAL | Iron transporter protein of the lipocalin family | Urine, serum | Proximal tubule (and distal tubule) | Identification of tubular damage, differentiation between prerenal azotaemia and acute tubular necrosis, early diagnosis of AKI |
| KIM-1 | Cell membrane glycoprotein with an extracellular, a transmembrane and an intracellular portion | Urine | Proximal tubule | Identification of tubular damage, early diagnosis, assessment of severity and prediction of mortality of AKI |
| NAG | Lysosomal enzyme | Urine | In lysosomes of proximal tubule | Identification of tubular damage, assessment of severity and prediction of mortality of AKI |
| IL-18 | Pro-inflammatory cytokine | Urine, serum | Proximal tubule, macrophages | Identification of tubular damage, early diagnosis and prediction of mortality of AKI (mediator of inflammation and immune response) |
| Cystatin C | Non-glycosylated protein belonging to cystatin protease inhibitors | Urine, serum | Low molecular weight protein expressed by all nucleated cells and freely filtered in the glomeruli | Urine cystatin C: identification of tubular damage, early diagnosis of AKI |
| Serum cystatin C: used to estimate GFR, increases when GFR is reduced | ||||
| L-FABP | Fatty acid–binding protein | Urine | Expressed in the tubules | Identification of tubular damage, early diagnosis of AKI |
| MIOX | Non-heme di-iron enzyme | Serum | Proximal tubule | Identification of tubular damage, early diagnosis of AKI |
| Biomarker . | Type . | Source . | Place of express . | Potential uses . |
|---|---|---|---|---|
| NGAL | Iron transporter protein of the lipocalin family | Urine, serum | Proximal tubule (and distal tubule) | Identification of tubular damage, differentiation between prerenal azotaemia and acute tubular necrosis, early diagnosis of AKI |
| KIM-1 | Cell membrane glycoprotein with an extracellular, a transmembrane and an intracellular portion | Urine | Proximal tubule | Identification of tubular damage, early diagnosis, assessment of severity and prediction of mortality of AKI |
| NAG | Lysosomal enzyme | Urine | In lysosomes of proximal tubule | Identification of tubular damage, assessment of severity and prediction of mortality of AKI |
| IL-18 | Pro-inflammatory cytokine | Urine, serum | Proximal tubule, macrophages | Identification of tubular damage, early diagnosis and prediction of mortality of AKI (mediator of inflammation and immune response) |
| Cystatin C | Non-glycosylated protein belonging to cystatin protease inhibitors | Urine, serum | Low molecular weight protein expressed by all nucleated cells and freely filtered in the glomeruli | Urine cystatin C: identification of tubular damage, early diagnosis of AKI |
| Serum cystatin C: used to estimate GFR, increases when GFR is reduced | ||||
| L-FABP | Fatty acid–binding protein | Urine | Expressed in the tubules | Identification of tubular damage, early diagnosis of AKI |
| MIOX | Non-heme di-iron enzyme | Serum | Proximal tubule | Identification of tubular damage, early diagnosis of AKI |
It is known that problems affecting renal function, including obstructive uropathy, kidney parenchyma injuries and urinary infections, are common complications of endourological procedures [8]. As a result, patients operated on for nephrolithiasis may be considered a risk group in terms of renal function impairment and AKI. As minimally invasive endourological operations are frequently successful and without complications, patients are often discharged on the same or the first post-operative day and generally renal function is not monitored even during the first post-operative week. Moreover, even when renal function is monitored in this early period, the changes of markers like serum urea and Cr may not fully represent the underlying renal injury and could be misleading in terms of long-term renal function.
EARLY DIAGNOSIS OF AKI: LIMITATIONS OF SCR AND THE ROLE OF NEW BIOMARKERS
The high incidence (13–18% in hospitalized patients) and the serious complications of AKI, combined with the inability of current markers to diagnose AKI in an early and reversible stage in order to take corrective measures, led nephrologists to examine possible biomarkers of kidney damage to improve risk assessment, early detection, differential diagnosis and prognosis of AKI [23]. Even though the definition and diagnosis of AKI are still based on serum creatine increase and/or diuresis decrease, certain characteristics limit their diagnostic value. Limitations of sCr as a marker of kidney injury include modulation by age, gender, hydration status, diet, muscle mass and medication [24]. Furthermore, in the AKI setting, there is a considerable time difference between actual renal injury, actual GFR changes and increases in sCr [25] that inhibit accurate estimation regarding the timing of renal injury and the associated reduction in renal function. Thus a severe renal injury may be combined with relatively small sCr changes in the first 24−48 h after AKI, leading to a false sense of security resulting in delayed therapeutic actions. All of the above decrease the utility of sCr as a tool for making treatment decisions in critically ill patients or in patients during the post-operative period, such as the decision to change nephrotoxic drugs or measures to increase renal perfusion [23].
As a result, in recent years, through the understanding of the biochemical, structural and functional changes caused by AKI, a set of new biomarkers for AKI was proposed. These substances are either low-molecular weight proteins that are freely filtered from the glomerulus and fully reabsorbed by the proximal tubule or proteins of normal tubular cells [24]. In cases of parenchymal kidney damage, the normal uptake and metabolism of these substances are substantially disturbed, thus their concentration in urine or even serum may increase rapidly and significantly. Also, this increase may occur long before that of the routine indicators used for renal function estimation, i.e. sCr. The main characteristics of the most widely used urine/serum biomarkers with proven correlation to AKI are presented in Table 2 [26].
| References . | Type of study . | Patients, n . | Method of assessment . | Time points of assessment . | Renal function . |
|---|---|---|---|---|---|
| Ünsal et al. [27] | RCT (three different dilatation techniques of percutaneous track) | 50 |
|
| No difference between groups and with baseline |
| Moosanejad et al. [28] | RCT (postop ureteral stenting or not) | 84 | sCr |
| No difference between groups |
| Chatham et al. [29] | Prospective observational | 19 |
|
| Stable or improved |
| Moskovitz et al. [30] | Prospective observational | 87 | Tc-99 QDMSAa |
| Stable |
| Kiliç et al. [31] | Prospective observational | 24 | CDUS |
| Stable |
| Bayrak et al. [32] | Prospective observational | 80 |
|
| Improved (P < 0.001) |
| Su et al. [33] | Prospective observational | 44 | eGFR (MDRD) |
| Stable or improved (P < 0.001) |
| Handa et al. [34] | Retrospective observational | 196 | sCr |
| Decreased (P < 0.001) |
| Hegarty and Desai[35] | Retrospective observational | 20 |
| NR |
|
| Bilen et al. [36] | Retrospective observational | 185 | eGFR (MDRD) |
| Improved (P = 0.02) |
| Nouralizadeh et al. [37] | Retrospective observational | 94 | eCrCl (Cockroft–Gault) |
|
|
| Bucuras et al. [38] | Retrospective observational | 189 | sCr |
| Stable |
| El-Tabey et al. [39] | Retrospective observational | 200 | eCrCl (Cockroft–Gault) | NR | Improved (P < 0.001) |
| References . | Type of study . | Patients, n . | Method of assessment . | Time points of assessment . | Renal function . |
|---|---|---|---|---|---|
| Ünsal et al. [27] | RCT (three different dilatation techniques of percutaneous track) | 50 |
|
| No difference between groups and with baseline |
| Moosanejad et al. [28] | RCT (postop ureteral stenting or not) | 84 | sCr |
| No difference between groups |
| Chatham et al. [29] | Prospective observational | 19 |
|
| Stable or improved |
| Moskovitz et al. [30] | Prospective observational | 87 | Tc-99 QDMSAa |
| Stable |
| Kiliç et al. [31] | Prospective observational | 24 | CDUS |
| Stable |
| Bayrak et al. [32] | Prospective observational | 80 |
|
| Improved (P < 0.001) |
| Su et al. [33] | Prospective observational | 44 | eGFR (MDRD) |
| Stable or improved (P < 0.001) |
| Handa et al. [34] | Retrospective observational | 196 | sCr |
| Decreased (P < 0.001) |
| Hegarty and Desai[35] | Retrospective observational | 20 |
| NR |
|
| Bilen et al. [36] | Retrospective observational | 185 | eGFR (MDRD) |
| Improved (P = 0.02) |
| Nouralizadeh et al. [37] | Retrospective observational | 94 | eCrCl (Cockroft–Gault) |
|
|
| Bucuras et al. [38] | Retrospective observational | 189 | sCr |
| Stable |
| El-Tabey et al. [39] | Retrospective observational | 200 | eCrCl (Cockroft–Gault) | NR | Improved (P < 0.001) |
Tc-99 QDMSA was used for assessment of renal defects, differential function and GFR (Gates method).
Tc-99 MAG3 was used for measurement of differential function.
Tc-99 QDMSA, quantitative SPECT of technetium-99 dimercaptosuccinic acid; Tc-99 MAG3, technetium-99m mercaptoacetyltriglycine; CDUS, colour Doppler ultrasonography; eCrCl, estimated creatinine clearance.
| References . | Type of study . | Patients, n . | Method of assessment . | Time points of assessment . | Renal function . |
|---|---|---|---|---|---|
| Ünsal et al. [27] | RCT (three different dilatation techniques of percutaneous track) | 50 |
|
| No difference between groups and with baseline |
| Moosanejad et al. [28] | RCT (postop ureteral stenting or not) | 84 | sCr |
| No difference between groups |
| Chatham et al. [29] | Prospective observational | 19 |
|
| Stable or improved |
| Moskovitz et al. [30] | Prospective observational | 87 | Tc-99 QDMSAa |
| Stable |
| Kiliç et al. [31] | Prospective observational | 24 | CDUS |
| Stable |
| Bayrak et al. [32] | Prospective observational | 80 |
|
| Improved (P < 0.001) |
| Su et al. [33] | Prospective observational | 44 | eGFR (MDRD) |
| Stable or improved (P < 0.001) |
| Handa et al. [34] | Retrospective observational | 196 | sCr |
| Decreased (P < 0.001) |
| Hegarty and Desai[35] | Retrospective observational | 20 |
| NR |
|
| Bilen et al. [36] | Retrospective observational | 185 | eGFR (MDRD) |
| Improved (P = 0.02) |
| Nouralizadeh et al. [37] | Retrospective observational | 94 | eCrCl (Cockroft–Gault) |
|
|
| Bucuras et al. [38] | Retrospective observational | 189 | sCr |
| Stable |
| El-Tabey et al. [39] | Retrospective observational | 200 | eCrCl (Cockroft–Gault) | NR | Improved (P < 0.001) |
| References . | Type of study . | Patients, n . | Method of assessment . | Time points of assessment . | Renal function . |
|---|---|---|---|---|---|
| Ünsal et al. [27] | RCT (three different dilatation techniques of percutaneous track) | 50 |
|
| No difference between groups and with baseline |
| Moosanejad et al. [28] | RCT (postop ureteral stenting or not) | 84 | sCr |
| No difference between groups |
| Chatham et al. [29] | Prospective observational | 19 |
|
| Stable or improved |
| Moskovitz et al. [30] | Prospective observational | 87 | Tc-99 QDMSAa |
| Stable |
| Kiliç et al. [31] | Prospective observational | 24 | CDUS |
| Stable |
| Bayrak et al. [32] | Prospective observational | 80 |
|
| Improved (P < 0.001) |
| Su et al. [33] | Prospective observational | 44 | eGFR (MDRD) |
| Stable or improved (P < 0.001) |
| Handa et al. [34] | Retrospective observational | 196 | sCr |
| Decreased (P < 0.001) |
| Hegarty and Desai[35] | Retrospective observational | 20 |
| NR |
|
| Bilen et al. [36] | Retrospective observational | 185 | eGFR (MDRD) |
| Improved (P = 0.02) |
| Nouralizadeh et al. [37] | Retrospective observational | 94 | eCrCl (Cockroft–Gault) |
|
|
| Bucuras et al. [38] | Retrospective observational | 189 | sCr |
| Stable |
| El-Tabey et al. [39] | Retrospective observational | 200 | eCrCl (Cockroft–Gault) | NR | Improved (P < 0.001) |
Tc-99 QDMSA was used for assessment of renal defects, differential function and GFR (Gates method).
Tc-99 MAG3 was used for measurement of differential function.
Tc-99 QDMSA, quantitative SPECT of technetium-99 dimercaptosuccinic acid; Tc-99 MAG3, technetium-99m mercaptoacetyltriglycine; CDUS, colour Doppler ultrasonography; eCrCl, estimated creatinine clearance.
Although evidence strongly suggests that these biomarkers are useful for assessing early renal damage, there are several issues potentially limiting their routine use. The ideal AKI biomarker is one that can predict and diagnose AKI; identify the location, type and aetiology of injury; predict outcomes and enable initiation and monitoring of therapeutic interventions [24]. Thus, presently, there is no single perfect AKI biomarker. The various biomarkers may indicate different mechanisms of injury, such as hypoxia [liver-type fatty acid binding protein (L-FABP)] or ischaemia [kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL)]. Moreover, the site of syntheses and also the kinetics of activation after kidney injury differ among these biomarkers [40–42]. Furthermore, factors such as time after exposure to injury, baseline renal function and subclinical underlying kidney disease may modify biomarker temporal profiles and should be considered when interpreting biomarker performance [43]. The post-operative changes in urine biomarkers, including peak level time point and the duration of increase above a certain threshold, as well as other issues, still need larger sample studies to be clarified [44]. Meanwhile, standardization of the kits used for measurements of these biomarkers is also a matter of concern. Therefore a combination of biomarkers may be necessary to provide the best diagnostic and prognostic information in a context-specific manner. It was reported that combining two to five biomarkers at different time points from 0 to 12 h post-operatively can improve the diagnostic accuracy [17, 45, 46].
EFFECTS OF ENDOUROLOGICAL PROCEDURES ON RENAL FUNCTION MEASURED WITH CONVENTIONAL INDEXES
Despite the extensive literature referring to the PCNL technique, its efficacy and possible complications, such as bleeding and sepsis [47–50], human studies examining the effect of PCNL on renal function are of low quality and with conflicting results [27–39] ( Table 3). The observed differences are mainly due to the different time periods after the operation (short-, medium- or long-term) and the different methods with which kidney function was assessed (Cr, eGFR and imaging techniques).
Basic characteristics of studies using biomarkers to assess the effect of endourological procedures on renal function
| References . | Study design . | Procedure . | Biomarker . | Patients, n . | Endpoints . | Urine sample time points . | Results . | Comments . |
|---|---|---|---|---|---|---|---|---|
| Daggülli et al. [5] | RCT | PCNL | KIM-1, NGAL, NAG and L-FABP (urine) | 76 (29 active versus 47 control) | Potential risk of renal injury after PCNL | 0, 2 and 24 h | KIM-1:Cr, NAG;Cr, NGAL:Cr increased at 24 h (P < 0.05) while L-FABP/Cr increased NS | Forty-seven control patients, unlike the study group, had not received anaesthesia |
| Dede et al. [51] | Prospective, control arm, non-randomized | RIRS | KIM-1, NGAL, NAG and L-FABP (urine) | 77 (30 active versus 47 control) | RIRS damage on kidney using biomarkers | −24, 2 and 24 h | KIM-1 + NGAL increased 2-h post-operatively and normalized at 24 h |
|
| Balasar et al. [52] | Prospective three-arm, non-randomized | Micro-PCNL, PCNL and RIRS | KIM-1 (urine) | 60 (20 versus 20 versus 20) | Levels of KIM-1 in stone disease and changes from surgery | 0, 4 and 14 days |
| KIM-1:Cr is correlated to stone size |
| Benli et al. [3] | Prospective | URS | NGAL, KIM-1, FABP and cystatin C (urine) | 30 | Whether URS has a negative effect on renal function | 0, 1, 3, 5 and 12 h | Only NGAL increase was significant (P = 0.001) | Markers increased in first and third hour and later reduced in a time-linked fashion |
| Mertoglu et al. [53] | Prospective | RIRS | MIOX, cystatin C (serum) | 27 | Change in MIOX and cystatin C post-operative values | 0, 6 and 24 h | MIOX and cystatin C increased NS at 24 h | RIRS has no negative effects on kidneys function |
| Jiang et al. [4] | Retrospective | Mini-PCNL (Fr18) | NAG (urine) | 90 | NAG as AKI predictor after PCNL | 0, 2, 4, 6, 12, 24, 48 and 72 h | Post-operative NAG levels were significantly higher at various time points compared with preoperative levels (P < 0.05) |
|
| References . | Study design . | Procedure . | Biomarker . | Patients, n . | Endpoints . | Urine sample time points . | Results . | Comments . |
|---|---|---|---|---|---|---|---|---|
| Daggülli et al. [5] | RCT | PCNL | KIM-1, NGAL, NAG and L-FABP (urine) | 76 (29 active versus 47 control) | Potential risk of renal injury after PCNL | 0, 2 and 24 h | KIM-1:Cr, NAG;Cr, NGAL:Cr increased at 24 h (P < 0.05) while L-FABP/Cr increased NS | Forty-seven control patients, unlike the study group, had not received anaesthesia |
| Dede et al. [51] | Prospective, control arm, non-randomized | RIRS | KIM-1, NGAL, NAG and L-FABP (urine) | 77 (30 active versus 47 control) | RIRS damage on kidney using biomarkers | −24, 2 and 24 h | KIM-1 + NGAL increased 2-h post-operatively and normalized at 24 h |
|
| Balasar et al. [52] | Prospective three-arm, non-randomized | Micro-PCNL, PCNL and RIRS | KIM-1 (urine) | 60 (20 versus 20 versus 20) | Levels of KIM-1 in stone disease and changes from surgery | 0, 4 and 14 days |
| KIM-1:Cr is correlated to stone size |
| Benli et al. [3] | Prospective | URS | NGAL, KIM-1, FABP and cystatin C (urine) | 30 | Whether URS has a negative effect on renal function | 0, 1, 3, 5 and 12 h | Only NGAL increase was significant (P = 0.001) | Markers increased in first and third hour and later reduced in a time-linked fashion |
| Mertoglu et al. [53] | Prospective | RIRS | MIOX, cystatin C (serum) | 27 | Change in MIOX and cystatin C post-operative values | 0, 6 and 24 h | MIOX and cystatin C increased NS at 24 h | RIRS has no negative effects on kidneys function |
| Jiang et al. [4] | Retrospective | Mini-PCNL (Fr18) | NAG (urine) | 90 | NAG as AKI predictor after PCNL | 0, 2, 4, 6, 12, 24, 48 and 72 h | Post-operative NAG levels were significantly higher at various time points compared with preoperative levels (P < 0.05) |
|
NS, not significant.
Basic characteristics of studies using biomarkers to assess the effect of endourological procedures on renal function
| References . | Study design . | Procedure . | Biomarker . | Patients, n . | Endpoints . | Urine sample time points . | Results . | Comments . |
|---|---|---|---|---|---|---|---|---|
| Daggülli et al. [5] | RCT | PCNL | KIM-1, NGAL, NAG and L-FABP (urine) | 76 (29 active versus 47 control) | Potential risk of renal injury after PCNL | 0, 2 and 24 h | KIM-1:Cr, NAG;Cr, NGAL:Cr increased at 24 h (P < 0.05) while L-FABP/Cr increased NS | Forty-seven control patients, unlike the study group, had not received anaesthesia |
| Dede et al. [51] | Prospective, control arm, non-randomized | RIRS | KIM-1, NGAL, NAG and L-FABP (urine) | 77 (30 active versus 47 control) | RIRS damage on kidney using biomarkers | −24, 2 and 24 h | KIM-1 + NGAL increased 2-h post-operatively and normalized at 24 h |
|
| Balasar et al. [52] | Prospective three-arm, non-randomized | Micro-PCNL, PCNL and RIRS | KIM-1 (urine) | 60 (20 versus 20 versus 20) | Levels of KIM-1 in stone disease and changes from surgery | 0, 4 and 14 days |
| KIM-1:Cr is correlated to stone size |
| Benli et al. [3] | Prospective | URS | NGAL, KIM-1, FABP and cystatin C (urine) | 30 | Whether URS has a negative effect on renal function | 0, 1, 3, 5 and 12 h | Only NGAL increase was significant (P = 0.001) | Markers increased in first and third hour and later reduced in a time-linked fashion |
| Mertoglu et al. [53] | Prospective | RIRS | MIOX, cystatin C (serum) | 27 | Change in MIOX and cystatin C post-operative values | 0, 6 and 24 h | MIOX and cystatin C increased NS at 24 h | RIRS has no negative effects on kidneys function |
| Jiang et al. [4] | Retrospective | Mini-PCNL (Fr18) | NAG (urine) | 90 | NAG as AKI predictor after PCNL | 0, 2, 4, 6, 12, 24, 48 and 72 h | Post-operative NAG levels were significantly higher at various time points compared with preoperative levels (P < 0.05) |
|
| References . | Study design . | Procedure . | Biomarker . | Patients, n . | Endpoints . | Urine sample time points . | Results . | Comments . |
|---|---|---|---|---|---|---|---|---|
| Daggülli et al. [5] | RCT | PCNL | KIM-1, NGAL, NAG and L-FABP (urine) | 76 (29 active versus 47 control) | Potential risk of renal injury after PCNL | 0, 2 and 24 h | KIM-1:Cr, NAG;Cr, NGAL:Cr increased at 24 h (P < 0.05) while L-FABP/Cr increased NS | Forty-seven control patients, unlike the study group, had not received anaesthesia |
| Dede et al. [51] | Prospective, control arm, non-randomized | RIRS | KIM-1, NGAL, NAG and L-FABP (urine) | 77 (30 active versus 47 control) | RIRS damage on kidney using biomarkers | −24, 2 and 24 h | KIM-1 + NGAL increased 2-h post-operatively and normalized at 24 h |
|
| Balasar et al. [52] | Prospective three-arm, non-randomized | Micro-PCNL, PCNL and RIRS | KIM-1 (urine) | 60 (20 versus 20 versus 20) | Levels of KIM-1 in stone disease and changes from surgery | 0, 4 and 14 days |
| KIM-1:Cr is correlated to stone size |
| Benli et al. [3] | Prospective | URS | NGAL, KIM-1, FABP and cystatin C (urine) | 30 | Whether URS has a negative effect on renal function | 0, 1, 3, 5 and 12 h | Only NGAL increase was significant (P = 0.001) | Markers increased in first and third hour and later reduced in a time-linked fashion |
| Mertoglu et al. [53] | Prospective | RIRS | MIOX, cystatin C (serum) | 27 | Change in MIOX and cystatin C post-operative values | 0, 6 and 24 h | MIOX and cystatin C increased NS at 24 h | RIRS has no negative effects on kidneys function |
| Jiang et al. [4] | Retrospective | Mini-PCNL (Fr18) | NAG (urine) | 90 | NAG as AKI predictor after PCNL | 0, 2, 4, 6, 12, 24, 48 and 72 h | Post-operative NAG levels were significantly higher at various time points compared with preoperative levels (P < 0.05) |
|
NS, not significant.
It has been theoretically suggested that during PCNL, the access to the kidney may lead to local injury of the renal parenchyma and renal ischaemia due to the induced vasoconstriction [34]. In relevant studies, hypotheses were presented that renal parenchyma injury is not exclusively limited to the needle entry point but could be expanding, possibly due to induced inflammation or vasoconstriction of adjacent renal vessels [27, 28, 30–39, 54]. An important factor possibly playing a central role in vasoconstriction is the needle puncture done for accessing the renal pelvis and possibly the subsequent dilation to create a working channel. The wound created is the stimulus to which the kidney responds by renin secretion, resulting in increased local concentration of angiotensin II, possibly resulting in vasoconstriction [55]. Other factors suggested to participate in renin secretion increase include oxidative stress induced by renal tissue damage [56] and activation of the sympathetic nervous system [57], the activity of which (via the renorenal reflex) has been implicated in vasoconstriction promotion to the contralateral kidney after PCNL, which has been observed in some studies [34, 58]. The reduction of kidney function due to the PCNL technique is reported to occur in the early post-operative period (first 48 h) [34, 37], while kidney function seems to recover in the long term [59, 60].
The effect of the RIRS technique on renal function is understudied; the only relevant study, using Cr and eGFR calculation [61], suggested that renal function is generally not adversely affected by RIRS and that reduction is only observed in repeated operations or in cases of pre-existing chronic kidney disease (CKD). Despite this, it has been shown that extremely high intrarenal pressures (IRPs; >300 mmHg) can be developed within the renal pelvis during RIRS [62]. The presence of high IRPs (>30 mmHg) is the primary cause of kidney failure in acute obstructive uropathy. As the increased pressure stretches renal tubular cells, macrophages are multiplied and accumulation of myofibroblasts occurs, as in the case of tubulointerstitial nephritis. The resulting increase in local cytokine and growth factor release may lead to tubular atrophy, nephron loss and accumulation of fibrotic interstitial tissue [63, 64]. Thus it could be hypothesized that increased IRPs frequently observed during RIRS could induce renal injury similar to the way in which elevated IRPs provoke renal injury in animal models of obstructive uropathy [65]; however, no study thus far has examined this specific hypothesis of possible RIRS-induced damage. The use of ureteral access sheath seems to be the most efficient measure to avoid IRPs that may be harmful during RIRS [6]; again, whether this can be protective against renal injury and a decrease in renal function is not known.
Miniaturized PCNL techniques (mini-PCNL and micro-PCNL) could theoretically also cause renal parenchyma injury, although smaller than in standard PCNL, and thus also affect renal function; however, as of this writing, there are no relevant studies available. Although the creation of a smaller-diameter access channel in the renal parenchyma is advantageous compared with conventional PCNL, it has been reported that higher IRPs are developed during miniaturized PCNL techniques than conventional PCNL, with all the accompanying risks concerning renal function [6]. Theoretically, IRPs during mini-PCNL can be controlled with the use of the vacuum-cleaner effect [66]. Therefore the relation between the nephroscope and the diameter of the inner sheath is crucial; the maximum effect can be achieved with a 12 F nephroscope and an inner sheath diameter of 15 Fr [6]. Examining the impact of miniaturized PCNL techniques on renal function and comparing it with that caused by conventional PCNL and RIRS is an important research question.
As mentioned above, the incidence of properly defined AKI after endourological procedures for the management of renal calculi was examined in only one study, in which the diagnostic value of urinary N-acetyl-β-d-glucosaminidase (NAG) was evaluated for the prediction of AKI in 90 patients who underwent mini-PCNL [4]. The authors used a modified KDIGO definition of AKI (sudden decrease in kidney function >48–72 h, evident as an absolute increase in sCr levels ≥0.3 mg/dL or a percentage increase of ≥50%). AKI occurred in 11 cases post-operatively, with NAG levels being significantly higher in the AKI compared with the non-AKI group after surgery (P < 0.05). No significant differences in age, gender ratio or baseline Cr levels were reported after comparison between the AKI and non-AKI groups. However, significant differences between the two groups were revealed regarding mini-PCNL duration, C-reactive protein levels, post-operative infection rate and hospitalization time.
Overall, endourological procedures to treat renal calculi may lead to reduced renal function in the early post-operative period, with possible recovery in the long term. This observation has been recorded in several studies, limited by the use of sCr evolution, Cr clearance and eGFR post-operatively at various time intervals [34, 37, 58, 67, 68]. However, Cr has proved to be a weak indicator of early renal injury assessment due to its aforementioned limitations [69], most important of which is that for it to be elevated, an important decline in renal function must have occurred [70]. Identification of early diagnostic AKI biomarkers that increase in the urine or blood even within hours after an endourological operation may help towards early detection and thus early management of this important complication.
EFFECTS OF ENDOUROLOGICAL PROCEDURES ON NOVEL AKI BIOMARKERS
In recent years, numerous studies have evaluated the epidemiology and prognostic importance of AKI, as well as the role of new biomarkers in its diagnosis in different settings and populations, including intensive care unit patients, general surgery patients, adults and children after cardiac surgery and other patient groups [71–76]. In most such studies, the novel biomarkers were shown to have high sensitivity and specificity for AKI diagnosis. In contrast, there is a relevant paucity of studies using these modern biomarkers for early diagnosis of renal impairment after endourological surgeries for kidney stones. To identify possible studies, a review of the literature was performed using PubMed up to January 2019. Original works restricted to the English language were identified referring to the correlation between endourological procedures for the management of kidney stones and urine biomarkers. The literature search, which was conducted by the first author by using the keywords percutaneous nephrolithotomy, PCNL, ureteroscopy, URS, RIRS, renal failure, AKI, urine biomarkers, serum biomarkers, renal function, NAG, NGAL, cystatin C, KIM -1, L-FABP and interleukin 18 (IL-18), resulted in a limited number of studies. Due to study heterogeneity and the non-standardized quality appraisal, a narrative synthesis was performed. The limitations of using a single database for review were taken into account [77].
Only six studies were found to investigate the effect of endourological procedures on renal function by using urine (n = 5) or serum (n = 1) biomarkers for early diagnosis of AKI [3–5, 51–53], while only two of them used modern definition criteria of AKI [4, 53]. The basic characteristics of these studies are presented in Table 4.
Ongoing registered clinical trials assessing the effect of endourological procedures on renal injury using biomarkers
| NCT number . | Study design . | Study population . | Endourological technique . | Biomarker . | Time points of renal function assessment . | Status and estimated completion date . |
|---|---|---|---|---|---|---|
| NCT02522689 | Two parallel-arms, open- label randomized clinical study | 60 participants (30 per group) with renal stone <2 cm | Ultra-mini PCNL versus micro-PCNL | Cystatin C (blood, urine) | Preoperative versus 12 h post-operative |
|
| NCT02522676 | Six parallel-arms, open-label randomized clinical study | 300 (50 per group) participants with lower pole and/or renal pelvis stone(s) | PCNL versus mini-PCNL versus ultramini-PCNL versus micro-PCNL versus RIRS versus ESWL |
| Preoperative versus 6-, 12-, 24-, 48- and 72-h post-operative |
|
| NCT03112499 | Three parallel-arms, open-label randomized clinical study | 75 (25 per group) participants with renal stone 1–3 cm | PCNL versus mini-PCNL versus RIRS |
| 2-h preoperative and 2, 6, 24 and 48 h post-operative |
|
| NCT number . | Study design . | Study population . | Endourological technique . | Biomarker . | Time points of renal function assessment . | Status and estimated completion date . |
|---|---|---|---|---|---|---|
| NCT02522689 | Two parallel-arms, open- label randomized clinical study | 60 participants (30 per group) with renal stone <2 cm | Ultra-mini PCNL versus micro-PCNL | Cystatin C (blood, urine) | Preoperative versus 12 h post-operative |
|
| NCT02522676 | Six parallel-arms, open-label randomized clinical study | 300 (50 per group) participants with lower pole and/or renal pelvis stone(s) | PCNL versus mini-PCNL versus ultramini-PCNL versus micro-PCNL versus RIRS versus ESWL |
| Preoperative versus 6-, 12-, 24-, 48- and 72-h post-operative |
|
| NCT03112499 | Three parallel-arms, open-label randomized clinical study | 75 (25 per group) participants with renal stone 1–3 cm | PCNL versus mini-PCNL versus RIRS |
| 2-h preoperative and 2, 6, 24 and 48 h post-operative |
|
Ongoing registered clinical trials assessing the effect of endourological procedures on renal injury using biomarkers
| NCT number . | Study design . | Study population . | Endourological technique . | Biomarker . | Time points of renal function assessment . | Status and estimated completion date . |
|---|---|---|---|---|---|---|
| NCT02522689 | Two parallel-arms, open- label randomized clinical study | 60 participants (30 per group) with renal stone <2 cm | Ultra-mini PCNL versus micro-PCNL | Cystatin C (blood, urine) | Preoperative versus 12 h post-operative |
|
| NCT02522676 | Six parallel-arms, open-label randomized clinical study | 300 (50 per group) participants with lower pole and/or renal pelvis stone(s) | PCNL versus mini-PCNL versus ultramini-PCNL versus micro-PCNL versus RIRS versus ESWL |
| Preoperative versus 6-, 12-, 24-, 48- and 72-h post-operative |
|
| NCT03112499 | Three parallel-arms, open-label randomized clinical study | 75 (25 per group) participants with renal stone 1–3 cm | PCNL versus mini-PCNL versus RIRS |
| 2-h preoperative and 2, 6, 24 and 48 h post-operative |
|
| NCT number . | Study design . | Study population . | Endourological technique . | Biomarker . | Time points of renal function assessment . | Status and estimated completion date . |
|---|---|---|---|---|---|---|
| NCT02522689 | Two parallel-arms, open- label randomized clinical study | 60 participants (30 per group) with renal stone <2 cm | Ultra-mini PCNL versus micro-PCNL | Cystatin C (blood, urine) | Preoperative versus 12 h post-operative |
|
| NCT02522676 | Six parallel-arms, open-label randomized clinical study | 300 (50 per group) participants with lower pole and/or renal pelvis stone(s) | PCNL versus mini-PCNL versus ultramini-PCNL versus micro-PCNL versus RIRS versus ESWL |
| Preoperative versus 6-, 12-, 24-, 48- and 72-h post-operative |
|
| NCT03112499 | Three parallel-arms, open-label randomized clinical study | 75 (25 per group) participants with renal stone 1–3 cm | PCNL versus mini-PCNL versus RIRS |
| 2-h preoperative and 2, 6, 24 and 48 h post-operative |
|
Jiang et al. [4] studied the diagnostic ability of the NAG biomarker in assessing AKI after mini-PCNL. The NAG:Cr ratio was measured in 90 patients before surgery (3.82) and at 2 (7.19), 4 (7.73), 6 (8.11), 12 (6.56), 24 (5.79), 48 (6.20) and 72 (4.78) h post-operatively. The NAG:Cr values were significantly higher at all the time points compared with pre-operative measurements (P < 0.01). The highest average value was observed at 6 h (8.11) post-operatively, almost double when compared with the preoperative value. In patients with AKI (n = 11), defined with the criterion of an absolute increase in sCr levels ≥0.3 mg/dL (≥26.4 mmol/L) or a percentage increase of ≥50% in the values of NAG, were statistically significantly higher compared with the group of patients who did not develop AKI (n = 79) (P < 0.01). The use of a single biomarker limits the study.
Dede et al. [51] studied the effect of RIRS on renal function immediately post-operatively by measuring the biomarkers KIM-1, NAG, NGAL, L-FABP and also urine Cr. Measurements were made in 30 patients with renal stones <2 cm preoperatively and at 2 and 24 h after surgery and in 47 healthy subjects who served as controls and were weighted on urinary Cr. The researchers concluded that RIRS is a safe for procedure with NGAL:Cr and KIM:Cr ratios increasing significantly at 2 h post-operatively (940 ± 171 and 5.16 ± 2.18) versus baseline values (575 ± 215 and 2.24 ± 1.14) (P < 0.05), but returning at 24 h (608 ± 296 and 2.42 ± 1.60) to baseline levels. Values of NAG:Cr increased, but not significantly from baseline (0.11 ± 0.08) to 2 h (0.16 ± 0.13) and 24 h (0.13 ± 0.09) post-operatively (P > 0.05). Values of biomarker L-FABP:Cr decreased slightly at 2 h (0.38 ± 0.32) and 24 h (0.40 ± 0.28) post-operatively compared with baseline (0.43 ± 0.17). Weaknesses of the study are the small number of patients and the fact that AKI biomarkers were measured at only two time points post-operatively. Also, the fact that all patients who underwent RIRS showed preoperative dilatation of the pyelocaliceal system, which has been proven to increase the biomarkers of AKI [78], and thus it is confounding as to the precise contribution of the intervention to the induced AKI.
Balasar et al. [52] conducted a prospective study to measure the effect of kidney stone size but also the different endourological techniques on the values of KIM-1. They included 60 patients with renal stones of 10–20 mm treated with RIRS (n = 20), PCNL (n = 20) and micro-PCNL (n = 20). Values of urinary Cr and KIM-1 were measured preoperatively and at 4 h and 14 days post-operatively. Levels of KIM-1 were weighted with the urinary Cr value. The results revealed a positive correlation between stone size and KIM-1:Cr (correlation coefficient = 0.3, P = 0.006). Values of the KIM-1:Cr ratio were significantly lower on Day 14 compared with preoperative values for individuals who underwent PCNL [1.03 ± 0.57 versus 3.25 ± 3.28 (P = 0.001)] and RIRS [0.79 ± 0.73 versus 1.56 ± 1.58 (P = 0.01)]. However, there was no difference for the micro-PCNL group [1.48 ± 1.04 versus 1.51 ± 0.73 (P = 0.212)]. The KIM:Cr ratio decreased in the PCNL group 4 h post-operatively (2.50 ± 2.73), while it increased in the RIRS (1.66 ± 1.09) and micro-PCNL (2.17 ± 2.12) groups. Weaknesses of the study are the lack of randomization of patients between the three techniques and the fact that the AKI biomarker was measured at only two time points post-operatively.
Daggülli et al. [5] conducted a prospective control study in order to examine the use of biomarkers KIM-1, NAG, NGAL, L-FABP as AKI indicators after PCNL. Biomarkers and Cr were measured in urine samples 2 h before and 2 and 24 h after surgery in 29 patients who underwent PCNL for renal stones >2 cm and in 47 healthy subjects who served as controls. Researchers concluded that the KIM-1:Cr, NAG:Cr and NGAL:Cr ratios increased significantly 24-h post-operatively (compared with the preoperative ratios, P < 0.05). Specifically, the values increased from 2.12 ± 0.98 to 4.47 ± 2.21 for KIM-1:Cr, from 0.08 ± 0.028 to 0.22 ± 0.11 for NAG:Cr and from 475 ± 173 to 1034 ± 662 for NGAL:Cr. The values of the L-FABP:Cr ratio at 2 h (0.55 ± 0.24) and 24 h (0.68 ± 0.36) post-operatively were increased compared with the preoperative value (0.37 ± 0.14), but this change did not reach significance. Limitations of the study are the small number of patients and the fact that the indicators were measured only two times post-operatively.
Benli et al. [3] included 30 patients undergoing ureterorenoscopy (URS) for ureter stones in a prospective observational study. Before the URS procedure (baseline) and at 1, 3, 5 and 12 h following URS, urine samples were collected. NGAL, cystatin C, L-FABP and KIM-1 levels were measured and compared with the baseline values. Following the URS procedure, NGAL, KIM-1, L-FABP and cystatin C levels increased, but only the NGAL increase reached statistical significance (P = 0.001). NGAL values were 34.59 ± 35.34, 62.72 ± 142.35, 47.15 ± 104.48, 45.23 ± 163.16 and 44.99 ± 60.79 ng/dL for baseline, 1, 3, 5 and 12 h, respectively (P = 0.001). KIM-1 values were 2.06 ± 3.71, 3.62 ± 6.43, 2.49 ± 2.49, 2.28 ± 2.75 and 2.26 ± 2.40 ng/mL, respectively (P = 0.707). L-FABP values were 128.59 ± 104.48, 214.50 ± 251.57, 192.25 ± 163.16, 192.76 ± 142.32 and 164.76 ± 218.90 pg/mL, respectively (P = 0.292). Cystatin C levels were 27.38 ± 25.20, 55.70 ± 81.53, 45.70 ± 71.17, 37.60 ± 46.37 and 33.49 ± 27.60 ng/mL, respectively (P = 0.095). The small number of patients limits the generalizability of the observations; nevertheless, this study remains the first to evaluate the effect of URS surgery on renal function in the early period using new urine biomarkers.
Recently, Mertoglu et al. [53] investigated, in a single-arm, open-label, prospective study including 27 patients, the effect of RIRS on renal function using serum myo-inositol oxygenase (MIOX) enzyme and cystatin C. MIOX is a renal tubular–specific novel biomarker used for the early diagnosis of AKI. Serum samples of MIOX, cystatin C and Cr levels were measured before RIRS and 6 and 24 h post-operatively. MIOX values were 157, 119 and 166 for baseline, 6th and 24th h post-operatively, respectively (P > 0.05). Cystatin C values were 0.71 ± 0.3, 0.61 ± 0.41 and 0.9 ± 0.34 (P > 0.05) for the predefined timepoints, while Cr values were 1.68 ± 0.3, 1.5 ± 0.3 and 1.6 ± 0.3 (P > 0.05), respectively. None of these markers increased significantly after RIRS, suggesting that this procedure is safe for the treatment of nephrolithiasis with no negative effect on kidney tissue and function, according to the authors.
ONGOING TRIALS
As of this writing, clinical trials are under way assessing the effect of endourological procedures on renal function with the use of the new biomarkers and are depicted in Table 5. The small number of relevant registered ongoing trials, the fact that only one has the effect on renal function as a primary outcome and the stagnation reported regarding their status points to the need for strengthening research efforts around this crucial but understudied scientific field.
Areas where future research is needed in the field of the effects of urological intervention for lithiasis treatment on renal injury and renal function
| Observational studies on the incidence of AKI, with proper definitions after each type of endourological procedure during the short-term post-operative period |
| Observational studies on the effects of each type of endourological procedure (and the possibly developed AKI) on medium- and long-term renal function |
| Observational studies on the course (with detailed timepoints) of different biomarker levels after different types of endourological procedures |
| Observational studies comparing the diagnostic accuracy of different biomarkers in the detection of tubular injury and/or AKI after each type of endourological procedures |
| Randomized controlled studies comparing the effects of different types of endourological procedures on renal injury using novel biomarkers and the incidence of AKI using a standard definition |
| Observational studies to establish factors predisposing to or protecting against the development of AKI after endourological procedures |
| Observational studies on the incidence of AKI, with proper definitions after each type of endourological procedure during the short-term post-operative period |
| Observational studies on the effects of each type of endourological procedure (and the possibly developed AKI) on medium- and long-term renal function |
| Observational studies on the course (with detailed timepoints) of different biomarker levels after different types of endourological procedures |
| Observational studies comparing the diagnostic accuracy of different biomarkers in the detection of tubular injury and/or AKI after each type of endourological procedures |
| Randomized controlled studies comparing the effects of different types of endourological procedures on renal injury using novel biomarkers and the incidence of AKI using a standard definition |
| Observational studies to establish factors predisposing to or protecting against the development of AKI after endourological procedures |
Areas where future research is needed in the field of the effects of urological intervention for lithiasis treatment on renal injury and renal function
| Observational studies on the incidence of AKI, with proper definitions after each type of endourological procedure during the short-term post-operative period |
| Observational studies on the effects of each type of endourological procedure (and the possibly developed AKI) on medium- and long-term renal function |
| Observational studies on the course (with detailed timepoints) of different biomarker levels after different types of endourological procedures |
| Observational studies comparing the diagnostic accuracy of different biomarkers in the detection of tubular injury and/or AKI after each type of endourological procedures |
| Randomized controlled studies comparing the effects of different types of endourological procedures on renal injury using novel biomarkers and the incidence of AKI using a standard definition |
| Observational studies to establish factors predisposing to or protecting against the development of AKI after endourological procedures |
| Observational studies on the incidence of AKI, with proper definitions after each type of endourological procedure during the short-term post-operative period |
| Observational studies on the effects of each type of endourological procedure (and the possibly developed AKI) on medium- and long-term renal function |
| Observational studies on the course (with detailed timepoints) of different biomarker levels after different types of endourological procedures |
| Observational studies comparing the diagnostic accuracy of different biomarkers in the detection of tubular injury and/or AKI after each type of endourological procedures |
| Randomized controlled studies comparing the effects of different types of endourological procedures on renal injury using novel biomarkers and the incidence of AKI using a standard definition |
| Observational studies to establish factors predisposing to or protecting against the development of AKI after endourological procedures |
FURTHER RESEARCH AGENDA
Further research on the effect of endourological procedures on renal injury and renal function is clearly needed. As discussed, different pathophysiological mechanisms leading to renal injury for each endourological procedure have been proposed [4, 6, 34, 65]; however, as of this writing, details on the nature and extent of renal injury during each of these procedures is still lacking, possibly because of the inappropriate structure of the studies (e.g. lack of a modern definition of AKI, use of only conventional indexes of renal function instead of markers of renal injury, incorrect timing of measurements, small sample size, short follow-up, incorrect or absent reporting of surgical parameters, etc.). Appropriate studies include observational efforts on the incidence of properly defined AKI after each type of endourological procedure during the short-term post-operative period as well as the effects of these procedures on long-term renal function (Table 6). Furthermore, as the field of novel biomarkers is currently expanding there are certain issues that should be addressed in some detail. For example, the associations (including positive and negative predictive values) between specific cut-off levels of these biomarkers and diagnosis of AKI, in general, are still being researched. In addition, in endourological procedures, a theoretical increase of biomarkers could be expected due to the effect of the surgery on renal tissue, but also a decrease of the same biomarkers should be seen due to stone removal, resulting in difficulty in quantification of these procedure’s overall effects on the kidney. Thus the optimal time points for biomarker sampling after endourological procedures and the optimal biomarker reflecting renal injury and aiding the early diagnosis of AKI are issues for future research. After these issues are clarified, proper randomized controlled trials comparing the extent of renal injury and AKI incidence between the various endourological procedures for stone treatment can be performed. Furthermore, larger, properly designed studies assessing renal function in the medium- and long-term are required to examine whether renal injury after the endourological procedures is associated with long-term renal function changes.
CONCLUSIONS
In recent years, a debate about the comparative efficacy and safety of different endourological surgical methods for the treatment of nephrolithiasis has occurred. The efficacy part (mainly by comparing stone-free rates among the treatment techniques) has been set as a primary outcome and thoroughly examined in several published studies. The safety part has been examined, in most relevant studies, as a secondary outcome only by comparing major complications (bleeding and sepsis). Endourological procedures for the treatment of lithiasis could theoretically affect renal function through various mechanisms, including direct mechanical kidney injury or indirect ways (high IRPs, inflammation or vasoconstriction of adjacent renal vessels) that promote renal parenchymal damage [4, 6, 34, 65]. However, the crucial question of the short- and long-term effects of these surgical procedures on renal tissue has not been extensively or properly studied. In the few existing studies, common indexes of renal function (including sCr, urea or GFR) are used to capture renal damage [34, 37, 58, 67, 68], which is not always appropriate. Furthermore, the incidence of AKI induced and its possible long-term detrimental effects on renal function are rarely examined.
Recently emerged biomarkers with higher sensitivity and specificity for the diagnosis of tubular injury have been used in some studies of endourological procedures [3–5, 51–53], but with no typically conclusive results. Therefore, future studies assessing in detail the time course of changes in these biomarkers, the possibility of renal injury and the incidence of AKI following different types of procedures, as well as the reflection of any possible injury on long-term renal function are warranted. If research in this field successfully identifies biomarkers that can be used to diagnose kidney parenchymal damage and AKI in this setting, then this may offer a new road regarding renal stone surgical treatment, helping urologists to make informed decisions on several issues, from which endourological technique to perform and the optimal duration of surgery to which drugs to administer during the post-operative period, in order to offer patients evidence-based care.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest regarding this article.
REFERENCES
National Institute for Health and Care Excellence.





Comments