-
PDF
- Split View
-
Views
-
Cite
Cite
Catherine H Bozio, Amy Blain, Karen Edge, Monica M Farley, Lee H Harrison, Tasha Poissant, William Schaffner, Tara Scheuer, Salina Torres, Lori Triden, Elizabeth Briere, Sara E Oliver, Clinical Characteristics and Adverse Clinical Outcomes of Invasive Haemophilus influenzae Serotype a Cases—United States, 2011–2015, Clinical Infectious Diseases, Volume 73, Issue 11, 1 December 2021, Pages e3670–e3676, https://doi.org/10.1093/cid/ciaa990
Close - Share Icon Share
Abstract
Incidence of invasive disease due to Haemophilus influenzae serotype a (Hia) increased an average of 13% annually from 2002 through 2015. We describe clinical characteristics and adverse clinical outcomes of US invasive Hia cases detected through multistate surveillance during 2011–2015.
Medical record data were abstracted for cases reported in 8 jurisdictions conducting active population- and laboratory-based surveillance for invasive Hia disease across the United States. Isolates from sterile sites were serotyped using real-time polymerase chain reaction. Adverse clinical outcomes were defined as any possible complication of meningitis, bacteremic pneumonia, or bacteremia (including hearing loss and developmental delay, but excluding death) and were assessed at hospital discharge and one-year post-disease onset.
During 2011–2015, 190 Hia cases were reported to the 8 participating sites; 169 (88.9%) had data abstracted. Many patients were aged <5 years (42.6%). Meningitis was the most common clinical presentation among those aged <1 year (71.4%); bacteremic pneumonia was the most common presentation among persons aged ≥50 years (78.7%). Overall, 95.9% of patients were hospitalized. Among those hospitalized, 47.5% were admitted to an intensive care unit and 6.2% died during hospitalization. At hospital discharge and one-year post-disease onset, adverse outcomes were identified in 17.7% and 17.8% of patients overall and in 43.9% and 48.5% of patients with meningitis (primarily children).
Hia infection can cause severe disease that requires hospitalization and may also cause short- and long-term adverse clinical outcomes, especially among children. Novel vaccines could prevent morbidity and mortality.
Haemophilus influenzae is a bacterial pathogen that can cause severe invasive disease, including meningitis, pneumonia, epiglottitis, and sepsis. In the United States, H. influenzae serotype b (Hib) was once the leading cause of bacterial meningitis among children aged <5 years [1]. However, following introduction of Hib vaccines, Hib been nearly eliminated [2]. However, the incidence of nonserotype b H. influenzae infections has been increasing, and no vaccines are available for these serotypes. In particular, the estimated mean annual incidence of invasive H. influenzae serotype a (Hia) disease increased from 0.04 per 100 000 in 2002–2008 to 0.10 per 100 000 in 2009–2015 for an average annual increase of 13% [2].
Given the increases in invasive Hia disease noted over recent years, understanding the clinical presentation, severity, and potential adverse clinical outcomes of this disease is important to inform appropriate public health interventions. Few population-based studies of Hia disease have been conducted in the United States. Existing studies have focused on American Indian/Alaska Native (AI/AN) populations [3–5] and have demonstrated invasive Hia disease severity to be similar to that of Hib, with meningitis as a predominant clinical presentation and young children primarily affected [4, 6–10]. Limited data are available on the clinical severity and short- and long-term adverse clinical outcomes of invasive Hia disease [5]. Here, we identify invasive Hia cases through active, population-based surveillance in sites across the United States during 2011–2015 and describe clinical characteristics, hospitalization course, and adverse clinical outcomes of the hospitalized cases at hospital discharge and at one-year post-disease onset.
METHODS
Surveillance
Active population- and laboratory-based surveillance for invasive H. influenzae disease was conducted as part of Active Bacterial Core surveillance (ABCs). ABCs is supported by the Centers for Disease Control and Prevention (CDC) as part of the Emerging Infections Program Network [11]. Data from 1 January 2011 through 31 December 2015 were included in this analysis.
The surveillance areas included California (3 San Francisco Bay area counties), Colorado (5 Denver area counties), Connecticut (statewide), Georgia (statewide), Maryland (statewide), Minnesota (statewide), New Mexico (statewide), New York (15 Rochester and Albany area counties), Oregon (statewide), and Tennessee (20 counties).
A case of invasive Hia disease was defined as isolation of Hia from a normally sterile site (eg, blood or cerebrospinal fluid [CSF]) in an ABCs surveillance area resident. Epidemiologic and clinical information was abstracted from medical records. Outcome (alive/dead) was based on the hospitalized patient’s status at hospital discharge and not at one-year post-disease onset; no information was collected to determine whether deaths were attributed to Hia infection.
State public health laboratories serotyped and sent H. influenzae isolates to CDC’s Bacterial Meningitis Laboratory, where species and serotyping were confirmed for all isolates using methods previously described [12, 13].
Cases of invasive Hia disease were categorized as meningitis if a clinical diagnosis of meningitis was recorded in the medical record and Hia was isolated from CSF or other sterile sites, as bacteremic pneumonia if pneumonia was recorded in the patient’s medical record and Hia was isolated from blood or pleural fluid, and as bacteremia if Hia was isolated from blood and the patient’s medical record did not note meningitis or bacteremic pneumonia. These clinical presentations were mutually exclusive, using the above hierarchy. Cases of invasive Hia disease were also categorized as septic arthritis if indicated in the patient’s medical record. Septic arthritis was evaluated separately and therefore was not mutually exclusive with the other clinical presentations. Information on presence of underlying conditions was systematically collected as part of routine ABCs surveillance.
Chart Abstraction
At 8 participating ABCs sites (California, Colorado, Georgia, Maryland, Minnesota, New Mexico, Oregon, and Tennessee), data on Hia cases were abstracted from medical records from the hospital where the patient was treated for invasive Hia disease using a standardized case report form to describe the clinical severity, hospital course, and adverse clinical outcomes at hospital discharge. For patients with invasive Hia disease who were not hospitalized, adverse outcomes were not assessed. Data on adverse outcomes 1 year after Hia disease onset were abstracted from medical records from the healthcare visit(s) that occurred around the 1-year mark. Adverse clinical outcomes were defined as any possible complication of meningitis, bacteremic pneumonia, or bacteremia, excluding death (eg, hearing loss, speech delay, seizures/seizure disorder). The full abstraction form is available in the Supplementary Materials. Preexisting health conditions were also assessed at the time of hospital admission to distinguish between conditions present before Hia diagnosis and adverse outcomes after Hia diagnosis.
Data Analyses
Race was categorized as white, black, AI/AN, or Asian/Pacific Islander. Age was categorized as <1, 1–4, 5–49, 50–64, or ≥65 years. Similar incidence was seen across ages 5–49 years; given small numbers across these ages, they were combined into a single age group. Overall and age-specific proportions of clinical characteristics and outcomes, including case-fatality ratios, were calculated among all responses; “no” and unknown responses were combined, as missing data were overall infrequent. Antibiotics were categorized according to class, except for vancomycin, which was the only glycopeptide of interest. Procedures and complications documented during hospitalization were categorized based on body system involved. For example, burr hole, craniotomy, and subdural drain placement were classified as cranial procedures, and complications related to the central nervous system included brain abscess, cerebral edema, seizures, and stroke.
The CDC Human Research Protection Office determined this activity was nonresearch and therefore not required to be fully reviewed by the CDC Institutional Review Board (IRB). At each ABCs site that participated in the chart abstraction for Hia cases, the work was deemed either a public health assessment or human subjects research, for which approval was granted by local IRBs.
RESULTS
Case Characteristics
From 2011 through 2015, 190 invasive Hia cases, including 169 (88.9%) with medical record abstraction data available, were reported by the 8 participating ABCs sites. The age distribution was similar between the 169 cases that had data abstracted and the 21 cases that did not have data abstracted. However, the racial distribution differed, as 61.9% (13/21) of cases that did not have data abstracted were AI/AN compared with 8.9% (15/169) of those that had data abstracted. Cases that did not have data abstracted had a lower proportion of hospitalization than cases that had data abstracted (81.0% [17/21] vs 95.9% [162/169], respectively). However, no differences were observed in the proportion admitted to the intensive care unit (ICU) or died during hospitalization. Among the 169 cases that had data abstracted, 115 (68.0%) patients were white, 20 (11.8%) were black, and 15 (8.9%) were AI/AN (Table 1). Many patients were aged <5 years (42.6%); 47.9% of patients were male. Overall, 107 (63.3%) Hia patients had ≥1 underlying medical condition, though this proportion differed by age group, most meaningfully between those aged <5 years and ≥5 years (26.4% and 90.7%, respectively). Examples of underlying conditions included developmental delay, seizure/seizure disorders, and cancer in children aged <5 years and chronic lung disease, atherosclerotic cardiovascular disease, and obesity in persons aged ≥5 years. Meningitis was the most common clinical presentation among persons aged <1 year (71.4%), whereas bacteremic pneumonia was the most common clinical presentation among persons aged ≥50 years (78.7%; Table 1).
Demographic and Hospitalization Characteristics of Haemophilus influenzae Serotype a Cases, Overall and by Age, 2011–2015
| . | . | . | . | . | Age . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics . | <1 Year (n = 35) . | . | 1–4 Years (n = 37) . | . | 5–49 Years (n = 26) . | . | 50–64 Years (n = 36) . | . | ≥65 Years (n = 35) . | . | All Ages (n = 169) . | . |
| . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . |
| Male sex | 18 | (51.4) | 21 | (56.8) | 14 | (53.9) | 18 | (50.0) | 10 | (28.6) | 81 | (47.9) |
| Race | ||||||||||||
| White | 24 | (68.6) | 19 | (51.4) | 17 | (65.4) | 29 | (80.6) | 26 | (74.3) | 115 | (68.0) |
| Black | 7 | (20.0) | 9 | (24.3) | 2 | (7.7) | 2 | (5.6) | 0 | (0.0) | 20 | (11.8) |
| American Indian/Alaskan Native | 3 | (8.6) | 8 | (21.6) | 1 | (3.8) | 0 | (0.0) | 3 | (8.6) | 15 | (8.9) |
| Asian | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (2.8) | 0 | (0.0) | 1 | (0.6) |
| Unknown | 1 | (2.9) | 1 | (2.7) | 6 | (23.1) | 4 | (11.1) | 6 | (17.1) | 18 | (10.7) |
| Hispanic ethnicity | 9 | (25.7) | 9 | (24.3) | 6 | (23.1) | 2 | (5.6) | 3 | (8.6) | 29 | (17.2) |
| Presence of ≥1 underlying medical condition | 8 | (22.9) | 11 | (29.7) | 23 | (88.5) | 35 | (97.2) | 30 | (85.7) | 107 | (63.3) |
| Clinical presentation | ||||||||||||
| Meningitis | 25 | (71.4) | 13 | (35.1) | 4 | (15.4) | 1 | (2.8) | 0 | (0.0) | 43 | (25.4) |
| Bacteremic pneumonia | 2 | (5.7) | 6 | (16.2) | 14 | (53.9) | 27 | (75.0) | 21 | (60.0) | 70 | (41.4) |
| Bacteremia | 7 | (20.0) | 15 | (40.5) | 8 | (30.8) | 8 | (22.2) | 14 | (40.0) | 52 | (30.8) |
| Othera | 1 | (2.9) | 3 | (8.1) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 4 | (2.4) |
| Hospitalized | 34 | (97.1) | 35 | (94.6) | 24 | (92.3) | 36 | (100.0) | 33 | (94.3) | 162 | (95.9) |
| Age | ||||||||||||
| Characteristics Among Hospitalized Cases | <1 Year (n = 34) | 1–4 Years (n = 35) | 5–49 Years (n = 24) | 50–64 Years (n = 36) | ≥65 Years (n = 33) | All Ages (n = 162) | ||||||
| Duration of hospitalization, median (interquartile range) | 11 | (6–17) | 7.5 | (5–12) | 6 | (3.5–10) | 6 | (4–9) | 5 | (4–10) | 7 | (4–12) |
| Intensive care unit admission | 23 | (67.7) | 10 | (28.6) | 14 | (58.3) | 18 | (50.0) | 12 | (36.4) | 77 | (47.5) |
| Mechanical ventilation | 11 | (32.4) | 2 | (5.7) | 7 | (29.2) | 14 | (38.9) | 7 | (21.2) | 41 | (25.3) |
| Died in hospital | 1 | (2.9) | 0 | (0.0) | 2 | (8.3) | 3 | (8.3) | 4 | (12.1) | 10 | (6.2) |
| . | . | . | . | . | Age . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics . | <1 Year (n = 35) . | . | 1–4 Years (n = 37) . | . | 5–49 Years (n = 26) . | . | 50–64 Years (n = 36) . | . | ≥65 Years (n = 35) . | . | All Ages (n = 169) . | . |
| . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . |
| Male sex | 18 | (51.4) | 21 | (56.8) | 14 | (53.9) | 18 | (50.0) | 10 | (28.6) | 81 | (47.9) |
| Race | ||||||||||||
| White | 24 | (68.6) | 19 | (51.4) | 17 | (65.4) | 29 | (80.6) | 26 | (74.3) | 115 | (68.0) |
| Black | 7 | (20.0) | 9 | (24.3) | 2 | (7.7) | 2 | (5.6) | 0 | (0.0) | 20 | (11.8) |
| American Indian/Alaskan Native | 3 | (8.6) | 8 | (21.6) | 1 | (3.8) | 0 | (0.0) | 3 | (8.6) | 15 | (8.9) |
| Asian | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (2.8) | 0 | (0.0) | 1 | (0.6) |
| Unknown | 1 | (2.9) | 1 | (2.7) | 6 | (23.1) | 4 | (11.1) | 6 | (17.1) | 18 | (10.7) |
| Hispanic ethnicity | 9 | (25.7) | 9 | (24.3) | 6 | (23.1) | 2 | (5.6) | 3 | (8.6) | 29 | (17.2) |
| Presence of ≥1 underlying medical condition | 8 | (22.9) | 11 | (29.7) | 23 | (88.5) | 35 | (97.2) | 30 | (85.7) | 107 | (63.3) |
| Clinical presentation | ||||||||||||
| Meningitis | 25 | (71.4) | 13 | (35.1) | 4 | (15.4) | 1 | (2.8) | 0 | (0.0) | 43 | (25.4) |
| Bacteremic pneumonia | 2 | (5.7) | 6 | (16.2) | 14 | (53.9) | 27 | (75.0) | 21 | (60.0) | 70 | (41.4) |
| Bacteremia | 7 | (20.0) | 15 | (40.5) | 8 | (30.8) | 8 | (22.2) | 14 | (40.0) | 52 | (30.8) |
| Othera | 1 | (2.9) | 3 | (8.1) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 4 | (2.4) |
| Hospitalized | 34 | (97.1) | 35 | (94.6) | 24 | (92.3) | 36 | (100.0) | 33 | (94.3) | 162 | (95.9) |
| Age | ||||||||||||
| Characteristics Among Hospitalized Cases | <1 Year (n = 34) | 1–4 Years (n = 35) | 5–49 Years (n = 24) | 50–64 Years (n = 36) | ≥65 Years (n = 33) | All Ages (n = 162) | ||||||
| Duration of hospitalization, median (interquartile range) | 11 | (6–17) | 7.5 | (5–12) | 6 | (3.5–10) | 6 | (4–9) | 5 | (4–10) | 7 | (4–12) |
| Intensive care unit admission | 23 | (67.7) | 10 | (28.6) | 14 | (58.3) | 18 | (50.0) | 12 | (36.4) | 77 | (47.5) |
| Mechanical ventilation | 11 | (32.4) | 2 | (5.7) | 7 | (29.2) | 14 | (38.9) | 7 | (21.2) | 41 | (25.3) |
| Died in hospital | 1 | (2.9) | 0 | (0.0) | 2 | (8.3) | 3 | (8.3) | 4 | (12.1) | 10 | (6.2) |
aOther clinical presentations included septic arthritis (n = 4) and abscess (n = 1).
Demographic and Hospitalization Characteristics of Haemophilus influenzae Serotype a Cases, Overall and by Age, 2011–2015
| . | . | . | . | . | Age . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics . | <1 Year (n = 35) . | . | 1–4 Years (n = 37) . | . | 5–49 Years (n = 26) . | . | 50–64 Years (n = 36) . | . | ≥65 Years (n = 35) . | . | All Ages (n = 169) . | . |
| . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . |
| Male sex | 18 | (51.4) | 21 | (56.8) | 14 | (53.9) | 18 | (50.0) | 10 | (28.6) | 81 | (47.9) |
| Race | ||||||||||||
| White | 24 | (68.6) | 19 | (51.4) | 17 | (65.4) | 29 | (80.6) | 26 | (74.3) | 115 | (68.0) |
| Black | 7 | (20.0) | 9 | (24.3) | 2 | (7.7) | 2 | (5.6) | 0 | (0.0) | 20 | (11.8) |
| American Indian/Alaskan Native | 3 | (8.6) | 8 | (21.6) | 1 | (3.8) | 0 | (0.0) | 3 | (8.6) | 15 | (8.9) |
| Asian | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (2.8) | 0 | (0.0) | 1 | (0.6) |
| Unknown | 1 | (2.9) | 1 | (2.7) | 6 | (23.1) | 4 | (11.1) | 6 | (17.1) | 18 | (10.7) |
| Hispanic ethnicity | 9 | (25.7) | 9 | (24.3) | 6 | (23.1) | 2 | (5.6) | 3 | (8.6) | 29 | (17.2) |
| Presence of ≥1 underlying medical condition | 8 | (22.9) | 11 | (29.7) | 23 | (88.5) | 35 | (97.2) | 30 | (85.7) | 107 | (63.3) |
| Clinical presentation | ||||||||||||
| Meningitis | 25 | (71.4) | 13 | (35.1) | 4 | (15.4) | 1 | (2.8) | 0 | (0.0) | 43 | (25.4) |
| Bacteremic pneumonia | 2 | (5.7) | 6 | (16.2) | 14 | (53.9) | 27 | (75.0) | 21 | (60.0) | 70 | (41.4) |
| Bacteremia | 7 | (20.0) | 15 | (40.5) | 8 | (30.8) | 8 | (22.2) | 14 | (40.0) | 52 | (30.8) |
| Othera | 1 | (2.9) | 3 | (8.1) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 4 | (2.4) |
| Hospitalized | 34 | (97.1) | 35 | (94.6) | 24 | (92.3) | 36 | (100.0) | 33 | (94.3) | 162 | (95.9) |
| Age | ||||||||||||
| Characteristics Among Hospitalized Cases | <1 Year (n = 34) | 1–4 Years (n = 35) | 5–49 Years (n = 24) | 50–64 Years (n = 36) | ≥65 Years (n = 33) | All Ages (n = 162) | ||||||
| Duration of hospitalization, median (interquartile range) | 11 | (6–17) | 7.5 | (5–12) | 6 | (3.5–10) | 6 | (4–9) | 5 | (4–10) | 7 | (4–12) |
| Intensive care unit admission | 23 | (67.7) | 10 | (28.6) | 14 | (58.3) | 18 | (50.0) | 12 | (36.4) | 77 | (47.5) |
| Mechanical ventilation | 11 | (32.4) | 2 | (5.7) | 7 | (29.2) | 14 | (38.9) | 7 | (21.2) | 41 | (25.3) |
| Died in hospital | 1 | (2.9) | 0 | (0.0) | 2 | (8.3) | 3 | (8.3) | 4 | (12.1) | 10 | (6.2) |
| . | . | . | . | . | Age . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics . | <1 Year (n = 35) . | . | 1–4 Years (n = 37) . | . | 5–49 Years (n = 26) . | . | 50–64 Years (n = 36) . | . | ≥65 Years (n = 35) . | . | All Ages (n = 169) . | . |
| . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . | No. . | (%) . |
| Male sex | 18 | (51.4) | 21 | (56.8) | 14 | (53.9) | 18 | (50.0) | 10 | (28.6) | 81 | (47.9) |
| Race | ||||||||||||
| White | 24 | (68.6) | 19 | (51.4) | 17 | (65.4) | 29 | (80.6) | 26 | (74.3) | 115 | (68.0) |
| Black | 7 | (20.0) | 9 | (24.3) | 2 | (7.7) | 2 | (5.6) | 0 | (0.0) | 20 | (11.8) |
| American Indian/Alaskan Native | 3 | (8.6) | 8 | (21.6) | 1 | (3.8) | 0 | (0.0) | 3 | (8.6) | 15 | (8.9) |
| Asian | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (2.8) | 0 | (0.0) | 1 | (0.6) |
| Unknown | 1 | (2.9) | 1 | (2.7) | 6 | (23.1) | 4 | (11.1) | 6 | (17.1) | 18 | (10.7) |
| Hispanic ethnicity | 9 | (25.7) | 9 | (24.3) | 6 | (23.1) | 2 | (5.6) | 3 | (8.6) | 29 | (17.2) |
| Presence of ≥1 underlying medical condition | 8 | (22.9) | 11 | (29.7) | 23 | (88.5) | 35 | (97.2) | 30 | (85.7) | 107 | (63.3) |
| Clinical presentation | ||||||||||||
| Meningitis | 25 | (71.4) | 13 | (35.1) | 4 | (15.4) | 1 | (2.8) | 0 | (0.0) | 43 | (25.4) |
| Bacteremic pneumonia | 2 | (5.7) | 6 | (16.2) | 14 | (53.9) | 27 | (75.0) | 21 | (60.0) | 70 | (41.4) |
| Bacteremia | 7 | (20.0) | 15 | (40.5) | 8 | (30.8) | 8 | (22.2) | 14 | (40.0) | 52 | (30.8) |
| Othera | 1 | (2.9) | 3 | (8.1) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 4 | (2.4) |
| Hospitalized | 34 | (97.1) | 35 | (94.6) | 24 | (92.3) | 36 | (100.0) | 33 | (94.3) | 162 | (95.9) |
| Age | ||||||||||||
| Characteristics Among Hospitalized Cases | <1 Year (n = 34) | 1–4 Years (n = 35) | 5–49 Years (n = 24) | 50–64 Years (n = 36) | ≥65 Years (n = 33) | All Ages (n = 162) | ||||||
| Duration of hospitalization, median (interquartile range) | 11 | (6–17) | 7.5 | (5–12) | 6 | (3.5–10) | 6 | (4–9) | 5 | (4–10) | 7 | (4–12) |
| Intensive care unit admission | 23 | (67.7) | 10 | (28.6) | 14 | (58.3) | 18 | (50.0) | 12 | (36.4) | 77 | (47.5) |
| Mechanical ventilation | 11 | (32.4) | 2 | (5.7) | 7 | (29.2) | 14 | (38.9) | 7 | (21.2) | 41 | (25.3) |
| Died in hospital | 1 | (2.9) | 0 | (0.0) | 2 | (8.3) | 3 | (8.3) | 4 | (12.1) | 10 | (6.2) |
aOther clinical presentations included septic arthritis (n = 4) and abscess (n = 1).
Hospital Course
Overall, 162 (95.9%) patients with invasive Hia disease were hospitalized, with a median length of stay of 7 days (interquartile range [IQR], 4–12). The proportion hospitalized and median length of stay were similar across all age groups, except in patients aged <1 year, who had a longer length of stay (11 days; IQR, 6–17; Table 1). Seven persons with invasive Hia disease presented to the emergency department but were not hospitalized; 5 persons were alive after leaving the emergency department, and 2 died in the emergency department (both of whom were aged ≥65 years). Among the 166 patients who had information on antibiotic treatment, 31 (18.7%) received 1 antibiotic, 130 (78.3%) received >1 antibiotics (Figure 1), and 5 (3.0%) did not receive an antibiotic. Cephalosporins were the most commonly administered antibiotics among patients of all ages combined; the highest proportions of patients who received cephalosporins were in the <1 year and 1–4 year age groups (97.1% [34/35] and 94.4% [34/36], respectively).
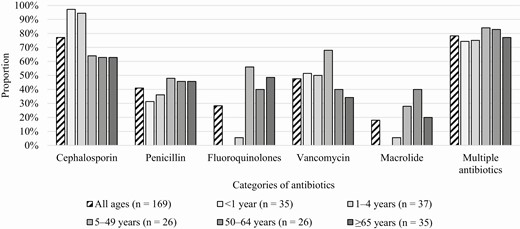
Types of antibiotics received during hospitalization among Haemophilus influenzae serotype a cases, 2011–2015.
During hospitalization, 31 (19.1%) of the 162 hospitalized Hia patients had procedures performed, which varied by presentation of invasive Hia disease (Table 2). Among 43 patients with meningitis (mostly aged <5 years), 11 (25.6%) had a procedure performed, including 5 cranial procedures (eg, shunt placement, drainage), and 4 required post-hospitalization rehabilitation. Additionally, 23 (53.5%) patients with meningitis experienced a complication during hospitalization, of which 21 were related to the central nervous system, including seizures (n = 17). Among 66 patients with bacteremic pneumonia, one-third (n = 21) experienced a complication during hospitalization. The most common complications in this group were septic shock (19.7%, 13/66) and renal complications (eg, acute kidney injury; 15.2%, 10/66), and all occurred among patients aged ≥5 years. Septic shock (8.2%, 4/49) and renal complications (14.3%, 7/49) were also the most common complications among the 49 patients with bacteremia and primarily occurred among patients aged ≥5 years.
Types of Procedures Performed and Complications During Hospitalization of Haemophilus influenzae Serotype a Cases, Overall and by Clinical Presentation, 2011–2015
| Characteristic . | Meningitis (n = 43), No. (%) . | Bacteremic Pneumonia (n = 66), No. (%) . | Bacteremia (n = 49), No. (%) . | All Clinical Presentationsa (n = 162), No. (%) . |
|---|---|---|---|---|
| Median age (interquartile range), y | 0 (0–1) | 56 (39–67) | 38 (2–65) | 32 (1–60) |
| Underlying conditionsb | 19 (44.2) | 57 (86.4) | 37 (75.5) | 115 (71.0) |
| Any procedurec | 11 (25.6) | 6 (9.1) | 11 (22.5) | 31 (19.1) |
| Type of procedure | ||||
| Cranial | 5 (11.6) | 0 (0) | 0 (0) | 5 (3.1) |
| Joint irrigationc | 2 (4.7) | 0 (0) | 7 (14.3) | 11 (6.8) |
| Tissue drainagec | 3 (7.0) | 1 (1.5) | 4 (8.2) | 9 (5.6) |
| Other proceduresd | 4 (9.3) | 5 (7.6) | 3 (6.1) | 12 (7.4) |
| Any complicatione | 23 (53.5) | 21 (31.8) | 13 (26.5) | 58 (35.8) |
| Type of complication | ||||
| Central nervous system | 21 (48.8) | 6 (9.1) | 1 (2.0) | 28 (17.3) |
| Hematological | 3 (7.0) | 1 (1.5) | 1 (2.0) | 5 (3.1) |
| Cardiac | 2 (4.7) | 1 (1.5) | 1 (2.0) | 4 (2.5) |
| Renal | 2 (4.7) | 10 (15.2) | 7 (14.3) | 19 (11.7) |
| Ear, Nose, and Throat/Pulmonary | 0 (0) | 3 (4.6) | 1 (2.0) | 4 (2.5) |
| Septic shock | 4 (9.3) | 13 (19.7) | 4 (8.2) | 21 (13.0) |
| Other complicationsf | 0 (0.0) | 0 (0) | 1 (2.0) | 2 (1.2) |
| Characteristic . | Meningitis (n = 43), No. (%) . | Bacteremic Pneumonia (n = 66), No. (%) . | Bacteremia (n = 49), No. (%) . | All Clinical Presentationsa (n = 162), No. (%) . |
|---|---|---|---|---|
| Median age (interquartile range), y | 0 (0–1) | 56 (39–67) | 38 (2–65) | 32 (1–60) |
| Underlying conditionsb | 19 (44.2) | 57 (86.4) | 37 (75.5) | 115 (71.0) |
| Any procedurec | 11 (25.6) | 6 (9.1) | 11 (22.5) | 31 (19.1) |
| Type of procedure | ||||
| Cranial | 5 (11.6) | 0 (0) | 0 (0) | 5 (3.1) |
| Joint irrigationc | 2 (4.7) | 0 (0) | 7 (14.3) | 11 (6.8) |
| Tissue drainagec | 3 (7.0) | 1 (1.5) | 4 (8.2) | 9 (5.6) |
| Other proceduresd | 4 (9.3) | 5 (7.6) | 3 (6.1) | 12 (7.4) |
| Any complicatione | 23 (53.5) | 21 (31.8) | 13 (26.5) | 58 (35.8) |
| Type of complication | ||||
| Central nervous system | 21 (48.8) | 6 (9.1) | 1 (2.0) | 28 (17.3) |
| Hematological | 3 (7.0) | 1 (1.5) | 1 (2.0) | 5 (3.1) |
| Cardiac | 2 (4.7) | 1 (1.5) | 1 (2.0) | 4 (2.5) |
| Renal | 2 (4.7) | 10 (15.2) | 7 (14.3) | 19 (11.7) |
| Ear, Nose, and Throat/Pulmonary | 0 (0) | 3 (4.6) | 1 (2.0) | 4 (2.5) |
| Septic shock | 4 (9.3) | 13 (19.7) | 4 (8.2) | 21 (13.0) |
| Other complicationsf | 0 (0.0) | 0 (0) | 1 (2.0) | 2 (1.2) |
aAll clinical presentations include meningitis (n = 43), bacteremic pneumonia (n = 66), bacteremia (n = 49), septic arthritis (n = 3), and septic arthritis and abscess (n = 1).
bAmong the 4 patients with septic arthritis, 2 (50.0%) had underlying conditions.
cThree (75.0%) patients with septic arthritis had any procedure performed. Two (50.0%) had joint irrigation performed, and 1 (25.0%) had tissue drainage performed.
dOther procedures include chest tube placement (n = 3), tracheostomy (n = 3), thoracoscopic decortication (n = 2), ear tube placement (n = 1), extracorporeal membrane oxygenation cannulation (n = 1), sinus irrigation (n = 1), pericardial drain insertion (n = 1), and peripherally inserted central catheter placement (n = 1).
eAmong the 4 patients who had septic arthritis and did not have meningitis, bacteremic pneumonia, or bacteremia, 1 (25.0%) had a complication reported.
fOther complications included pyomyositis (n = 1) and scrotal abscess (n = 1), of which 1 was reported in a patient with septic arthritis.
Types of Procedures Performed and Complications During Hospitalization of Haemophilus influenzae Serotype a Cases, Overall and by Clinical Presentation, 2011–2015
| Characteristic . | Meningitis (n = 43), No. (%) . | Bacteremic Pneumonia (n = 66), No. (%) . | Bacteremia (n = 49), No. (%) . | All Clinical Presentationsa (n = 162), No. (%) . |
|---|---|---|---|---|
| Median age (interquartile range), y | 0 (0–1) | 56 (39–67) | 38 (2–65) | 32 (1–60) |
| Underlying conditionsb | 19 (44.2) | 57 (86.4) | 37 (75.5) | 115 (71.0) |
| Any procedurec | 11 (25.6) | 6 (9.1) | 11 (22.5) | 31 (19.1) |
| Type of procedure | ||||
| Cranial | 5 (11.6) | 0 (0) | 0 (0) | 5 (3.1) |
| Joint irrigationc | 2 (4.7) | 0 (0) | 7 (14.3) | 11 (6.8) |
| Tissue drainagec | 3 (7.0) | 1 (1.5) | 4 (8.2) | 9 (5.6) |
| Other proceduresd | 4 (9.3) | 5 (7.6) | 3 (6.1) | 12 (7.4) |
| Any complicatione | 23 (53.5) | 21 (31.8) | 13 (26.5) | 58 (35.8) |
| Type of complication | ||||
| Central nervous system | 21 (48.8) | 6 (9.1) | 1 (2.0) | 28 (17.3) |
| Hematological | 3 (7.0) | 1 (1.5) | 1 (2.0) | 5 (3.1) |
| Cardiac | 2 (4.7) | 1 (1.5) | 1 (2.0) | 4 (2.5) |
| Renal | 2 (4.7) | 10 (15.2) | 7 (14.3) | 19 (11.7) |
| Ear, Nose, and Throat/Pulmonary | 0 (0) | 3 (4.6) | 1 (2.0) | 4 (2.5) |
| Septic shock | 4 (9.3) | 13 (19.7) | 4 (8.2) | 21 (13.0) |
| Other complicationsf | 0 (0.0) | 0 (0) | 1 (2.0) | 2 (1.2) |
| Characteristic . | Meningitis (n = 43), No. (%) . | Bacteremic Pneumonia (n = 66), No. (%) . | Bacteremia (n = 49), No. (%) . | All Clinical Presentationsa (n = 162), No. (%) . |
|---|---|---|---|---|
| Median age (interquartile range), y | 0 (0–1) | 56 (39–67) | 38 (2–65) | 32 (1–60) |
| Underlying conditionsb | 19 (44.2) | 57 (86.4) | 37 (75.5) | 115 (71.0) |
| Any procedurec | 11 (25.6) | 6 (9.1) | 11 (22.5) | 31 (19.1) |
| Type of procedure | ||||
| Cranial | 5 (11.6) | 0 (0) | 0 (0) | 5 (3.1) |
| Joint irrigationc | 2 (4.7) | 0 (0) | 7 (14.3) | 11 (6.8) |
| Tissue drainagec | 3 (7.0) | 1 (1.5) | 4 (8.2) | 9 (5.6) |
| Other proceduresd | 4 (9.3) | 5 (7.6) | 3 (6.1) | 12 (7.4) |
| Any complicatione | 23 (53.5) | 21 (31.8) | 13 (26.5) | 58 (35.8) |
| Type of complication | ||||
| Central nervous system | 21 (48.8) | 6 (9.1) | 1 (2.0) | 28 (17.3) |
| Hematological | 3 (7.0) | 1 (1.5) | 1 (2.0) | 5 (3.1) |
| Cardiac | 2 (4.7) | 1 (1.5) | 1 (2.0) | 4 (2.5) |
| Renal | 2 (4.7) | 10 (15.2) | 7 (14.3) | 19 (11.7) |
| Ear, Nose, and Throat/Pulmonary | 0 (0) | 3 (4.6) | 1 (2.0) | 4 (2.5) |
| Septic shock | 4 (9.3) | 13 (19.7) | 4 (8.2) | 21 (13.0) |
| Other complicationsf | 0 (0.0) | 0 (0) | 1 (2.0) | 2 (1.2) |
aAll clinical presentations include meningitis (n = 43), bacteremic pneumonia (n = 66), bacteremia (n = 49), septic arthritis (n = 3), and septic arthritis and abscess (n = 1).
bAmong the 4 patients with septic arthritis, 2 (50.0%) had underlying conditions.
cThree (75.0%) patients with septic arthritis had any procedure performed. Two (50.0%) had joint irrigation performed, and 1 (25.0%) had tissue drainage performed.
dOther procedures include chest tube placement (n = 3), tracheostomy (n = 3), thoracoscopic decortication (n = 2), ear tube placement (n = 1), extracorporeal membrane oxygenation cannulation (n = 1), sinus irrigation (n = 1), pericardial drain insertion (n = 1), and peripherally inserted central catheter placement (n = 1).
eAmong the 4 patients who had septic arthritis and did not have meningitis, bacteremic pneumonia, or bacteremia, 1 (25.0%) had a complication reported.
fOther complications included pyomyositis (n = 1) and scrotal abscess (n = 1), of which 1 was reported in a patient with septic arthritis.
Mechanical ventilation was provided to 25.3% of hospitalized patients with invasive Hia disease; the highest proportions of patients who received mechanical ventilation were in the 50–64 years, <1 year, and 5–49 years age groups (38.9%, 32.4%, and 29.2%, respectively; Table 1). Seventy-seven (47.5%) patients were admitted to the ICU; this ranged from 28.6% of patients aged 1–4 years to 67.7% of patients aged <1 year. By clinical presentation, patients with meningitis had the highest proportion of ICU admission (76.7%, 33/43) compared with patients with bacteremic pneumonia (40.9%, 27/66) and with bacteremia (32.7%, 16/49). Among the 70 patients admitted to the ICU with a known length of stay, 18 (25.7%) stayed 1 week or longer. Ten (6.2%) of 162 patients with invasive Hia died during hospitalization; 8 patients who had Hia bacteremia pneumonia were aged ≥5 years, whereas 2 patients who had meningitis were aged <1 year or 5–49 years. All patients had underlying conditions, of which diabetes (n = 5) and lung disease (n = 7) were the most common. Case-fatality ratios were highest among patients aged ≥65 years, 50–64 years, and 5–49 years (12.1% [4/33], 8.3% [3/36], and 8.3% [2/24], respectively). Notably, case-fatality was lower among patients aged <1 year and 1–4 years (2.9% and 0.0%, respectively).
Thirteen (8.0%) patients hospitalized with invasive Hia disease were also noted to have septic arthritis; 10 occurred in patients aged 1–4 years and 3 occurred in patients aged <1, 5–49, and 50–64 years. Notably, among Hia patients aged 1–4 years, 28.6% (10/35) had septic arthritis. Of these 13 patients, 7 had bacteremia and 2 had meningitis. Patients with septic arthritis had the highest proportion of procedures performed (92.3%, 12/13), of which joint irrigation (84.6%) was the most common. No deaths were reported among patients with septic arthritis.
Adverse Clinical Outcomes of Invasive Hia Disease Among Patients Alive at Hospital Discharge
At the time of hospital discharge, adverse clinical outcomes were recorded for 27 (17.7%) of the 152 surviving invasive Hia disease patients. Patients with meningitis had the highest proportion of adverse outcomes recorded (18/41, 43.9%); among 18 meningitis patients with adverse outcomes, all were aged <5 years. The proportions of adverse outcomes were much lower for patients with bacteremic pneumonia (8/58, 13.8%) and bacteremia (1/49, 2.0%). Of patients with bacteremic pneumonia and adverse outcomes, 62.5% were aged >50 years, whereas the patient with bacteremia and an adverse outcome was aged 5–49 years.
To assess clinical outcomes 1 year after Hia disease onset, charts were available for 118 (77.6%) of the 152 patients who were alive at hospital discharge; among these, 21 (17.8%) had documented adverse outcomes 1 year after disease onset. Among 33 patients with meningitis, 16 (48.5%) had adverse outcomes documented and all were in children aged <5 years at the time of Hia disease onset. The most common adverse outcomes were hearing loss, developmental delay, and speech delay (30.3%, 12.1%, and 12.1%, respectively; Table 3). Seven patients with meningitis required hearing aids (n = 3) or cochlear implants (n = 4) within the year after Hia disease onset, and 9 required occupational, speech, and/or physical therapy; all of these patients were aged <5 years at the time of Hia disease onset. The proportion of adverse clinical outcomes 1 year after disease onset was similar among patients with bacteremia (3/42, 7.1%) and bacteremic pneumonia (2/42, 4.8%); all adverse outcomes were among patients aged ≥50 years at the time of Hia disease onset. Among the 118 patients with invasive Hia disease who had a chart available at both time points, 10 (8.5%) had the same outcome reported, likely indicating that a condition identified at the time of hospital discharge had not yet resolved 1 year after Hia disease onset. Nine of these patients presented with meningitis at age ≤2 years; among them, adverse clinical outcomes included hearing loss (n = 6), seizures/seizure disorder (n = 3), developmental delay (n = 1), and cranial nerve dysfunction/palsy (n = 1). A 45-year-old Hispanic patient who presented with bacteremic pneumonia had hearing loss reported at both time points.
Adverse Clinical Outcomes Recorded at Time of Hospital Discharge and 1 Year After Haemophilus influenzae Serotype a Disease Onset Among Patients Who Had Adverse Clinical Outcomes by Medical Record Review, Overall and by Clinical Presentation, 2011–2015
| Outcomes at Hospital Discharge (No. of Surviving Patients With Invasive Hia) . | Meningitis (n = 41) . | . | Bacteremic Pneumonia (n = 58) . | . | Bacteremiaa (n = 49) . | . | All Clinical Presentationsb (n = 152) . | . |
|---|---|---|---|---|---|---|---|---|
| . | No. . | % . | No. . | % . | No. . | % . | No. . | % . |
| Any clinical adverse outcome | 18 | 43.9 | 8 | 13.8 | 1 | 2.0 | 27 | 17.7 |
| Seizures/seizure disorders | 10 | 24.4 | 0 | 0.0 | 0 | 0.0 | 10 | 6.5 |
| Hearing loss | 9 | 22.0 | 0 | 0.0 | 0 | 0.0 | 9 | 5.9 |
| Oxygen dependence | 0 | 0.0 | 8 | 13.8 | 0 | 0.0 | 8 | 5.2 |
| Neurological deficits | 4 | 9.8 | 0 | 0.0 | 0 | 0.0 | 4 | 2.6 |
| Motor deficits | 1 | 2.4 | 1 | 1.7 | 0 | 0.0 | 2 | 1.3 |
| Cognitive impairments | 0 | 0.0 | 2 | 3.5 | 0 | 0.0 | 2 | 1.3 |
| Developmental delay | 2 | 4.9 | 0 | 0.0 | 0 | 0.0 | 2 | 1.3 |
| Paralysis | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Cranial nerve dysfunction/palsy | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Joint or bone abnormality | 0 | 0.0 | 0 | 0.0 | 1 | 2.0 | 1 | 0.7 |
| Ventriculomegaly | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Outcomes 1 Year After Hia Disease Onset (No. Who Had Chart Available 1 Year After Hia Disease Onset) | Meningitisc(n = 33) | Bacteremic Pneumonia (n = 42) | Bacteremiad(n = 42) | All Clinical Presentationse(n = 118) | ||||
| No. | % | No. | % | No. | % | No. | % | |
| Any adverse clinical outcome | 16 | 48.5 | 2 | 4.8 | 3 | 7.1 | 21 | 17.8 |
| Hearing loss | 10 | 30.3 | 0 | 0.0 | 0 | 0.0 | 10 | 8.5 |
| Developmental delay | 4 | 12.1 | 0 | 0.0 | 0 | 0.0 | 4 | 3.4 |
| Speech delay | 4 | 12.1 | 0 | 0.0 | 0 | 0.0 | 4 | 3.4 |
| Seizures/seizure disorders | 3 | 9.1 | 0 | 0.0 | 0 | 0.0 | 3 | 2.5 |
| Cranial nerve dysfunction/palsy | 2 | 6.1 | 0 | 0.0 | 0 | 0.0 | 2 | 1.7 |
| Motor deficits | 1 | 3.0 | 0 | 0.0 | 1 | 2.4 | 2 | 1.7 |
| Recurrent pneumonia | 0 | 0.0 | 1 | 2.4 | 1 | 2.4 | 2 | 1.7 |
| Neurological deficits | 0 | 0.0 | 0 | 0.0 | 1 | 2.4 | 1 | 0.9 |
| Ventriculomegaly | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 |
| Other long-term sequelae | 0 | 0.0 | 1 | 2.4 | 0 | 0.0 | 1 | 0.9 |
| Vision loss | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 |
| Outcomes at Hospital Discharge (No. of Surviving Patients With Invasive Hia) . | Meningitis (n = 41) . | . | Bacteremic Pneumonia (n = 58) . | . | Bacteremiaa (n = 49) . | . | All Clinical Presentationsb (n = 152) . | . |
|---|---|---|---|---|---|---|---|---|
| . | No. . | % . | No. . | % . | No. . | % . | No. . | % . |
| Any clinical adverse outcome | 18 | 43.9 | 8 | 13.8 | 1 | 2.0 | 27 | 17.7 |
| Seizures/seizure disorders | 10 | 24.4 | 0 | 0.0 | 0 | 0.0 | 10 | 6.5 |
| Hearing loss | 9 | 22.0 | 0 | 0.0 | 0 | 0.0 | 9 | 5.9 |
| Oxygen dependence | 0 | 0.0 | 8 | 13.8 | 0 | 0.0 | 8 | 5.2 |
| Neurological deficits | 4 | 9.8 | 0 | 0.0 | 0 | 0.0 | 4 | 2.6 |
| Motor deficits | 1 | 2.4 | 1 | 1.7 | 0 | 0.0 | 2 | 1.3 |
| Cognitive impairments | 0 | 0.0 | 2 | 3.5 | 0 | 0.0 | 2 | 1.3 |
| Developmental delay | 2 | 4.9 | 0 | 0.0 | 0 | 0.0 | 2 | 1.3 |
| Paralysis | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Cranial nerve dysfunction/palsy | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Joint or bone abnormality | 0 | 0.0 | 0 | 0.0 | 1 | 2.0 | 1 | 0.7 |
| Ventriculomegaly | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Outcomes 1 Year After Hia Disease Onset (No. Who Had Chart Available 1 Year After Hia Disease Onset) | Meningitisc(n = 33) | Bacteremic Pneumonia (n = 42) | Bacteremiad(n = 42) | All Clinical Presentationse(n = 118) | ||||
| No. | % | No. | % | No. | % | No. | % | |
| Any adverse clinical outcome | 16 | 48.5 | 2 | 4.8 | 3 | 7.1 | 21 | 17.8 |
| Hearing loss | 10 | 30.3 | 0 | 0.0 | 0 | 0.0 | 10 | 8.5 |
| Developmental delay | 4 | 12.1 | 0 | 0.0 | 0 | 0.0 | 4 | 3.4 |
| Speech delay | 4 | 12.1 | 0 | 0.0 | 0 | 0.0 | 4 | 3.4 |
| Seizures/seizure disorders | 3 | 9.1 | 0 | 0.0 | 0 | 0.0 | 3 | 2.5 |
| Cranial nerve dysfunction/palsy | 2 | 6.1 | 0 | 0.0 | 0 | 0.0 | 2 | 1.7 |
| Motor deficits | 1 | 3.0 | 0 | 0.0 | 1 | 2.4 | 2 | 1.7 |
| Recurrent pneumonia | 0 | 0.0 | 1 | 2.4 | 1 | 2.4 | 2 | 1.7 |
| Neurological deficits | 0 | 0.0 | 0 | 0.0 | 1 | 2.4 | 1 | 0.9 |
| Ventriculomegaly | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 |
| Other long-term sequelae | 0 | 0.0 | 1 | 2.4 | 0 | 0.0 | 1 | 0.9 |
| Vision loss | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 |
Abbreviation: Hia, Haemophilus influenzae serotype a.
aThe patient who had the adverse outcome at hospital discharge had both bacteremia and septic arthritis.
bAll clinical presentations include meningitis (n = 41), bacteremic pneumonia (n = 58), bacteremia (n = 49), septic arthritis (n = 3), and septic arthritis and abscess (n = 1).
cOne patient with both meningitis and septic arthritis had hearing loss reported 1 year after Hia disease onset.
dOne patient with both bacteremia and septic arthritis had motor deficits reported 1 year after Hia disease onset.
eAll clinical presentations include meningitis (n = 33), bacteremic pneumonia (n = 42), bacteremia (n = 42), and septic arthritis (n = 1).
Adverse Clinical Outcomes Recorded at Time of Hospital Discharge and 1 Year After Haemophilus influenzae Serotype a Disease Onset Among Patients Who Had Adverse Clinical Outcomes by Medical Record Review, Overall and by Clinical Presentation, 2011–2015
| Outcomes at Hospital Discharge (No. of Surviving Patients With Invasive Hia) . | Meningitis (n = 41) . | . | Bacteremic Pneumonia (n = 58) . | . | Bacteremiaa (n = 49) . | . | All Clinical Presentationsb (n = 152) . | . |
|---|---|---|---|---|---|---|---|---|
| . | No. . | % . | No. . | % . | No. . | % . | No. . | % . |
| Any clinical adverse outcome | 18 | 43.9 | 8 | 13.8 | 1 | 2.0 | 27 | 17.7 |
| Seizures/seizure disorders | 10 | 24.4 | 0 | 0.0 | 0 | 0.0 | 10 | 6.5 |
| Hearing loss | 9 | 22.0 | 0 | 0.0 | 0 | 0.0 | 9 | 5.9 |
| Oxygen dependence | 0 | 0.0 | 8 | 13.8 | 0 | 0.0 | 8 | 5.2 |
| Neurological deficits | 4 | 9.8 | 0 | 0.0 | 0 | 0.0 | 4 | 2.6 |
| Motor deficits | 1 | 2.4 | 1 | 1.7 | 0 | 0.0 | 2 | 1.3 |
| Cognitive impairments | 0 | 0.0 | 2 | 3.5 | 0 | 0.0 | 2 | 1.3 |
| Developmental delay | 2 | 4.9 | 0 | 0.0 | 0 | 0.0 | 2 | 1.3 |
| Paralysis | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Cranial nerve dysfunction/palsy | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Joint or bone abnormality | 0 | 0.0 | 0 | 0.0 | 1 | 2.0 | 1 | 0.7 |
| Ventriculomegaly | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Outcomes 1 Year After Hia Disease Onset (No. Who Had Chart Available 1 Year After Hia Disease Onset) | Meningitisc(n = 33) | Bacteremic Pneumonia (n = 42) | Bacteremiad(n = 42) | All Clinical Presentationse(n = 118) | ||||
| No. | % | No. | % | No. | % | No. | % | |
| Any adverse clinical outcome | 16 | 48.5 | 2 | 4.8 | 3 | 7.1 | 21 | 17.8 |
| Hearing loss | 10 | 30.3 | 0 | 0.0 | 0 | 0.0 | 10 | 8.5 |
| Developmental delay | 4 | 12.1 | 0 | 0.0 | 0 | 0.0 | 4 | 3.4 |
| Speech delay | 4 | 12.1 | 0 | 0.0 | 0 | 0.0 | 4 | 3.4 |
| Seizures/seizure disorders | 3 | 9.1 | 0 | 0.0 | 0 | 0.0 | 3 | 2.5 |
| Cranial nerve dysfunction/palsy | 2 | 6.1 | 0 | 0.0 | 0 | 0.0 | 2 | 1.7 |
| Motor deficits | 1 | 3.0 | 0 | 0.0 | 1 | 2.4 | 2 | 1.7 |
| Recurrent pneumonia | 0 | 0.0 | 1 | 2.4 | 1 | 2.4 | 2 | 1.7 |
| Neurological deficits | 0 | 0.0 | 0 | 0.0 | 1 | 2.4 | 1 | 0.9 |
| Ventriculomegaly | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 |
| Other long-term sequelae | 0 | 0.0 | 1 | 2.4 | 0 | 0.0 | 1 | 0.9 |
| Vision loss | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 |
| Outcomes at Hospital Discharge (No. of Surviving Patients With Invasive Hia) . | Meningitis (n = 41) . | . | Bacteremic Pneumonia (n = 58) . | . | Bacteremiaa (n = 49) . | . | All Clinical Presentationsb (n = 152) . | . |
|---|---|---|---|---|---|---|---|---|
| . | No. . | % . | No. . | % . | No. . | % . | No. . | % . |
| Any clinical adverse outcome | 18 | 43.9 | 8 | 13.8 | 1 | 2.0 | 27 | 17.7 |
| Seizures/seizure disorders | 10 | 24.4 | 0 | 0.0 | 0 | 0.0 | 10 | 6.5 |
| Hearing loss | 9 | 22.0 | 0 | 0.0 | 0 | 0.0 | 9 | 5.9 |
| Oxygen dependence | 0 | 0.0 | 8 | 13.8 | 0 | 0.0 | 8 | 5.2 |
| Neurological deficits | 4 | 9.8 | 0 | 0.0 | 0 | 0.0 | 4 | 2.6 |
| Motor deficits | 1 | 2.4 | 1 | 1.7 | 0 | 0.0 | 2 | 1.3 |
| Cognitive impairments | 0 | 0.0 | 2 | 3.5 | 0 | 0.0 | 2 | 1.3 |
| Developmental delay | 2 | 4.9 | 0 | 0.0 | 0 | 0.0 | 2 | 1.3 |
| Paralysis | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Cranial nerve dysfunction/palsy | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Joint or bone abnormality | 0 | 0.0 | 0 | 0.0 | 1 | 2.0 | 1 | 0.7 |
| Ventriculomegaly | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 |
| Outcomes 1 Year After Hia Disease Onset (No. Who Had Chart Available 1 Year After Hia Disease Onset) | Meningitisc(n = 33) | Bacteremic Pneumonia (n = 42) | Bacteremiad(n = 42) | All Clinical Presentationse(n = 118) | ||||
| No. | % | No. | % | No. | % | No. | % | |
| Any adverse clinical outcome | 16 | 48.5 | 2 | 4.8 | 3 | 7.1 | 21 | 17.8 |
| Hearing loss | 10 | 30.3 | 0 | 0.0 | 0 | 0.0 | 10 | 8.5 |
| Developmental delay | 4 | 12.1 | 0 | 0.0 | 0 | 0.0 | 4 | 3.4 |
| Speech delay | 4 | 12.1 | 0 | 0.0 | 0 | 0.0 | 4 | 3.4 |
| Seizures/seizure disorders | 3 | 9.1 | 0 | 0.0 | 0 | 0.0 | 3 | 2.5 |
| Cranial nerve dysfunction/palsy | 2 | 6.1 | 0 | 0.0 | 0 | 0.0 | 2 | 1.7 |
| Motor deficits | 1 | 3.0 | 0 | 0.0 | 1 | 2.4 | 2 | 1.7 |
| Recurrent pneumonia | 0 | 0.0 | 1 | 2.4 | 1 | 2.4 | 2 | 1.7 |
| Neurological deficits | 0 | 0.0 | 0 | 0.0 | 1 | 2.4 | 1 | 0.9 |
| Ventriculomegaly | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 |
| Other long-term sequelae | 0 | 0.0 | 1 | 2.4 | 0 | 0.0 | 1 | 0.9 |
| Vision loss | 1 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 |
Abbreviation: Hia, Haemophilus influenzae serotype a.
aThe patient who had the adverse outcome at hospital discharge had both bacteremia and septic arthritis.
bAll clinical presentations include meningitis (n = 41), bacteremic pneumonia (n = 58), bacteremia (n = 49), septic arthritis (n = 3), and septic arthritis and abscess (n = 1).
cOne patient with both meningitis and septic arthritis had hearing loss reported 1 year after Hia disease onset.
dOne patient with both bacteremia and septic arthritis had motor deficits reported 1 year after Hia disease onset.
eAll clinical presentations include meningitis (n = 33), bacteremic pneumonia (n = 42), bacteremia (n = 42), and septic arthritis (n = 1).
DISCUSSION
Invasive Hia disease is an important cause of serious morbidity and mortality. Invasive Hia disease can affect persons of all ages, though the burden is greatest in the youngest and oldest age groups. Meningitis was the predominant clinical presentation in infants and young children, whereas older adults primarily had bacteremic pneumonia or bacteremia. The spectrum and distribution of clinical presentations are similar for Hia and Hib [4, 6–10]. Additionally, invasive Hia disease can be clinically severe; most patients across all ages were hospitalized, with approximately half requiring intensive care. Survivors of invasive Hia disease may experience adverse clinical outcomes. Patients with Hia meningitis experienced the most adverse outcomes at both hospital discharge and 1 year after Hia disease onset, with outcomes that were primary neurological and affected almost exclusively patients aged <5 years at the time of Hia disease onset.
Patient age and clinical presentation in this analysis are consistent with previous studies that, because of the higher burden of Hia disease in indigenous populations, included only AI/AN populations [3–5, 8, 14]. Consistent with our analysis, children aged <5 years represented the largest proportion of Hia cases in AI/AN populations [3, 4, 8, 14]. Children with invasive Hia disease predominantly present with meningitis [3–5, 8, 14, 15], though septic arthritis has also been observed. Plumb et al described septic arthritis in 13.9% of children aged <10 years with invasive Hia disease [5]. Similarly, we observed 14.7% of septic arthritis among children of the same age (data not shown). Despite the predominance of Hia among young children, case-fatality was low. In contrast, we also observed that persons aged ≥5 years primarily presented with bacteremia pneumonia or bacteremia, as generally seen with invasive H. influenzae disease [2], and had a relatively higher case-fatality.
The clinical severity, complications, and adverse clinical outcomes of invasive Hia disease noted in this study are consistent with previous work, which reported that most Hia patients across all ages were hospitalized [3, 4, 8, 14] and case-fatality ratios ranged from 5.4% to 6.6% [8, 14]. Further, neurological complications, particularly cerebral empyema and stroke, have been noted among patients with Hia meningitis [5]. Of patients with Hia meningitis in our analysis, almost 90% were aged <5 years and half had neurological complications, of which seizures, subdural effusion, and subdural empyema were the most common. Additionally, adverse clinical outcomes at discharge were most common in children aged <5 years with invasive Hia disease (25.0%) in our analysis, which was higher than Millar et al’s estimate of 6.6% in the same age group [4]. Consistent with our analysis, commonly reported adverse outcomes among children with invasive Hia disease described in previous studies included hearing loss at hospital discharge, along with motor or speech deficits and developmental delay 1 year after Hia disease onset [4, 5, 15].
Our analysis had some limitations. First, despite the importance of describing any sequelae from invasive Hia disease, we were unable to determine whether the Hia infection caused the reported adverse clinical outcomes. However, the need for a procedure suggests the presence of disease in the relevant organ system that is severe enough to have produced adverse outcomes, particularly those at hospital discharge. It is also possible that complications and/or adverse clinical outcomes may have occurred, in part, because of underlying conditions. However, compared with other age groups, children aged <5 years had the lowest proportion of underlying conditions, yet had higher frequencies of procedures and complications during hospitalization and of adverse outcomes at discharge and 1 year after Hia disease onset, suggesting that the Hia infection, rather than underlying conditions, led to these phenomena. Additionally, adverse clinical outcomes were assessed by chart abstraction 1 year after Hia disease onset, though it is possible that not all medical records from healthcare encounters around the 1-year mark were accessible and therefore available for abstraction. This may have contributed to records being unavailable for 22% of hospitalized Hia cases for the assessment of adverse outcomes 1 year after disease onset. Relying on abstracted data at this time point, rather than systematic assessments, increases the potential for misclassification of outcomes due to inconsistencies of diagnosis and coding. Finally, chart reviews could not be completed for 13 AI/AN patients with invasive Hia disease, though our findings were consistent with those observed in AI/AN populations.
Invasive Hia disease primarily affects young children and older adults. Clinical presentations and severity are similar to those of Hib, predominantly presenting as meningitis in young children and bacteremic pneumonia or bacteremia in adults. Likewise, invasive Hia disease frequently results in hospitalization and may cause short- and long-term adverse clinical outcomes. As no prevention strategies currently exist for Hia, a better understanding of the extent of adverse clinical outcomes of and their attribution to invasive Hia disease could inform guidance for public health interventions. With a conjugate Hia vaccine in development [16], these data on clinical characteristics and outcomes help to describe the potential severe disease caused by invasive Hia infection and demonstrate the utility of an Hia vaccine, especially in young children.
Notes
Acknowledgments. The authors thank everyone in the Active Bacterial Core surveillance (ABCs) areas who are involved in surveillance and maintenance of the system at the 10 sites. We also thank the laboratorians and technicians who isolate the ABCs pathogens and make it possible to track these infections and the surveillance and laboratory personnel at the Centers for Disease Control and Prevention (CDC) for their careful work characterizing the isolates. We acknowledge the following members of the ABCs team and others for their contributions at the study sites: Amy Tunali (Georgia Emerging Infections Program), Teressa Carter, Kathleen Shutt, Rosemary Hollick (Maryland Emerging Infections Program), and Brenda Barnes and Anise Elie (Tennessee Emerging Infections Program).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by a cooperative agreement with the Emerging Infections Program of the Centers for Disease Control and Prevention (CDC-RFA-CK17-1701).
Potential conflicts of interest. L. H. H. reports personal fees from Merck, Sanofi Pasteur, Pfizer, and GSK outside the submitted work. W. S. has received personal fees from Merck, Pfizer, and Roche Diagnostics and has received grants from the CDC outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References




