-
PDF
- Split View
-
Views
-
Cite
Cite
Heidi M Soeters, Sara E Oliver, Ian D Plumb, Amy E Blain, Tammy Zulz, Brenna C Simons, Meghan Barnes, Monica M Farley, Lee H Harrison, Ruth Lynfield, Stephanie Massay, Joseph McLaughlin, Alison G Muse, Susan Petit, William Schaffner, Ann Thomas, Salina Torres, James Watt, Tracy Pondo, Melissa J Whaley, Fang Hu, Xin Wang, Elizabeth C Briere, Michael G Bruce, Epidemiology of Invasive Haemophilus influenzae Serotype a Disease—United States, 2008–2017, Clinical Infectious Diseases, Volume 73, Issue 2, 15 July 2021, Pages e371–e379, https://doi.org/10.1093/cid/ciaa875
Close - Share Icon Share
Abstract
Haemophilus influenzae serotype a (Hia) can cause invasive disease similar to serotype b; no Hia vaccine is available. We describe the epidemiology of invasive Hia disease in the United States overall and specifically in Alaska during 2008–2017.
Active population- and laboratory-based surveillance for invasive Hia disease was conducted through Active Bacterial Core surveillance sites and from Alaska statewide invasive bacterial disease surveillance. Sterile-site isolates were serotyped via slide agglutination or real-time polymerase chain reaction. Incidences in cases per 100 000 were calculated.
From 2008 to 2017, an estimated average of 306 invasive Hia disease cases occurred annually in the United States (estimated annual incidence: 0.10); incidence increased by an average of 11.1% annually. Overall, 42.7% of cases were in children aged <5 years (incidence: 0.64), with highest incidence among children aged <1 year (1.60). Case fatality was 7.8% overall and was highest among adults aged ≥65 years (15.1%). Among children aged <5 years, the incidence was 17 times higher among American Indian and Alaska Native (AI/AN) children (8.29) than among children of all other races combined (0.49). In Alaska, incidences among all ages (0.68) and among children aged <1 year (24.73) were nearly 6 and 14 times higher, respectively, than corresponding US incidences. Case fatality in Alaska was 10.2%, and the vast majority (93.9%) of cases occurred among AI/AN.
Incidence of invasive Hia disease has increased since 2008, with the highest burden among AI/AN children. These data can inform prevention strategies, including Hia vaccine development.
(See the Editorial Commentary by Ulanova on pages e380–2.)
Invasive Haemophilus influenzae disease is an important cause of morbidity and mortality in young children and older adults and in those with certain underlying medical conditions [1–3]. Haemophilus influenzae bacteria may be either encapsulated (typeable) or nonencapsulated (nontypeable), with 6 encapsulated serotypes designated a through f according to their distinct capsular polysaccharides. Haemophilus influenzae can cause asymptomatic nasopharyngeal carriage or clinical disease, including meningitis, bacteremia, and pneumonia. The greatest burden of disease occurs at both ends of the life spectrum—in infants and in older adults [1, 2, 4].
After the introduction of H. influenzae serotype b (Hib) vaccines in the 1980s–1990s, the incidence of invasive H. influenzae disease among children aged younger than 5 years decreased by more than 99% in the United States [5–8]. Following this dramatic reduction, the serotype distribution of invasive H. influenzae cases shifted [9]. Although nontypeable H. influenzae and H. influenzae serotype f now cause the majority of invasive disease in the United States, H. influenzae serotype a (Hia) is of particular concern because incidence increased by an average of 13% annually from 0.02 per 100 000 in 2002 to 0.14 per 100 000 in 2015 [9], and elevated incidence has been reported among children and indigenous populations in the United States and Canada [10–17]. In Alaska, the epidemiology of Hia is distinct from that in the rest of the United States, and multiple invasive Hia disease outbreaks have occurred [10, 17, 18]. Haemophilus influenzae serotype a can cause disease of similar clinical presentation and severity as Hib [10], and Hib vaccines offer no cross-protection against Hia. We analyzed data from active, population- and laboratory-based surveillance during 2008–2017 to describe the current epidemiology of invasive Hia disease in the United States overall and in Alaska specifically.
METHODS
Active Bacterial Core Surveillance and Laboratory Methods
Active, population- and laboratory-based surveillance for invasive H. influenzae disease was conducted as part of Active Bacterial Core surveillance (ABCs) [9, 21]. ABCs is supported by the Centers for Disease Control and Prevention (CDC) as part of the Emerging Infections Program Network [20]. The ABCs surveillance areas included California (3 San Francisco Bay area counties, 2008–2017), Colorado (5 Denver area counties, 2008–2017), Connecticut (statewide, 2008–2017), Georgia (20 Atlanta area counties, 2008–2009; statewide, 2010–2017), Maryland (statewide, 2008–2017), Minnesota (statewide, 2008–2017), New Mexico (statewide, 2008–2017), New York (15 Rochester and Albany area counties, 2008–2017), Oregon (statewide, 2008–2017), and Tennessee (11 counties, 2008–2009; 20 counties, 2010–2017). The population under surveillance represented 11.9% and 13.7% of the US population in 2008 and 2017, respectively [21].
A case of invasive Hia disease was defined as isolation of Hia from a normally sterile site (eg, blood or cerebrospinal fluid [CSF]) in an ABCs surveillance area resident. Epidemiologic and clinical information was abstracted from medical records. Outcome (alive/dead) was based on patient status at discharge. Infants with a gestational age of 22 weeks or less were excluded from ABCs.
State public health laboratories serotyped and sent H. influenzae isolates to CDC, where species and serotyping were confirmed for all isolates and whole-genome sequencing was performed on select isolates [9, 22–24].
Alaska Surveillance and Laboratory Methods
Statewide laboratory-based surveillance for invasive H. influenzae disease in Alaska was conducted by CDC’s Arctic Investigations Program (AIP) in Anchorage, Alaska [10, 17]. Invasive H. influenzae cases are reportable by clinicians and laboratories to the Alaska Section of Epidemiology (AKSOE). The AIP and ASKOE participate in reciprocal notification of cases. Case information is routinely abstracted from medical records using a standardized form. Clinical laboratories send H. influenzae isolates recovered from a normally sterile site in Alaska residents to AIP for laboratory confirmation and characterization. Prior to 2017, isolates received by AIP were confirmed by culture (first by growth on chocolate agar, and then by X,V-factor requirement testing and aminolevulinic acid [ALA]-porphyrin testing); serotype was confirmed by slide agglutination. Since 2017, species confirmation and serotyping has been performed by real-time polymerase chain reaction [23, 24].
Statistical Analysis
Data from 1 January 2008 through 31 December 2017 were included in this analysis. Cases of invasive Hia disease were categorized as meningitis if a clinical diagnosis of meningitis was recorded in the medical record and Hia was isolated from CSF or other sterile sites, as bacteremic pneumonia if pneumonia was recorded in the patient’s medical record and Hia was isolated from a blood or pleural fluid, and as isolated bacteremia if Hia was isolated from blood and the patient did not have another clinical syndrome. All other clinical syndromes were classified based upon source of isolate or information noted in the medical record. Clinical syndromes were not considered mutually exclusive in this analysis. Race was categorized as White, Black, American Indian and Alaska Native (AI/AN), or Asian/Pacific Islander; however, within the AI/AN category, the distribution of persons who identify as AI versus AN likely varied between the ABCs and Alaska data. Case-fatality ratios were calculated using the proportion of cases with known outcomes as the denominator. Wilcoxon rank-sum tests were used to compare continuous variables, and Pearson’s χ 2 test was used for categorical variables.
For the ABCs sites, incidence rates were reported as cases per 100 000 population and calculated using National Center for Health Statistics’ bridged-race postcensal population estimates [21]; nationwide estimates were calculated by directly standardizing to the age and race distribution of the US population. For race-stratified nationwide incidence estimates, missing race was multiply imputed using sequential regression multiple imputation [25] via IVEware software (Institute for Social Research, University of Michigan, Ann Arbor, MI). Variance estimates were calculated using standard combining rules for multiply imputed data. The 95% confidence intervals (CIs) around the directly standardized rates were calculated using a method derived from the relationship between the Poisson distribution and the gamma distribution, while estimated age-, race-, and serotype-specific 95% CIs were calculated using exact CI for a Poisson random variable [26]. Theoretically, the projected nationwide estimates include Alaska; however, the epidemiology of Hia in Alaska is distinct from that in the rest of the United States and is therefore presented separately. Incidence in Alaska was calculated using State of Alaska population estimates [27]. Incidence trends over time were assessed using Cochrane-Armitage tests for trend. A negative binomial model with 95% CIs was used to estimate annual percentage changes in incidence from 2008 to 2017. To identify potential secondary cases of Hia, all instances where 2 Hia cases were reported to ABCs from the same county within less than 60 days were reviewed for epidemiologic links.
This project was reviewed in accordance with CDC human research protection procedures and was determined to be nonresearch public health surveillance. At each ABCs site, it was deemed either a public health assessment or human subjects research, for which approval was granted by local institutional review boards.
RESULTS
US Haemophilus influenzae Serotype a Epidemiology
During 2008–2017, 386 cases of invasive Hia disease were reported from ABCs sites; 30 (7.8%) were fatal (Table 1). The median annual incidence among ABCs sites was 0.10 cases per 100 000, with site-specific incidences ranging from 0.01 (Connecticut) to 0.47 (New Mexico). Nationwide, the estimated annual number of cases ranged from 160 in 2008 to 459 in 2017 (estimated annual average: 306), with an estimated average annual national incidence of 0.10 cases per 100 000 (Table 1). Haemophilus influenzae serotype a incidence increased by an average of 11.1% annually in the United States, from 0.05 in 2008 to 0.14 in 2017 (Figure 1).
Average Annual Incidence, Average Annual Percentage Change in Incidence, and Case-fatality Ratios for Invasive Haemophilus influenzae Serotype a Disease, by Age Group—United States and Alaska, 2008–2017
| Age (Years) . | United States . | Alaska . | ||||
|---|---|---|---|---|---|---|
| . | Estimated Incidence (95% CI) . | Average Annual Percentage Change in Incidence (95% CI) . | CFR, % . | Incidence (95% CI) . | Average Annual Percentage Change in Incidence (95% CI) . | CFR, % . |
| <1 | 1.60 (1.46–1.75) | 14.6 (11.4–17.8) | 6.2 | 24.73 (16.30–36.00) | 1.2 (−11.2 to 15.3) | 7.4 |
| 1–4 | .41 (.36–.45) | 5.6 (2.8–8.5) | 1.1 | 4.14 (2.45–6.54) | 17.7 (−.8 to 39.5) | 11.1 |
| 5–17 | .03 (.03–.03) | 7.8 (2.0–14.0) | 5.0 | .07 (.00–.41) | −1.0 (−29.8 to 39.5)a | 0.0 |
| 18–34 | .02 (.01–.02) | 16.4 (8.3–25.1) | 14.3 | .00 (.00–.00) | 0.0 | |
| 35–49 | .05 (.04–.05) | 4.0 (−.1 to 8.3) | 11.1 | .07 (.00–.41) | 0.0 | |
| 50–64 | .10 (.10–.11) | 23.4 (19.5–27.3) | 7.9 | .07 (.00–.41) | 0.0 | |
| ≥65 | .14 (.14–.15) | 7.9 (5.0–10.9) | 15.1 | .15 (.00–.85) | 100.0 | |
| Total | .10 (.10–.10) | 11.1 (9.7–12.6) | 7.8 | .68 (.50–.89) | 4.9 (−5.0 to 15.8) | 10.2 |
| Age (Years) . | United States . | Alaska . | ||||
|---|---|---|---|---|---|---|
| . | Estimated Incidence (95% CI) . | Average Annual Percentage Change in Incidence (95% CI) . | CFR, % . | Incidence (95% CI) . | Average Annual Percentage Change in Incidence (95% CI) . | CFR, % . |
| <1 | 1.60 (1.46–1.75) | 14.6 (11.4–17.8) | 6.2 | 24.73 (16.30–36.00) | 1.2 (−11.2 to 15.3) | 7.4 |
| 1–4 | .41 (.36–.45) | 5.6 (2.8–8.5) | 1.1 | 4.14 (2.45–6.54) | 17.7 (−.8 to 39.5) | 11.1 |
| 5–17 | .03 (.03–.03) | 7.8 (2.0–14.0) | 5.0 | .07 (.00–.41) | −1.0 (−29.8 to 39.5)a | 0.0 |
| 18–34 | .02 (.01–.02) | 16.4 (8.3–25.1) | 14.3 | .00 (.00–.00) | 0.0 | |
| 35–49 | .05 (.04–.05) | 4.0 (−.1 to 8.3) | 11.1 | .07 (.00–.41) | 0.0 | |
| 50–64 | .10 (.10–.11) | 23.4 (19.5–27.3) | 7.9 | .07 (.00–.41) | 0.0 | |
| ≥65 | .14 (.14–.15) | 7.9 (5.0–10.9) | 15.1 | .15 (.00–.85) | 100.0 | |
| Total | .10 (.10–.10) | 11.1 (9.7–12.6) | 7.8 | .68 (.50–.89) | 4.9 (−5.0 to 15.8) | 10.2 |
Note: Incidence is presented in cases per 100 000 persons per year.
Abbreviations: CFR, case-fatality ratio; CI, confidence interval.
aDue to small numbers, age groups were combined. This average annual percentage change in incidence is for persons aged ≥5 years in Alaska.
Average Annual Incidence, Average Annual Percentage Change in Incidence, and Case-fatality Ratios for Invasive Haemophilus influenzae Serotype a Disease, by Age Group—United States and Alaska, 2008–2017
| Age (Years) . | United States . | Alaska . | ||||
|---|---|---|---|---|---|---|
| . | Estimated Incidence (95% CI) . | Average Annual Percentage Change in Incidence (95% CI) . | CFR, % . | Incidence (95% CI) . | Average Annual Percentage Change in Incidence (95% CI) . | CFR, % . |
| <1 | 1.60 (1.46–1.75) | 14.6 (11.4–17.8) | 6.2 | 24.73 (16.30–36.00) | 1.2 (−11.2 to 15.3) | 7.4 |
| 1–4 | .41 (.36–.45) | 5.6 (2.8–8.5) | 1.1 | 4.14 (2.45–6.54) | 17.7 (−.8 to 39.5) | 11.1 |
| 5–17 | .03 (.03–.03) | 7.8 (2.0–14.0) | 5.0 | .07 (.00–.41) | −1.0 (−29.8 to 39.5)a | 0.0 |
| 18–34 | .02 (.01–.02) | 16.4 (8.3–25.1) | 14.3 | .00 (.00–.00) | 0.0 | |
| 35–49 | .05 (.04–.05) | 4.0 (−.1 to 8.3) | 11.1 | .07 (.00–.41) | 0.0 | |
| 50–64 | .10 (.10–.11) | 23.4 (19.5–27.3) | 7.9 | .07 (.00–.41) | 0.0 | |
| ≥65 | .14 (.14–.15) | 7.9 (5.0–10.9) | 15.1 | .15 (.00–.85) | 100.0 | |
| Total | .10 (.10–.10) | 11.1 (9.7–12.6) | 7.8 | .68 (.50–.89) | 4.9 (−5.0 to 15.8) | 10.2 |
| Age (Years) . | United States . | Alaska . | ||||
|---|---|---|---|---|---|---|
| . | Estimated Incidence (95% CI) . | Average Annual Percentage Change in Incidence (95% CI) . | CFR, % . | Incidence (95% CI) . | Average Annual Percentage Change in Incidence (95% CI) . | CFR, % . |
| <1 | 1.60 (1.46–1.75) | 14.6 (11.4–17.8) | 6.2 | 24.73 (16.30–36.00) | 1.2 (−11.2 to 15.3) | 7.4 |
| 1–4 | .41 (.36–.45) | 5.6 (2.8–8.5) | 1.1 | 4.14 (2.45–6.54) | 17.7 (−.8 to 39.5) | 11.1 |
| 5–17 | .03 (.03–.03) | 7.8 (2.0–14.0) | 5.0 | .07 (.00–.41) | −1.0 (−29.8 to 39.5)a | 0.0 |
| 18–34 | .02 (.01–.02) | 16.4 (8.3–25.1) | 14.3 | .00 (.00–.00) | 0.0 | |
| 35–49 | .05 (.04–.05) | 4.0 (−.1 to 8.3) | 11.1 | .07 (.00–.41) | 0.0 | |
| 50–64 | .10 (.10–.11) | 23.4 (19.5–27.3) | 7.9 | .07 (.00–.41) | 0.0 | |
| ≥65 | .14 (.14–.15) | 7.9 (5.0–10.9) | 15.1 | .15 (.00–.85) | 100.0 | |
| Total | .10 (.10–.10) | 11.1 (9.7–12.6) | 7.8 | .68 (.50–.89) | 4.9 (−5.0 to 15.8) | 10.2 |
Note: Incidence is presented in cases per 100 000 persons per year.
Abbreviations: CFR, case-fatality ratio; CI, confidence interval.
aDue to small numbers, age groups were combined. This average annual percentage change in incidence is for persons aged ≥5 years in Alaska.
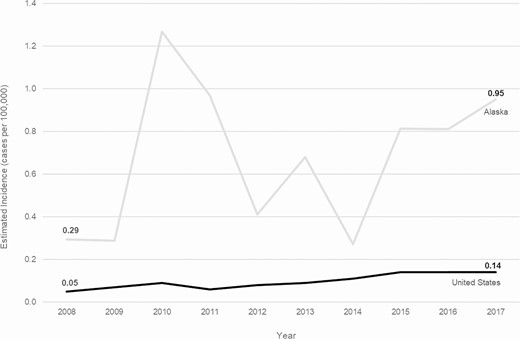
Trends in incidence of invasive Haemophilus influenzae serotype a disease in the United States and Alaska, 2008–2017.
Estimated national invasive Hia disease incidence was highest among children aged younger than 1 year (1.60) and lowest among adults aged 18–34 years (0.02) (Table 1). Case-fatality ratio was highest among adults aged 65 years or older (15.1%), with 80.0% of deaths occurring in adults. Overall, exactly half of patients were male; 59.3% were White, 14.8% Black, 14.8% AI/AN, 1.3% Asian/Pacific Islander, and 9.8% of unknown race (Table 2). Incidence was highest in AI/AN populations (1.01), although steady incidence increases were also seen in White and Black populations (Supplementary Figure 1). The median patient age was 30 years (range: 0–92 years). Information on clinical syndrome was available for 99.7% of cases: 41.3% had bacteremic pneumonia, 32.0% had bacteremia, 23.9% had meningitis, 7.8% had septic arthritis, 4.4% had cellulitis, and 3.9% had epiglottitis (syndromes were not mutually exclusive). The median age of patients with bacteremic pneumonia was 56 years (interquartile range [IQR]: 36–66 years), whereas the median age was 30 years (IQR: 1–62 years) among patients with bacteremia and 0 years (IQR: 0–1 years) among those with meningitis (P < .0001); all of the patients with epiglottitis were aged older than 50 years.
Epidemiologic and Clinical Characteristics of Patients With Invasive Haemophilus influenzae Serotype a Disease—United States and Alaska, 2008–2017
| Characteristics . | United Statesa (N = 386) . | Alaska(N = 49) . |
|---|---|---|
| Age in years, median (range) | 30 (0–92) | 1 (0–77) |
| Sex, % | ||
| Male | 50.0 | 61.2 |
| Race, % | ||
| White | 59.3 | 4.1 |
| Black | 14.8 | 0.0 |
| AI/AN | 14.8 | 93.9 |
| Asian/PI | 1.3 | 2.0 |
| Unknown | 9.8 | 0.0 |
| Ethnicity, % | ||
| Hispanic/Latino | 15.0 | N/A |
| Non-Hispanic/Latino | 68.1 | N/A |
| Unknown | 16.8 | N/A |
| Clinical syndrome,b % | ||
| Bacteremic pneumonia | 41.3 | 18.4 |
| Bacteremia | 32.0 | 12.2 |
| Meningitis | 23.9 | 38.8 |
| Septic arthritis | 7.8 | 16.3 |
| Cellulitis | 4.4 | 12.2 |
| Epiglottitis | 3.9 | 0.0 |
| Other invasive disease | 0.5 | 2.1 |
| Hospitalized, % | 94.8 | 87.8 |
| Duration of hospitalization in days, median (range) | 7 (0–126) | 8 (0–68) |
| Died,c % | 7.8 | 10.2 |
| Characteristics . | United Statesa (N = 386) . | Alaska(N = 49) . |
|---|---|---|
| Age in years, median (range) | 30 (0–92) | 1 (0–77) |
| Sex, % | ||
| Male | 50.0 | 61.2 |
| Race, % | ||
| White | 59.3 | 4.1 |
| Black | 14.8 | 0.0 |
| AI/AN | 14.8 | 93.9 |
| Asian/PI | 1.3 | 2.0 |
| Unknown | 9.8 | 0.0 |
| Ethnicity, % | ||
| Hispanic/Latino | 15.0 | N/A |
| Non-Hispanic/Latino | 68.1 | N/A |
| Unknown | 16.8 | N/A |
| Clinical syndrome,b % | ||
| Bacteremic pneumonia | 41.3 | 18.4 |
| Bacteremia | 32.0 | 12.2 |
| Meningitis | 23.9 | 38.8 |
| Septic arthritis | 7.8 | 16.3 |
| Cellulitis | 4.4 | 12.2 |
| Epiglottitis | 3.9 | 0.0 |
| Other invasive disease | 0.5 | 2.1 |
| Hospitalized, % | 94.8 | 87.8 |
| Duration of hospitalization in days, median (range) | 7 (0–126) | 8 (0–68) |
| Died,c % | 7.8 | 10.2 |
Abbreviations: AI/AN, American Indian and Alaska Native; N/A, not available; PI, Pacific Islander.
aNote: data shown in this column are from Active Bacterial Core surveillance sites and do not include all cases in the United States.
bInformation on clinical syndrome was available for 385/386 (99.7%) cases in the United States and 49/49 (100%) cases in Alaska. Clinical syndrome was not mutually exclusive.
cInformation on outcome was available for 384/386 (99.5%) cases in the United States and 49/49 (100%) cases in Alaska.
Epidemiologic and Clinical Characteristics of Patients With Invasive Haemophilus influenzae Serotype a Disease—United States and Alaska, 2008–2017
| Characteristics . | United Statesa (N = 386) . | Alaska(N = 49) . |
|---|---|---|
| Age in years, median (range) | 30 (0–92) | 1 (0–77) |
| Sex, % | ||
| Male | 50.0 | 61.2 |
| Race, % | ||
| White | 59.3 | 4.1 |
| Black | 14.8 | 0.0 |
| AI/AN | 14.8 | 93.9 |
| Asian/PI | 1.3 | 2.0 |
| Unknown | 9.8 | 0.0 |
| Ethnicity, % | ||
| Hispanic/Latino | 15.0 | N/A |
| Non-Hispanic/Latino | 68.1 | N/A |
| Unknown | 16.8 | N/A |
| Clinical syndrome,b % | ||
| Bacteremic pneumonia | 41.3 | 18.4 |
| Bacteremia | 32.0 | 12.2 |
| Meningitis | 23.9 | 38.8 |
| Septic arthritis | 7.8 | 16.3 |
| Cellulitis | 4.4 | 12.2 |
| Epiglottitis | 3.9 | 0.0 |
| Other invasive disease | 0.5 | 2.1 |
| Hospitalized, % | 94.8 | 87.8 |
| Duration of hospitalization in days, median (range) | 7 (0–126) | 8 (0–68) |
| Died,c % | 7.8 | 10.2 |
| Characteristics . | United Statesa (N = 386) . | Alaska(N = 49) . |
|---|---|---|
| Age in years, median (range) | 30 (0–92) | 1 (0–77) |
| Sex, % | ||
| Male | 50.0 | 61.2 |
| Race, % | ||
| White | 59.3 | 4.1 |
| Black | 14.8 | 0.0 |
| AI/AN | 14.8 | 93.9 |
| Asian/PI | 1.3 | 2.0 |
| Unknown | 9.8 | 0.0 |
| Ethnicity, % | ||
| Hispanic/Latino | 15.0 | N/A |
| Non-Hispanic/Latino | 68.1 | N/A |
| Unknown | 16.8 | N/A |
| Clinical syndrome,b % | ||
| Bacteremic pneumonia | 41.3 | 18.4 |
| Bacteremia | 32.0 | 12.2 |
| Meningitis | 23.9 | 38.8 |
| Septic arthritis | 7.8 | 16.3 |
| Cellulitis | 4.4 | 12.2 |
| Epiglottitis | 3.9 | 0.0 |
| Other invasive disease | 0.5 | 2.1 |
| Hospitalized, % | 94.8 | 87.8 |
| Duration of hospitalization in days, median (range) | 7 (0–126) | 8 (0–68) |
| Died,c % | 7.8 | 10.2 |
Abbreviations: AI/AN, American Indian and Alaska Native; N/A, not available; PI, Pacific Islander.
aNote: data shown in this column are from Active Bacterial Core surveillance sites and do not include all cases in the United States.
bInformation on clinical syndrome was available for 385/386 (99.7%) cases in the United States and 49/49 (100%) cases in Alaska. Clinical syndrome was not mutually exclusive.
cInformation on outcome was available for 384/386 (99.5%) cases in the United States and 49/49 (100%) cases in Alaska.
The majority (94.8%) of patients were hospitalized; the median duration of hospitalization was 7 days (range: 0–126 days). The duration of hospitalization also varied by syndrome, with a median of 6 days (IQR: 3–10 days) for bacteremic pneumonia, 5 days (IQR: 3–8 days) for bacteremia, and 14 days (IQR: 9–18 days) for meningitis (P < .0001). Case-fatality ratio did not significantly vary by syndrome.
During 2008–2017, 165 (42.7%) cases of invasive Hia disease in children younger than 5 years were reported from ABCs sites, corresponding to an estimated national incidence of 0.64 cases per 100 000: 1.60 among infants younger than 1 year, 0.86 among children aged 1 year, 0.25 among children aged 2–4 years (Table 3). Of the 81 (21.0%) infants younger than 1 year with invasive Hia disease, 7 (8.6%) were younger than 2 months, 5 (6.2%) were aged 2 to less than 4 months, 22 (27.2%) were aged 4 to less than 6 months, and 47 (58.0%) were aged 6 to less than 12 months. Among invasive Hia disease cases in children younger than 5 years reported to ABCs, 3.6% were fatal (of which 83.3% were in infants aged 3–11 months and 16.7% were in children aged 1–4 years); clinical syndromes included meningitis (47.9%), bacteremia (32.7%), septic arthritis (13.9%), bacteremic pneumonia (12.1%), and cellulitis (3.0%).
Average Annual Incidence (95% Confidence Interval) of Haemophilus influenzae Serotype a Disease, by Age and Race, Among Children Aged <5 years—United States and Alaska, 2008–2017
| . | United States . | Alaska . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) . | White . | Black . | AI/AN . | Asian/PI . | Total . | White . | Black . | AI/AN . | Asian/PI . | Total . |
| <1 | 1.45 (1.28–1.61) | 1.22 (1.01–1.45) | 17.78 (11.95–23.56) | .00 (.00–.00) | 1.60 (1.46–1.75) | .00 (.00–.00) | .00 (.00–.00) | 82.39 (53.82–120.72) | 8.64 (.22–48.15) | 24.73 (16.3–35.99) |
| 1 | .52 (.39–.64) | 1.05 (.76–1.33) | 16.07 (9.18–22.89) | .00 (.00–.00) | .86 (.72–1.00) | .00 (.00–.00) | .00 (.00–.00) | 41.96 (22.34–71.75) | .00 (.00–.00) | 12.02 (6.40–20.55) |
| 2–4 | .18 (.15–.21) | .29 (.21–.37) | 3.20 (1.68–4.71) | .10 (.03–.16) | .25 (.22–.29) | .44 (.01–2.46) | .00 (.00–.00) | 4.31 (1.18–11.05) | .00 (.00–.00) | 1.53 (.50–3.57) |
| <5 | .50 (.45–.54) | .64 (.55–.71) | 8.29 (6.76–10.73) | .06 (.04–.08) | .64 (.60–.69) | .27 (.01–1.49) | .00 (.00–.00) | 27.74 (20.07–37.40) | 1.70 (.04–9.45) | 8.27 (6.03–11.07) |
| . | United States . | Alaska . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) . | White . | Black . | AI/AN . | Asian/PI . | Total . | White . | Black . | AI/AN . | Asian/PI . | Total . |
| <1 | 1.45 (1.28–1.61) | 1.22 (1.01–1.45) | 17.78 (11.95–23.56) | .00 (.00–.00) | 1.60 (1.46–1.75) | .00 (.00–.00) | .00 (.00–.00) | 82.39 (53.82–120.72) | 8.64 (.22–48.15) | 24.73 (16.3–35.99) |
| 1 | .52 (.39–.64) | 1.05 (.76–1.33) | 16.07 (9.18–22.89) | .00 (.00–.00) | .86 (.72–1.00) | .00 (.00–.00) | .00 (.00–.00) | 41.96 (22.34–71.75) | .00 (.00–.00) | 12.02 (6.40–20.55) |
| 2–4 | .18 (.15–.21) | .29 (.21–.37) | 3.20 (1.68–4.71) | .10 (.03–.16) | .25 (.22–.29) | .44 (.01–2.46) | .00 (.00–.00) | 4.31 (1.18–11.05) | .00 (.00–.00) | 1.53 (.50–3.57) |
| <5 | .50 (.45–.54) | .64 (.55–.71) | 8.29 (6.76–10.73) | .06 (.04–.08) | .64 (.60–.69) | .27 (.01–1.49) | .00 (.00–.00) | 27.74 (20.07–37.40) | 1.70 (.04–9.45) | 8.27 (6.03–11.07) |
Note: Incidence is presented in cases per 100 000 persons per year. Incidence in the United States is an estimated national incidence.
Abbreviations: AI/AN, American Indian and Alaska Native; PI, Pacific Islander.
Average Annual Incidence (95% Confidence Interval) of Haemophilus influenzae Serotype a Disease, by Age and Race, Among Children Aged <5 years—United States and Alaska, 2008–2017
| . | United States . | Alaska . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) . | White . | Black . | AI/AN . | Asian/PI . | Total . | White . | Black . | AI/AN . | Asian/PI . | Total . |
| <1 | 1.45 (1.28–1.61) | 1.22 (1.01–1.45) | 17.78 (11.95–23.56) | .00 (.00–.00) | 1.60 (1.46–1.75) | .00 (.00–.00) | .00 (.00–.00) | 82.39 (53.82–120.72) | 8.64 (.22–48.15) | 24.73 (16.3–35.99) |
| 1 | .52 (.39–.64) | 1.05 (.76–1.33) | 16.07 (9.18–22.89) | .00 (.00–.00) | .86 (.72–1.00) | .00 (.00–.00) | .00 (.00–.00) | 41.96 (22.34–71.75) | .00 (.00–.00) | 12.02 (6.40–20.55) |
| 2–4 | .18 (.15–.21) | .29 (.21–.37) | 3.20 (1.68–4.71) | .10 (.03–.16) | .25 (.22–.29) | .44 (.01–2.46) | .00 (.00–.00) | 4.31 (1.18–11.05) | .00 (.00–.00) | 1.53 (.50–3.57) |
| <5 | .50 (.45–.54) | .64 (.55–.71) | 8.29 (6.76–10.73) | .06 (.04–.08) | .64 (.60–.69) | .27 (.01–1.49) | .00 (.00–.00) | 27.74 (20.07–37.40) | 1.70 (.04–9.45) | 8.27 (6.03–11.07) |
| . | United States . | Alaska . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) . | White . | Black . | AI/AN . | Asian/PI . | Total . | White . | Black . | AI/AN . | Asian/PI . | Total . |
| <1 | 1.45 (1.28–1.61) | 1.22 (1.01–1.45) | 17.78 (11.95–23.56) | .00 (.00–.00) | 1.60 (1.46–1.75) | .00 (.00–.00) | .00 (.00–.00) | 82.39 (53.82–120.72) | 8.64 (.22–48.15) | 24.73 (16.3–35.99) |
| 1 | .52 (.39–.64) | 1.05 (.76–1.33) | 16.07 (9.18–22.89) | .00 (.00–.00) | .86 (.72–1.00) | .00 (.00–.00) | .00 (.00–.00) | 41.96 (22.34–71.75) | .00 (.00–.00) | 12.02 (6.40–20.55) |
| 2–4 | .18 (.15–.21) | .29 (.21–.37) | 3.20 (1.68–4.71) | .10 (.03–.16) | .25 (.22–.29) | .44 (.01–2.46) | .00 (.00–.00) | 4.31 (1.18–11.05) | .00 (.00–.00) | 1.53 (.50–3.57) |
| <5 | .50 (.45–.54) | .64 (.55–.71) | 8.29 (6.76–10.73) | .06 (.04–.08) | .64 (.60–.69) | .27 (.01–1.49) | .00 (.00–.00) | 27.74 (20.07–37.40) | 1.70 (.04–9.45) | 8.27 (6.03–11.07) |
Note: Incidence is presented in cases per 100 000 persons per year. Incidence in the United States is an estimated national incidence.
Abbreviations: AI/AN, American Indian and Alaska Native; PI, Pacific Islander.
Alaska Haemophilus influenzae Serotype a Epidemiology
During 2008–2017, 49 cases of invasive Hia disease were reported in Alaska; 5 (10.2%) were fatal, with 4 of 5 fatalities occurring among children younger than 5 years (Tables 1 and 2). In comparison to the United States overall, the incidence of invasive Hia disease in Alaska was nearly 6 times higher among all ages (0.68 cases per 100 000) and 14 times higher among children younger than 1 year (24.73) (Figures 1 and 2, Table 1). Incidence increased by an average of 4.9% annually. The vast majority (93.9%) of invasive Hia disease cases in Alaska occurred in AI/AN people. The median patient age in Alaska was much younger than that in the United States overall (1 vs 30 years, respectively). The most common clinical syndrome observed in Alaska was meningitis (38.8%), followed by bacteremic pneumonia (18.4%) and septic arthritis (16.3%). Similar to the United States overall, most (87.8%) patients were hospitalized; the median duration of hospitalization was 8 days (range: 0–68 days).
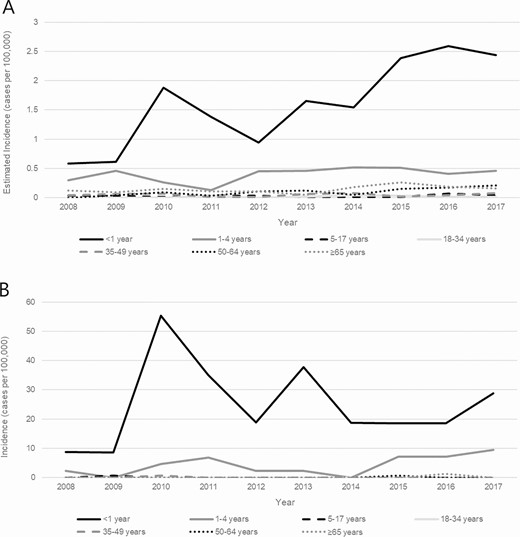
Trends in incidence of invasive Haemophilus influenzae serotype a disease, by age group—United States (A) and Alaska (B), 2008–2017.
In Alaska, 45 cases of invasive Hia disease in children younger than 5 years were reported during 2008–2017, with an incidence of 8.27: 24.73 among infants younger than 1 year, 12.02 among children aged 1 year, and 1.53 among children aged 2–4 years (Table 3). Of the 4 fatal cases in children younger than 5 years, 2 were in infants and 2 were in children aged 1–4 years. The age distribution of the 27 Alaskan infants younger than 1 year with invasive Hia disease was slightly older than that in the United States overall: 1 (3.7%) was younger than 2 months, 3 (11.1%) were aged 2 to less than 4 months, 4 (14.8%) were aged 4 to less than 6 months, and 19 (70.4%) were aged 6 to less than 12 months (Figure 3). All but 1 of the cases in infants younger than 1 year were in the AI/AN population.
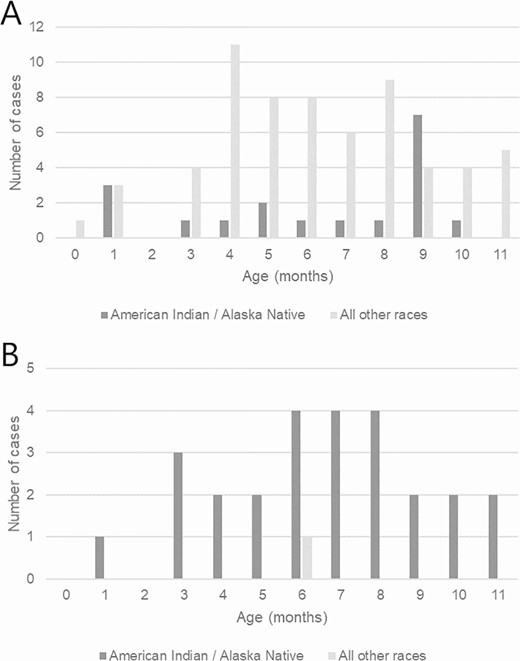
Infants younger than 1 year with invasive Haemophilus influenzae serotype a disease, by age in months and race—Active Bacterial Core surveillance sites (A) and Alaska (B), 2008–2017.
Haemophilus influenzae Serotype a Epidemiology in American Indian and Alaska Native Children
In the United States overall, the burden of invasive Hia disease among AI/AN children is much greater than that in the general US population, with an estimated incidence of 8.29 per 100 000 among AI/AN children younger than 5 years (16.9 times the incidence among all other races combined) and 17.78 among AI/AN infants younger than 1 year (13.9 times the incidence among all other races combined) (Table 4). These incidence rate ratios are even more pronounced in Alaska, with an incidence of 27.74 among AI/AN children younger than 5 years (54.4 times the incidence among all other races combined) and 82.39 among AI/AN infants younger than 1 year (63.9 times the incidence among all other races combined). Stratifying by both age (<5 vs ≥5 years) and race (AI/AN vs all other races), the incidence of invasive Hia disease was markedly higher among AI/AN children younger than 5 years than among all other age-race subgroups, both in the United States overall and in Alaska (Figure 4).
Average Annual Incidence and Incidence Rate Ratios for Haemophilus influenzae Serotype a Disease Among American Indian and Alaska Native Persons Compared With Persons of All Other Races—United States and Alaska, 2008–2017
| . | United States . | Alaska . | ||||
|---|---|---|---|---|---|---|
| . | Estimated Cases . | Estimated Incidence . | Incidence Rate Ratio . | Cases . | Incidence . | Incidence Rate Ratio . |
| All ages | ||||||
| AI/AN | 433 | 1.01 | 11.2 | 46 | 3.22 | 4.7 |
| All other races | 2700 | 0.09 | Ref | 3 | 0.68 | Ref |
| Children aged <5 years | ||||||
| AI/AN | 313 | 8.29 | 16.9 | 43 | 27.74 | 54.4 |
| All other races | 968 | 0.49 | Ref | 2 | 0.51 | Ref |
| Infants aged <1 year | ||||||
| AI/AN | 136 | 17.78 | 13.9 | 26 | 82.39 | 63.9 |
| All other races | 504 | 1.28 | Ref | 1 | 1.29 | Ref |
| . | United States . | Alaska . | ||||
|---|---|---|---|---|---|---|
| . | Estimated Cases . | Estimated Incidence . | Incidence Rate Ratio . | Cases . | Incidence . | Incidence Rate Ratio . |
| All ages | ||||||
| AI/AN | 433 | 1.01 | 11.2 | 46 | 3.22 | 4.7 |
| All other races | 2700 | 0.09 | Ref | 3 | 0.68 | Ref |
| Children aged <5 years | ||||||
| AI/AN | 313 | 8.29 | 16.9 | 43 | 27.74 | 54.4 |
| All other races | 968 | 0.49 | Ref | 2 | 0.51 | Ref |
| Infants aged <1 year | ||||||
| AI/AN | 136 | 17.78 | 13.9 | 26 | 82.39 | 63.9 |
| All other races | 504 | 1.28 | Ref | 1 | 1.29 | Ref |
Note: Incidence is presented in cases per 100 000 population.
Abbreviation: AI/AN, American Indian and Alaska Native.
Average Annual Incidence and Incidence Rate Ratios for Haemophilus influenzae Serotype a Disease Among American Indian and Alaska Native Persons Compared With Persons of All Other Races—United States and Alaska, 2008–2017
| . | United States . | Alaska . | ||||
|---|---|---|---|---|---|---|
| . | Estimated Cases . | Estimated Incidence . | Incidence Rate Ratio . | Cases . | Incidence . | Incidence Rate Ratio . |
| All ages | ||||||
| AI/AN | 433 | 1.01 | 11.2 | 46 | 3.22 | 4.7 |
| All other races | 2700 | 0.09 | Ref | 3 | 0.68 | Ref |
| Children aged <5 years | ||||||
| AI/AN | 313 | 8.29 | 16.9 | 43 | 27.74 | 54.4 |
| All other races | 968 | 0.49 | Ref | 2 | 0.51 | Ref |
| Infants aged <1 year | ||||||
| AI/AN | 136 | 17.78 | 13.9 | 26 | 82.39 | 63.9 |
| All other races | 504 | 1.28 | Ref | 1 | 1.29 | Ref |
| . | United States . | Alaska . | ||||
|---|---|---|---|---|---|---|
| . | Estimated Cases . | Estimated Incidence . | Incidence Rate Ratio . | Cases . | Incidence . | Incidence Rate Ratio . |
| All ages | ||||||
| AI/AN | 433 | 1.01 | 11.2 | 46 | 3.22 | 4.7 |
| All other races | 2700 | 0.09 | Ref | 3 | 0.68 | Ref |
| Children aged <5 years | ||||||
| AI/AN | 313 | 8.29 | 16.9 | 43 | 27.74 | 54.4 |
| All other races | 968 | 0.49 | Ref | 2 | 0.51 | Ref |
| Infants aged <1 year | ||||||
| AI/AN | 136 | 17.78 | 13.9 | 26 | 82.39 | 63.9 |
| All other races | 504 | 1.28 | Ref | 1 | 1.29 | Ref |
Note: Incidence is presented in cases per 100 000 population.
Abbreviation: AI/AN, American Indian and Alaska Native.
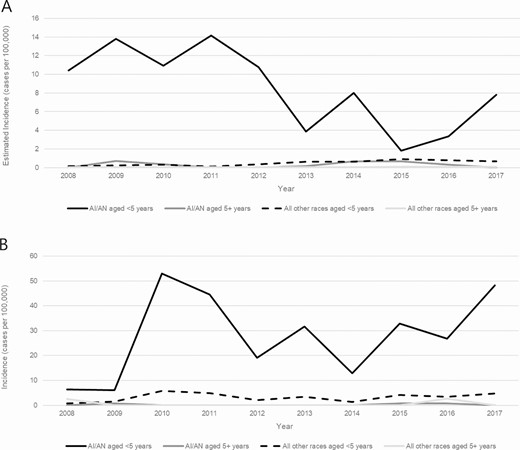
Trends in incidence of invasive Haemophilus influenzae serotype a disease, by age group and race—United States (A) and Alaska (B), 2008–2017. Abbreviation: AI/AN, American Indian and Alaska Native.
Potential Secondary Case of Haemophilus influenzae Serotype a
Only 1 potential secondary case was identified in ABCs or Alaska data. In Georgia, a 15-year-old boy and his 56-year-old mother, neither of whom had underlying conditions, became ill several days apart and were diagnosed with Hia bacteremic pneumonia. Isolates from both patients were found to be sequence type 56, with only 1 single nucleotide polymorphism difference between them.
Discussion
Although the incidence of invasive Hia disease was 5-fold higher in Alaska than in the United States overall, in both settings the incidence increased from 2008 to 2017 and was highest among AI/AN children younger than 5 years. In total, an estimated 11% of reported invasive Hia disease cases occurred in AI/AN children younger than 5 years, who experienced a disease incidence 17 and 54 times that of children younger than 5 years of all other races in the United States and Alaska, respectively.
Other recent reports from the United States have highlighted Hia as a pathogen of increasing importance [11, 28]. In 2018, an Hia outbreak involving 4 children younger than 2 years occurred in a remote Alaskan village. In response, chemoprophylaxis was administered to village residents younger than 10 years and a community-wide carriage study was conducted. A similar Alaskan village outbreak was reported in 2003 [18]. Moreover, Hia hospitalization rates have increased in Texas and Utah in recent years, with Hia as the predominant cause of H. influenzae meningitis [11, 16].
Most reports of Hia have been from North America and Brazil [9, 29, 30]; surveillance elsewhere in the world has rarely detected invasive Hia disease [31–33]. However, reporting of H. influenzae in many locations is still limited to Hib versus non–serotype b, with no serotyping capacity for Hia. Therefore, the true geographic range and disease burden of Hia globally is unknown.
Numerous reports have shown that Hia can cause disease with clinical severity similar to Hib [10, 11], perhaps influenced by the close similarity between the Hia and Hib capsular polysaccharides [31, 34]. A chart review of a subset of ABCs Hia cases from 2011 to 2015 found evidence of severe disease, and that children younger than 1 year had the highest proportion of cases with both short- and long-term adverse clinical outcomes, including hearing loss, developmental delay, and speech delay (CDC, unpublished data). In the present analysis, the case-fatality ratio was higher in Alaska than in ABCs, possibly because a higher proportion of cases presented with meningitis and occurred among young children living in remote locations. Additionally, we showed a notable proportion of Hia cases presenting with septic arthritis (7.7% in ABCs, 16.3% in Alaska), mainly among children younger than 5 years. Haemophilus influenzae serotype a septic arthritis has been previously reported [11, 35, 36] but is not a clinical presentation historically associated with invasive H. influenzae disease.
To prevent secondary cases of invasive Hib disease, the Advisory Committee on Immunization Practices (ACIP) recommends antibiotic chemoprophylaxis for household and daycare contacts of cases with specific circumstances [37]. However, the ACIP recommendations also state that “chemoprophylaxis is not recommended for contacts of persons with invasive disease caused by nontype b H. influenzae because cases of secondary transmission of disease have not been documented” [37]. The mother–son pair of Hia cases reported here, as well as 2 genetically indistinguishable Hia cases reported within the same daycare center in Texas [11], represent an important documentation of potential secondary transmission of a non-b serotype and may indicate a need to revisit chemoprophylaxis recommendations. In fact, the 2018 Red Book updated language around chemoprophylaxis to state that “clinicians may consider prophylaxis of contacts of index cases of Hia invasive disease, using the same criteria as that recommended … for Hib disease” [38]. Following an Hia village outbreak in Alaska in 2018, Alaska issued a bulletin indicating that “chemoprophylaxis should be considered” for certain contacts of patients with invasive Hia [39]. To note, as serotyping is often performed at a state or reference public health laboratory, results are often not available in time to inform serotype-specific chemoprophylaxis interventions.
Given the recent increases in Hia incidence, clear burden of disease among AI/AN children, clinical disease severity similar to Hib, and lack of cross-protection of Hib vaccines against Hia, improved Hia disease prevention measures are needed. The National Research Council of Canada and the Public Health Agency of Canada recently developed a vaccine with the Hia capsular polysaccharide conjugated to a cross reacting material (CRM) protein [40, 41]. Proof of concept has been established, as all tested Hia strains were killed by conjugated vaccine–derived sera. There are ongoing efforts to move this vaccine candidate towards phase 1 clinical trials, with the hopes that the vaccine could become available to high-risk populations at a reasonable cost. Because 44% of AI/AN infants with invasive Hia disease were aged 2–6 months, any Hia vaccine would need careful consideration of which carrier protein would be needed to provide early protection (ie, after 1 dose). Although there are acknowledged challenges to license and introduce an Hia vaccine for a relatively small subpopulation, increasing incidence in non–AI/AN populations suggest that an Hia vaccine could potentially have broader appeal by the time it completes the licensure pathway.
The incidence of invasive Hia disease is increasing in the United States overall and Alaska in particular, with the highest burden among AI/AN infants and children. In the context of increasing Hia incidence and clinical severity similar to Hib, new prevention strategies, including development of an Hia vaccine, could prevent morbidity and mortality among these vulnerable populations. Additional questions remain regarding the frequency with which secondary Hia transmission occurs, the potential need for updated chemoprophylaxis recommendations, and appropriate target populations for a potential Hia vaccine.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to the following individuals for their contributions to the establishment and maintenance of the ABCs system—California Emerging Infections Program: Susan Brooks and Hallie Randel; Colorado Emerging Infections Program: Benjamin White, Deborah Aragon, Meghan Barnes, and Jennifer Sadlowski; Connecticut Department of Public Health: Matt Cartter, Carmen Marquez, and Michelle Wilson; Georgia Emerging Infections Program: Stephanie Thomas, Amy Tunali, Wendy Baughman, Ashley Moore, Lauren Lorentzson, and Melissa Tobin-D’Angelo; Maryland Emerging Infections Program: Joanne Benton, Terresa Carter, Rosemary Hollick, Kim Holmes, and Andrea Riner; Minnesota Emerging Infections Program: Kathryn Como-Sabetti, Lori Triden, Corinne Holtzman, Richard Danila, and Kerry MacInnes; New Mexico Emerging Infections Program: Kathy Angeles, Joseph Bareta, Lisa Butler, Sarah Khanlian, Robert Mansmann, and Megin Nichols; New York Emerging Infections Program: Kari Burlzaff, Suzanne McGuire, Glenda Smith, Nancy Spina, and Rachel Wester; Oregon Emerging Infections Program: Mark Schmidt, Jamie Thompson, and Tasha Poissant; Tennessee Emerging Infections Program: Brenda Barnes, Karen Leib, Katie Dyer, Tiffanie Markus, and Lura McKnight; Arctic Investigations Program, CDC: Debby Hurlburt, Danielle Lecy, Gail Thompson, Sara Seeman, Alisa Reasonover, and Carolynn Debyle; Division of Bacterial Diseases, CDC: Melissa Arvay, Olivia Almendares, Huong Pham, the Bacterial Meningitis Laboratory.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by a cooperative agreement with the Emerging Infections Program of the Centers for Disease Control and Prevention (grant number CDC-RFA-CK17-1701).
Potential conflicts of interest. L. H. H. has served as a consultant to GSK, Merck, Pfizer, and Sanofi Pasteur. W. S. has served as a consultant to Roche Diagnostics and as a member of data safety monitoring boards for Merck and Pfizer. R. L. served as a co-editor on a book on Infectious Disease Surveillance, Public Health and Preventive Medicine and American Academy of Pediatrics Committee on Infectious Disease, with all proceeds donated to the Minnesota Department of Health. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




