-
PDF
- Split View
-
Views
-
Cite
Cite
Okko Savonius, Emilie Rugemalira, Irmeli Roine, Manuel Leite Cruzeiro, Heikki Peltola, Tuula Pelkonen, Extended Continuous β-Lactam Infusion With Oral Acetaminophen in Childhood Bacterial Meningitis: A Randomized, Double-blind Clinical Trial, Clinical Infectious Diseases, Volume 72, Issue 10, 15 May 2021, Pages 1738–1744, https://doi.org/10.1093/cid/ciaa341
Close - Share Icon Share
Abstract
In our previous study in Luanda, Angola, initial continuous β-lactam infusion for 24 hours combined with oral acetaminophen for 48 hours showed promising results as a new treatment for childhood bacterial meningitis. We investigated whether extending this treatment regimen to 4 days would improve the outcomes further.
We conducted a randomized, double-blind, parallel-group study at the same hospital in Luanda. Children aged 2 months to 15 years presenting to hospital with symptoms and signs of bacterial meningitis were randomized to receive, for the first 4 days, a continuous infusion of cefotaxime (250 mg/kg/day) with simultaneous oral acetaminophen (first dose 30 mg/kg, then 20 mg/kg every 6 hours), or cefotaxime conventionally as boluses (62.5 mg/kg, 4 times per day) with placebo orally. All children received also glycerol orally. The primary outcome was mortality by day 7.
In all, 375 patients were included in the study between 22 January 2012 and 21 January 2017. As 2 children succumbed before treatment initiation, 187 vs 186 participants remained in the intervention and control groups, respectively. On day 7, 61 of 187 (32.6%) children in the intervention group vs 64 of 186 (34.4%) in the control group had died (risk ratio, 0.95 [95% confidence interval {CI}, .71–1.26]; absolute risk difference, 1.8% [95% CI, −7.8 to 11.4]). At discharge from hospital, the corresponding numbers were 71 of 187 (38.0%) and 75 of 186 (40.3%), respectively.
Prolonged continuous β-lactam infusion combined with oral acetaminophen did not improve the gloomy outcomes of childhood bacterial meningitis in Angola.
NCT01540838.
Bacterial meningitis (BM) remains a serious threat to global child health, being the 10th most common cause of death in children aged < 5 years [1]. The disease burden falls in particular on children in resource-poor settings, where the mortality rates may still rise up to 50%, survivors being often left with disabling neurological or audiological sequelae. Although vaccination against Haemophilus influenzae type b (Hib), several types of Streptococcus pneumoniae, and the essential serogroups of Neisseria meningitidis have reduced the burden [2–4], the poor disease outcomes urgently call for more effective treatment modalities.
Dexamethasone and, to a lesser extent, glycerol have been evaluated as adjunctive treatments for childhood BM. The results have been controversial [5–8], as their effects likely relate to the study environment, causative bacteria, patient age, and likely most importantly, to the clinical condition on admission [9]. Neither treatment has, however, significantly reduced the high mortality rate of this potentially devastating disease. This said, oral glycerol prevented severe neurological sequelae in a large (N = 654) randomized placebo-controlled study in Latin America, the odds ratio for glycerol being 0.31 (95% confidence interval [CI], .13–.76) compared to the placebo recipients [7]. Although a similar effect was not obtained later in a smaller study in Malawi [8], the clear results from Latin America prevented us, on ethical grounds, from withdrawal of the utilization of glycerol in our further BM studies in Africa.
As β-lactam antibiotics exert time-dependent killing of bacteria, prolonged or continuous infusions may optimize the pharmacodynamic performance of these compounds [10]. A continuous infusion may also produce other benefits, such as a better controlled inflammatory response due to less fluctuating target antibiotic concentrations. We therefore performed a randomized, double-blind, placebo-controlled study at the Hospital Pediátrico David Bernardino in Luanda, Angola in 2005–2008, in which the effects of initial continuous infusion of cefotaxime were examined [11]. In addition, large-dose oral acetaminophen (paracetamol) was administered, the basis for this intervention deriving from a large retrospective analysis of bacteremic adult patients in Finland [12]. Our main finding from this series of 723 BM patients was that the children receiving cefotaxime infusion for 24 hours and oral acetaminophen for 48 hours showed significantly lower mortality, this effect starting within the first 24 hours from treatment initiation and lasting for several days [11]. Although there was insufficient evidence of a difference at discharge from hospital, this encouraging result prompted us to examine our finding further in another study.
With this background, a new trial was carried out at the same institution in Angola, now with a prolongation of our modified treatment. We here report the results of this investigation.
METHODS
Study Design and Participants
This was a single-center, randomized, double-blind, placebo-controlled parallel-group study conducted at the Hospital Pediátrico David Bernardino in Luanda, Angola. With its 300 beds and 250 daily attendees, of whom 70 are hospitalized, the institute is the largest pediatric referral center and teaching hospital in Angola. The ethics committee of the hospital approved the study protocol on 19 January 2012.
The study entry was assessed for all children aged 2 months to 15 years presenting with the symptoms and signs suggestive of BM and who underwent lumbar puncture. The child was enrolled if the cerebrospinal fluid (CSF) appeared cloudy, was positive by Gram staining, or showed at least 50 leukocytes/mm3. Exclusion criteria included previous known neurological abnormalities or hearing impairment, immunosuppression except for HIV infection, active tuberculosis, known hepatic disease, or pretreatment with > 1 dose of parenteral antibiotic. Before enrollment, the attending physician explained the study to the child’s legal guardian, whose consent was expressed by a signature or, in case of illiteracy, a fingerprint. If participation was refused, the child was treated as routine in the hospital.
The intention-to-treat population comprised all enrolled children, excluding the patients who died before the initiation of treatment. The per-protocol analysis included the patients who received the allocated treatment and had confirmed BM, defined as a positive CSF culture or polymerase chain reaction (PCR); those with compatible symptoms and signs of BM and a positive blood culture; or the children with compatible symptoms and at least 2 of the following criteria: CSF pleocytosis ≥100 cells/mm3 (predominantly polymorphs), a positive CSF Gram stain result, a positive CSF latex agglutination test, or serum C-reactive protein level ≥ 40 mg/L.
Basic laboratory analyses, bacterial cultures, and sensitivity tests were performed at the hospital’s laboratory with standard techniques. As of October 2016, CSF samples were also sent to the National Institute for Communicable Diseases in Johannesburg, South Africa, for identification of N. meningitidis, S. pneumoniae, and H.influenzae by PCR [13].
Randomization and Masking
Randomization was done using a computer-generated list in fixed blocks of 20, which allocated the participants in a 1:1 ratio. The code was kept in a sealed envelope in the office of the head of the hospital, and a copy was sent to the external data and safety monitoring board (DSMB) for the interim analyses. For emergency purposes, the opening key was also kept sealed in Helsinki.
The cards disclosing the specific treatment were kept in sealed envelopes in a box at the hospital ward. Once the attending physician had included a patient in the study, an otherwise nonparticipating study nurse opened the next envelope, prepared the medications and placebos as advised, and gave the preparations for 96 hours (4 days). Thus, the patients, guardians, attending physicians, and the investigators were kept blinded to the intervention.
For further masking, all children received both infusions and boluses intravenously for the entire 4-day course. Since the ready-to-use cefotaxime is slightly yellowish liquid, yellow intravenous lines (Becton-Dickinson GmbH, product number 300326) and black 50-mL syringes (B Braun, reference number 8728828F) were used with a foil covering on the 3-way stopcock’s (B Braun Smallbore T-Port Extension Set, reference number 471954) short end, which leads to the skin-penetrating needle. This setup allowed the same line for the antimicrobial and placebo administrations, each always given as infusion or boluses.
As black syringes and yellow intravenous lines were used, saline could be used as a placebo preparation for cefotaxime, no matter how it was administered. The oral acetaminophen solution from effervescent tablets looked like plain water once the bubbles had disappeared. Being practically tasteless, regular drinking water served as its placebo.
Procedures
All children were treated with cefotaxime in a dose of 250 mg/kg/24 hours for 7 days. For the first 4 days, the children were randomized to receive the agent as a continuous intravenous infusion with high-dose acetaminophen orally (first dose 30 mg/kg, then 20 mg/kg every 6 hours for 4 full days), or as conventional intravenous boluses (62.5 mg/kg every 6 hours) with placebo orally. To prevent degradation of the antibiotic, the daily dose of cefotaxime was administered as two 12-hour infusions (125 mg/kg each). The dissolved acetaminophen (Panadol) tablets formed a solution with 10 mg/mL of acetaminophen.
Based on our previous studies [7, 11], all children were given oral glycerol (6.0 g/kg per day divided into 4 equal doses, maximum dose of 25 g) for 2 days. Antipyretics (besides scheduled acetaminophen as part of the intervention) were avoided. If hyperpyrexia or pain required medication, ibuprofen was given at a dose of 15 mg/kg twice daily. Seizures were treated as was the routine in the hospital, most commonly with diazepam, while for malaria, quinine and artemisinin derivatives were used. A blood transfusion (10 mL/kg) was administered to children with a hemoglobin level < 50 g/L, and sometimes also if considered clinically relevant. Fluid therapy was aimed at normovolemia. Blood glucose was monitored 4 times daily, and hypoglycemia was corrected with intravenous 10% glucose solution. All treatments were recorded in the specifically designed follow-up sheets.
Outcomes
The primary outcome measure was mortality by day 7 in hospital. The secondary outcome measures included later in-hospital mortality, the composite outcomes of death or severe neurological sequelae (hydrocephalus, severe psychomotor retardation, quadriparesis, or blindness), and death or any neurological sequelae (hemi- or monoparesis, ataxia, or psychomotor retardation of any degree, besides the above-mentioned sequelae). The secondary neurological outcomes were recorded on day 7 and at discharge from hospital.
We also aimed at measuring the hearing impairment quantitatively, but due to economical and practical constraints, precise audiological measurements were eventually performed only in a handful of survivors. Adverse events and the safety of our treatment were assessed as part of the detailed follow-up of all participants.
Statistical Analysis
Based on our previous study in Angola [11], we assumed to attain a 13% decrease in mortality with our intervention in patients with confirmed BM. Accepting a 5% error after adjustment for multiple testing in 1-tailed test with a power of 80%, at least 165 patients were required in each treatment group. Because of several confounding factors, such as dissimilar age, severity on admission, malnutrition, and malaria, we intended to enroll 400 patients. An external DSMB obtained the 7-day results after each 100 patients in total had been evaluated. Had an indisputable significance between the treatment modalities been found, the board would have interrupted enrollment for ethical reasons.
Differences between the study groups regarding the in-hospital clinical course were assessed using χ 2 test or Mann-Whitney U test, as appropriate. Potential differences between the study groups in terms of the primary and secondary outcomes were analyzed by χ 2 test, or in the case of low expected cell values in the 2 × 2 table, Fisher exact test. The treatment effects on the primary and secondary outcomes were presented as risk ratios with 95% CIs; the absolute risk difference and its CIs were obtained using a 2-sample z test. Kaplan-Meier analysis and the log-rank test were used when comparing the time of death and potential differences in survival, while treatment interactions between subgroups were examined with logistic regression analysis. All subgroup analyses were predefined in the study protocol. P values < .05 were considered statistically significant, and to detect potential adverse effects of the treatments, all analyses were conducted using a 2-sided hypothesis. Furthermore, differences between the participants of the current study and our previous trial in Luanda were assessed using χ 2 test or Mann-Whitney U test.
RESULTS
Recently commenced Hib and pneumococcal vaccinations in Angola had reduced the incidence of BM to the extent that patient enrollment was extended to the predefined maximum of 5 years (from 22 January 2012 to 21 January 2017). In all, 1128 children were assessed for eligibility, 753 were excluded, and 375 children were randomized into the treatment groups. Since 2 patients died before treatment initiation, 373 children comprised the intention-to-treat population (Figure 1).
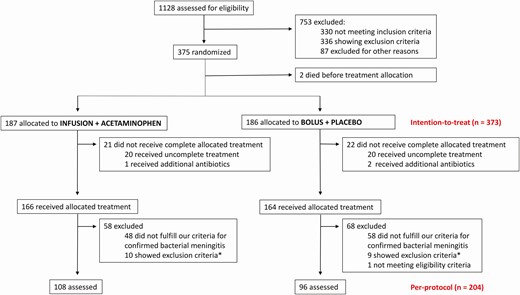
Flowchart of patient inclusion. *Of the patients with confirmed bacterial meningitis.
Of those, 43 had protocol violations: In 16 cases, paralytic ileus prevented oral administration, while the remaining 27 treatment failures were due to insurmountable practical difficulties such as dysfunctional intravenous lines. The number of patients failing treatment was similar in both treatment arms (Figure 1). Finally, our strict criteria for confirmed BM excluded additional 126 participants from the per-protocol population, which ultimately comprised 204 children (Figure 1).
As summarized in Table 1, the patient characteristics and laboratory results proved similar in both groups. Since the median prehospital duration of illness was 5 days, the child was usually severely ill: 67% had suffered from seizures and some one-third showed focal neurological signs on admission. Malnutrition was common, and according to the World Health Organization reference [14], 26% of the children presented with a weight-for-age z score under −2 standard deviations. The causative agent was identified in 152 of 373 (41%) cases, S. pneumoniae being the most common bacterium (Table 1). Interestingly, other not-so-common agents such as Klebsiella species (n = 6) and Proteus species (n = 6) were also detected.
| Characteristic . | Bolus + Placebo(n = 186) . | Infusion + Acetaminophen (n = 187) . |
|---|---|---|
| Age, mo (range), median age (range) | 31 (1–161) | 28 (2–177) |
| Male sex | 115 (62) | 101 (54) |
| Weight for age < −2 SDa | 48/185 (26) | 48/186 (26) |
| Duration of symptoms > 3 da | 116/181 (64) | 136/184 (74) |
| Previous antibioticsa | 73/165 (44) | 95/170 (56) |
| Seizures before admissiona | 122/175 (70) | 116/178 (65) |
| Glasgow Coma Scale score, median (IQR) | 11 (8–15) | 12 (9–15) |
| Focal neurological signsa | 53/181 (29) | 60/176 (34) |
| Another focus of infectiona | 46/169 (27) | 62/178 (35) |
| Dyspneaa | 111/177 (63) | 100/181 (55) |
| Dehydrationa | 43/183 (23) | 46/182 (25) |
| Blood test results | ||
| Hb, g/L, median (IQR)b | 73 (60–92) | 74 (62–89) |
| Leukocyte count, ×109/L, median (IQR)b | 15.1 (9.0–21.0) | 13.7 (8.8–19.7) |
| CRP, mg/L, median (IQR)b | 160 (96–160) | 160 (91 to > 160) |
| Malaria test positivec | 51/186 (27) | 40/187 (21) |
| HIV test positive | 4/98 (4) | 11/93 (12) |
| CSF test results, median (IQR) | ||
| Leukocyte count, ×106/L | 495 (130–2770) | 800 (140–2289) |
| Protein level, mg/dL | 197 (123–265) | 187 (110–250) |
| Glucose level, mmol/L | 0.9 (0.5–2.7) | 1.1 (0.5–2.4) |
| Causative agent | ||
| Streptococcus pneumoniae (n = 73) | 38 (20) | 35 (19) |
| Neisseria meningitidis (n = 37) | 21 (11) | 16 (9) |
| Haemophilus influenzae (n = 7) | 4 (2) | 3 (2) |
| Other bacteria (n = 39) | 16 (9) | 23 (12) |
| Unknown (n = 217) | 107 (58) | 110 (59) |
| Characteristic . | Bolus + Placebo(n = 186) . | Infusion + Acetaminophen (n = 187) . |
|---|---|---|
| Age, mo (range), median age (range) | 31 (1–161) | 28 (2–177) |
| Male sex | 115 (62) | 101 (54) |
| Weight for age < −2 SDa | 48/185 (26) | 48/186 (26) |
| Duration of symptoms > 3 da | 116/181 (64) | 136/184 (74) |
| Previous antibioticsa | 73/165 (44) | 95/170 (56) |
| Seizures before admissiona | 122/175 (70) | 116/178 (65) |
| Glasgow Coma Scale score, median (IQR) | 11 (8–15) | 12 (9–15) |
| Focal neurological signsa | 53/181 (29) | 60/176 (34) |
| Another focus of infectiona | 46/169 (27) | 62/178 (35) |
| Dyspneaa | 111/177 (63) | 100/181 (55) |
| Dehydrationa | 43/183 (23) | 46/182 (25) |
| Blood test results | ||
| Hb, g/L, median (IQR)b | 73 (60–92) | 74 (62–89) |
| Leukocyte count, ×109/L, median (IQR)b | 15.1 (9.0–21.0) | 13.7 (8.8–19.7) |
| CRP, mg/L, median (IQR)b | 160 (96–160) | 160 (91 to > 160) |
| Malaria test positivec | 51/186 (27) | 40/187 (21) |
| HIV test positive | 4/98 (4) | 11/93 (12) |
| CSF test results, median (IQR) | ||
| Leukocyte count, ×106/L | 495 (130–2770) | 800 (140–2289) |
| Protein level, mg/dL | 197 (123–265) | 187 (110–250) |
| Glucose level, mmol/L | 0.9 (0.5–2.7) | 1.1 (0.5–2.4) |
| Causative agent | ||
| Streptococcus pneumoniae (n = 73) | 38 (20) | 35 (19) |
| Neisseria meningitidis (n = 37) | 21 (11) | 16 (9) |
| Haemophilus influenzae (n = 7) | 4 (2) | 3 (2) |
| Other bacteria (n = 39) | 16 (9) | 23 (12) |
| Unknown (n = 217) | 107 (58) | 110 (59) |
Data are presented as no. of patients (%) unless otherwise indicated.
Abbreviations: CRP, C-reactive protein; Hb, hemoglobin; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation.
aDenominator indicates the number of patients for whom the information was available.
bHighest (CRP, leukocyte count) and lowest (Hb) value of days 1–2.
cPositive malaria thick smear within first week in hospital.
| Characteristic . | Bolus + Placebo(n = 186) . | Infusion + Acetaminophen (n = 187) . |
|---|---|---|
| Age, mo (range), median age (range) | 31 (1–161) | 28 (2–177) |
| Male sex | 115 (62) | 101 (54) |
| Weight for age < −2 SDa | 48/185 (26) | 48/186 (26) |
| Duration of symptoms > 3 da | 116/181 (64) | 136/184 (74) |
| Previous antibioticsa | 73/165 (44) | 95/170 (56) |
| Seizures before admissiona | 122/175 (70) | 116/178 (65) |
| Glasgow Coma Scale score, median (IQR) | 11 (8–15) | 12 (9–15) |
| Focal neurological signsa | 53/181 (29) | 60/176 (34) |
| Another focus of infectiona | 46/169 (27) | 62/178 (35) |
| Dyspneaa | 111/177 (63) | 100/181 (55) |
| Dehydrationa | 43/183 (23) | 46/182 (25) |
| Blood test results | ||
| Hb, g/L, median (IQR)b | 73 (60–92) | 74 (62–89) |
| Leukocyte count, ×109/L, median (IQR)b | 15.1 (9.0–21.0) | 13.7 (8.8–19.7) |
| CRP, mg/L, median (IQR)b | 160 (96–160) | 160 (91 to > 160) |
| Malaria test positivec | 51/186 (27) | 40/187 (21) |
| HIV test positive | 4/98 (4) | 11/93 (12) |
| CSF test results, median (IQR) | ||
| Leukocyte count, ×106/L | 495 (130–2770) | 800 (140–2289) |
| Protein level, mg/dL | 197 (123–265) | 187 (110–250) |
| Glucose level, mmol/L | 0.9 (0.5–2.7) | 1.1 (0.5–2.4) |
| Causative agent | ||
| Streptococcus pneumoniae (n = 73) | 38 (20) | 35 (19) |
| Neisseria meningitidis (n = 37) | 21 (11) | 16 (9) |
| Haemophilus influenzae (n = 7) | 4 (2) | 3 (2) |
| Other bacteria (n = 39) | 16 (9) | 23 (12) |
| Unknown (n = 217) | 107 (58) | 110 (59) |
| Characteristic . | Bolus + Placebo(n = 186) . | Infusion + Acetaminophen (n = 187) . |
|---|---|---|
| Age, mo (range), median age (range) | 31 (1–161) | 28 (2–177) |
| Male sex | 115 (62) | 101 (54) |
| Weight for age < −2 SDa | 48/185 (26) | 48/186 (26) |
| Duration of symptoms > 3 da | 116/181 (64) | 136/184 (74) |
| Previous antibioticsa | 73/165 (44) | 95/170 (56) |
| Seizures before admissiona | 122/175 (70) | 116/178 (65) |
| Glasgow Coma Scale score, median (IQR) | 11 (8–15) | 12 (9–15) |
| Focal neurological signsa | 53/181 (29) | 60/176 (34) |
| Another focus of infectiona | 46/169 (27) | 62/178 (35) |
| Dyspneaa | 111/177 (63) | 100/181 (55) |
| Dehydrationa | 43/183 (23) | 46/182 (25) |
| Blood test results | ||
| Hb, g/L, median (IQR)b | 73 (60–92) | 74 (62–89) |
| Leukocyte count, ×109/L, median (IQR)b | 15.1 (9.0–21.0) | 13.7 (8.8–19.7) |
| CRP, mg/L, median (IQR)b | 160 (96–160) | 160 (91 to > 160) |
| Malaria test positivec | 51/186 (27) | 40/187 (21) |
| HIV test positive | 4/98 (4) | 11/93 (12) |
| CSF test results, median (IQR) | ||
| Leukocyte count, ×106/L | 495 (130–2770) | 800 (140–2289) |
| Protein level, mg/dL | 197 (123–265) | 187 (110–250) |
| Glucose level, mmol/L | 0.9 (0.5–2.7) | 1.1 (0.5–2.4) |
| Causative agent | ||
| Streptococcus pneumoniae (n = 73) | 38 (20) | 35 (19) |
| Neisseria meningitidis (n = 37) | 21 (11) | 16 (9) |
| Haemophilus influenzae (n = 7) | 4 (2) | 3 (2) |
| Other bacteria (n = 39) | 16 (9) | 23 (12) |
| Unknown (n = 217) | 107 (58) | 110 (59) |
Data are presented as no. of patients (%) unless otherwise indicated.
Abbreviations: CRP, C-reactive protein; Hb, hemoglobin; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation.
aDenominator indicates the number of patients for whom the information was available.
bHighest (CRP, leukocyte count) and lowest (Hb) value of days 1–2.
cPositive malaria thick smear within first week in hospital.
After 7 days of treatment, 125 of the 373 (34%) patients had died. The composite outcomes of death or severe neurological sequelae, and of death or any neurological sequelae, were observed in 45% (155/345) and 54% (182/334) of the series, respectively (Table 2). As 21 patients died after the first week, the final fatality rate increased to 39%. At discharge, 47% (175/369) of patients had either died or survived with severe neurological sequelae. Adding any neurological sequelae to the calculation, the proportion rose to 54% (193/360) (Table 2).
The 2 Treatment Modalities Versus the Risk of Adverse Outcomes in the Intention-to-Treat and Per-Protocol Populations
| Outcome . | Infusion + Acetaminophen, % (No.) . | Bolus + Placebo, % (No.) . | Risk Ratio (95% CI) . | Absolute Risk Difference, % (95% CI)a . |
|---|---|---|---|---|
| Intention-to-treat | ||||
| Day 7 death | 32.6 (61/187) | 34.4 (64/186) | 0.95 (.71–1.26) | 1.8 (−7.8 to 11.4) |
| Death at discharge | 38.0 (71/187) | 40.3 (75/186) | 0.94 (.73–1.21) | 2.3 (−7.6 to 12.2) |
| Severe neurological sequelae or death, day 7 | 46.5 (80/172) | 43.4 (75/173) | 1.07 (.85–1.36) | 3.1 (−7.4 to 13.6) |
| Severe neurological sequelae or death, at discharge | 48.4 (90/186) | 46.4 (85/183) | 1.04 (.84–1.29) | 2.0 (−8.2 to 12.2) |
| Any neurological sequelae or death, day 7 | 57.8 (96/166) | 51.2 (86/168) | 1.13 (.93–1.38) | 6.6 (−4.1 to 17.3) |
| Any neurological sequelae or death, at discharge | 57.5 (104/181) | 49.7 (89/179) | 1.16 (.95–1.40) | 7.8 (−2.5 to 18.1) |
| Per-protocol | ||||
| Day 7 death | 30.6 (33/108) | 34.4 (33/96) | 0.89 (.60–1.32) | 3.8 (−9.1 to 16.7) |
| Death at discharge | 33.3 (36/108) | 37.5 (36/96) | 0.89 (.61–1.29) | 4.2 (−8.9 to 17.3) |
| Severe neurological sequelae or death, day 7 | 40.6 (41/101) | 44.8 (39/87) | 0.91 (.65–1.26) | 4.2 (−10.0 to 18.4) |
| Severe neurological sequelae or death, at discharge | 42.6 (46/108) | 45.2 (42/93) | 0.94 (.69–1.29) | 2.6 (−11.2 to 16.4) |
| Any neurological sequelae or death, day 7 | 53.1 (51/96) | 54.8 (46/84) | 0.97 (.74–1.27) | 1.7 (−12.9 to 16.3) |
| Any neurological sequelae or death, at discharge | 50.0 (52/104) | 50.6 (45/89) | 0.99 (.75–1.31) | 0.6 (−13.6 to 14.8) |
| Outcome . | Infusion + Acetaminophen, % (No.) . | Bolus + Placebo, % (No.) . | Risk Ratio (95% CI) . | Absolute Risk Difference, % (95% CI)a . |
|---|---|---|---|---|
| Intention-to-treat | ||||
| Day 7 death | 32.6 (61/187) | 34.4 (64/186) | 0.95 (.71–1.26) | 1.8 (−7.8 to 11.4) |
| Death at discharge | 38.0 (71/187) | 40.3 (75/186) | 0.94 (.73–1.21) | 2.3 (−7.6 to 12.2) |
| Severe neurological sequelae or death, day 7 | 46.5 (80/172) | 43.4 (75/173) | 1.07 (.85–1.36) | 3.1 (−7.4 to 13.6) |
| Severe neurological sequelae or death, at discharge | 48.4 (90/186) | 46.4 (85/183) | 1.04 (.84–1.29) | 2.0 (−8.2 to 12.2) |
| Any neurological sequelae or death, day 7 | 57.8 (96/166) | 51.2 (86/168) | 1.13 (.93–1.38) | 6.6 (−4.1 to 17.3) |
| Any neurological sequelae or death, at discharge | 57.5 (104/181) | 49.7 (89/179) | 1.16 (.95–1.40) | 7.8 (−2.5 to 18.1) |
| Per-protocol | ||||
| Day 7 death | 30.6 (33/108) | 34.4 (33/96) | 0.89 (.60–1.32) | 3.8 (−9.1 to 16.7) |
| Death at discharge | 33.3 (36/108) | 37.5 (36/96) | 0.89 (.61–1.29) | 4.2 (−8.9 to 17.3) |
| Severe neurological sequelae or death, day 7 | 40.6 (41/101) | 44.8 (39/87) | 0.91 (.65–1.26) | 4.2 (−10.0 to 18.4) |
| Severe neurological sequelae or death, at discharge | 42.6 (46/108) | 45.2 (42/93) | 0.94 (.69–1.29) | 2.6 (−11.2 to 16.4) |
| Any neurological sequelae or death, day 7 | 53.1 (51/96) | 54.8 (46/84) | 0.97 (.74–1.27) | 1.7 (−12.9 to 16.3) |
| Any neurological sequelae or death, at discharge | 50.0 (52/104) | 50.6 (45/89) | 0.99 (.75–1.31) | 0.6 (−13.6 to 14.8) |
Abbreviation: CI, confidence interval.
aThe absolute risk difference and its CIs were obtained using a 2-sample z test.
The 2 Treatment Modalities Versus the Risk of Adverse Outcomes in the Intention-to-Treat and Per-Protocol Populations
| Outcome . | Infusion + Acetaminophen, % (No.) . | Bolus + Placebo, % (No.) . | Risk Ratio (95% CI) . | Absolute Risk Difference, % (95% CI)a . |
|---|---|---|---|---|
| Intention-to-treat | ||||
| Day 7 death | 32.6 (61/187) | 34.4 (64/186) | 0.95 (.71–1.26) | 1.8 (−7.8 to 11.4) |
| Death at discharge | 38.0 (71/187) | 40.3 (75/186) | 0.94 (.73–1.21) | 2.3 (−7.6 to 12.2) |
| Severe neurological sequelae or death, day 7 | 46.5 (80/172) | 43.4 (75/173) | 1.07 (.85–1.36) | 3.1 (−7.4 to 13.6) |
| Severe neurological sequelae or death, at discharge | 48.4 (90/186) | 46.4 (85/183) | 1.04 (.84–1.29) | 2.0 (−8.2 to 12.2) |
| Any neurological sequelae or death, day 7 | 57.8 (96/166) | 51.2 (86/168) | 1.13 (.93–1.38) | 6.6 (−4.1 to 17.3) |
| Any neurological sequelae or death, at discharge | 57.5 (104/181) | 49.7 (89/179) | 1.16 (.95–1.40) | 7.8 (−2.5 to 18.1) |
| Per-protocol | ||||
| Day 7 death | 30.6 (33/108) | 34.4 (33/96) | 0.89 (.60–1.32) | 3.8 (−9.1 to 16.7) |
| Death at discharge | 33.3 (36/108) | 37.5 (36/96) | 0.89 (.61–1.29) | 4.2 (−8.9 to 17.3) |
| Severe neurological sequelae or death, day 7 | 40.6 (41/101) | 44.8 (39/87) | 0.91 (.65–1.26) | 4.2 (−10.0 to 18.4) |
| Severe neurological sequelae or death, at discharge | 42.6 (46/108) | 45.2 (42/93) | 0.94 (.69–1.29) | 2.6 (−11.2 to 16.4) |
| Any neurological sequelae or death, day 7 | 53.1 (51/96) | 54.8 (46/84) | 0.97 (.74–1.27) | 1.7 (−12.9 to 16.3) |
| Any neurological sequelae or death, at discharge | 50.0 (52/104) | 50.6 (45/89) | 0.99 (.75–1.31) | 0.6 (−13.6 to 14.8) |
| Outcome . | Infusion + Acetaminophen, % (No.) . | Bolus + Placebo, % (No.) . | Risk Ratio (95% CI) . | Absolute Risk Difference, % (95% CI)a . |
|---|---|---|---|---|
| Intention-to-treat | ||||
| Day 7 death | 32.6 (61/187) | 34.4 (64/186) | 0.95 (.71–1.26) | 1.8 (−7.8 to 11.4) |
| Death at discharge | 38.0 (71/187) | 40.3 (75/186) | 0.94 (.73–1.21) | 2.3 (−7.6 to 12.2) |
| Severe neurological sequelae or death, day 7 | 46.5 (80/172) | 43.4 (75/173) | 1.07 (.85–1.36) | 3.1 (−7.4 to 13.6) |
| Severe neurological sequelae or death, at discharge | 48.4 (90/186) | 46.4 (85/183) | 1.04 (.84–1.29) | 2.0 (−8.2 to 12.2) |
| Any neurological sequelae or death, day 7 | 57.8 (96/166) | 51.2 (86/168) | 1.13 (.93–1.38) | 6.6 (−4.1 to 17.3) |
| Any neurological sequelae or death, at discharge | 57.5 (104/181) | 49.7 (89/179) | 1.16 (.95–1.40) | 7.8 (−2.5 to 18.1) |
| Per-protocol | ||||
| Day 7 death | 30.6 (33/108) | 34.4 (33/96) | 0.89 (.60–1.32) | 3.8 (−9.1 to 16.7) |
| Death at discharge | 33.3 (36/108) | 37.5 (36/96) | 0.89 (.61–1.29) | 4.2 (−8.9 to 17.3) |
| Severe neurological sequelae or death, day 7 | 40.6 (41/101) | 44.8 (39/87) | 0.91 (.65–1.26) | 4.2 (−10.0 to 18.4) |
| Severe neurological sequelae or death, at discharge | 42.6 (46/108) | 45.2 (42/93) | 0.94 (.69–1.29) | 2.6 (−11.2 to 16.4) |
| Any neurological sequelae or death, day 7 | 53.1 (51/96) | 54.8 (46/84) | 0.97 (.74–1.27) | 1.7 (−12.9 to 16.3) |
| Any neurological sequelae or death, at discharge | 50.0 (52/104) | 50.6 (45/89) | 0.99 (.75–1.31) | 0.6 (−13.6 to 14.8) |
Abbreviation: CI, confidence interval.
aThe absolute risk difference and its CIs were obtained using a 2-sample z test.
Day 7 mortality and the death rate at discharge were similar in the treatment groups (Table 2). Nor did their composite outcomes differ, albeit the effect of the interventional treatment seemed somewhat more consistent (all risk ratios staying below 1) in the per-protocol population (Table 2). Overall, our prolonged modified treatment did not show significant benefits (Figure 2).
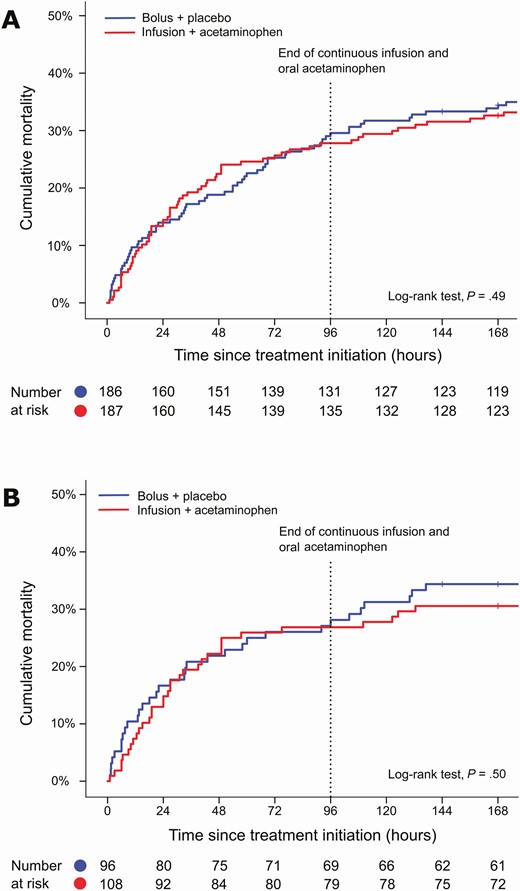
Mortality curves for the intention-to-treat (A) and per-protocol (B) populations, shown for the first 7 days. Ticks (in the blue and red curves) indicate censored participants.
Since the prognosis of BM differs substantially in different populations, we also analyzed certain predefined subgroups (Table 3). While the overall mortality varied largely, the given treatment did not relate to improved survival in any subgroup. Regarding the course of illness on the ward, ibuprofen was given more often in the control group, likely due to the higher need of antipyresis among these children (Table 4).
| Variable . | Bolus + Placebo, % (No.) . | Infusion + Acetaminophen, % (No.) . | Subgroup-Specific P Valuea . | Test for Interaction P Valueb . |
|---|---|---|---|---|
| Age | ||||
| <12 mo (n = 107) | 33 (18/54) | 34 (18/53) | .95 | .72 |
| ≥12 mo (n = 265) | 35 (46/132) | 32 (42/133) | .57 | |
| Presenting conditionc | ||||
| GCS score < 12 (n = 178) | 49 (46/94) | 49 (41/84) | .99 | .74 |
| GCS score ≥ 12 (n = 181) | 20 (17/87) | 17 (16/94) | .66 | |
| Duration of illness before hospital admissiond | ||||
| ≤3 d (n = 113) | 22 (14/65) | 19 (9/48) | .72 | .93 |
| >3 d (n = 252) | 41 (47/116) | 38 (51/136) | .62 | |
| Weight-for-age z scoree | ||||
| Slightly malnourished or normal (≥ −2 SD) (n = 275) | 31 (43/137) | 29 (40/138) | .66 | .82 |
| Severely malnourished (< −2 SD) (n = 96) | 42 (20/48) | 42 (20/48) | 1.00 | |
| Bacterial etiology | ||||
| Streptococcus pneumoniae (n = 73) | 45 (17/38) | 34 (12/35) | .36 | .40 |
| Nonpneumococcal or unknown (n = 300) | 32 (47/148) | 32 (49/152) | .93 |
| Variable . | Bolus + Placebo, % (No.) . | Infusion + Acetaminophen, % (No.) . | Subgroup-Specific P Valuea . | Test for Interaction P Valueb . |
|---|---|---|---|---|
| Age | ||||
| <12 mo (n = 107) | 33 (18/54) | 34 (18/53) | .95 | .72 |
| ≥12 mo (n = 265) | 35 (46/132) | 32 (42/133) | .57 | |
| Presenting conditionc | ||||
| GCS score < 12 (n = 178) | 49 (46/94) | 49 (41/84) | .99 | .74 |
| GCS score ≥ 12 (n = 181) | 20 (17/87) | 17 (16/94) | .66 | |
| Duration of illness before hospital admissiond | ||||
| ≤3 d (n = 113) | 22 (14/65) | 19 (9/48) | .72 | .93 |
| >3 d (n = 252) | 41 (47/116) | 38 (51/136) | .62 | |
| Weight-for-age z scoree | ||||
| Slightly malnourished or normal (≥ −2 SD) (n = 275) | 31 (43/137) | 29 (40/138) | .66 | .82 |
| Severely malnourished (< −2 SD) (n = 96) | 42 (20/48) | 42 (20/48) | 1.00 | |
| Bacterial etiology | ||||
| Streptococcus pneumoniae (n = 73) | 45 (17/38) | 34 (12/35) | .36 | .40 |
| Nonpneumococcal or unknown (n = 300) | 32 (47/148) | 32 (49/152) | .93 |
These subgroup analyses were predefined and conducted within the intention-to-treat population.
Abbreviations: GCS, Glasgow Coma Scale; SD, standard deviation.
aObtained using χ 2 test within the subgroup.
bCalculated, using logistic regression analysis, for interaction between each variable and the treatment.
cData on the GCS score were missing from 14 participants.
dDuration of illness unknown in 8 participants.
eInformation missing from 2 participants.
| Variable . | Bolus + Placebo, % (No.) . | Infusion + Acetaminophen, % (No.) . | Subgroup-Specific P Valuea . | Test for Interaction P Valueb . |
|---|---|---|---|---|
| Age | ||||
| <12 mo (n = 107) | 33 (18/54) | 34 (18/53) | .95 | .72 |
| ≥12 mo (n = 265) | 35 (46/132) | 32 (42/133) | .57 | |
| Presenting conditionc | ||||
| GCS score < 12 (n = 178) | 49 (46/94) | 49 (41/84) | .99 | .74 |
| GCS score ≥ 12 (n = 181) | 20 (17/87) | 17 (16/94) | .66 | |
| Duration of illness before hospital admissiond | ||||
| ≤3 d (n = 113) | 22 (14/65) | 19 (9/48) | .72 | .93 |
| >3 d (n = 252) | 41 (47/116) | 38 (51/136) | .62 | |
| Weight-for-age z scoree | ||||
| Slightly malnourished or normal (≥ −2 SD) (n = 275) | 31 (43/137) | 29 (40/138) | .66 | .82 |
| Severely malnourished (< −2 SD) (n = 96) | 42 (20/48) | 42 (20/48) | 1.00 | |
| Bacterial etiology | ||||
| Streptococcus pneumoniae (n = 73) | 45 (17/38) | 34 (12/35) | .36 | .40 |
| Nonpneumococcal or unknown (n = 300) | 32 (47/148) | 32 (49/152) | .93 |
| Variable . | Bolus + Placebo, % (No.) . | Infusion + Acetaminophen, % (No.) . | Subgroup-Specific P Valuea . | Test for Interaction P Valueb . |
|---|---|---|---|---|
| Age | ||||
| <12 mo (n = 107) | 33 (18/54) | 34 (18/53) | .95 | .72 |
| ≥12 mo (n = 265) | 35 (46/132) | 32 (42/133) | .57 | |
| Presenting conditionc | ||||
| GCS score < 12 (n = 178) | 49 (46/94) | 49 (41/84) | .99 | .74 |
| GCS score ≥ 12 (n = 181) | 20 (17/87) | 17 (16/94) | .66 | |
| Duration of illness before hospital admissiond | ||||
| ≤3 d (n = 113) | 22 (14/65) | 19 (9/48) | .72 | .93 |
| >3 d (n = 252) | 41 (47/116) | 38 (51/136) | .62 | |
| Weight-for-age z scoree | ||||
| Slightly malnourished or normal (≥ −2 SD) (n = 275) | 31 (43/137) | 29 (40/138) | .66 | .82 |
| Severely malnourished (< −2 SD) (n = 96) | 42 (20/48) | 42 (20/48) | 1.00 | |
| Bacterial etiology | ||||
| Streptococcus pneumoniae (n = 73) | 45 (17/38) | 34 (12/35) | .36 | .40 |
| Nonpneumococcal or unknown (n = 300) | 32 (47/148) | 32 (49/152) | .93 |
These subgroup analyses were predefined and conducted within the intention-to-treat population.
Abbreviations: GCS, Glasgow Coma Scale; SD, standard deviation.
aObtained using χ 2 test within the subgroup.
bCalculated, using logistic regression analysis, for interaction between each variable and the treatment.
cData on the GCS score were missing from 14 participants.
dDuration of illness unknown in 8 participants.
eInformation missing from 2 participants.
| Variable . | Bolus + Placebo . | Infusion + Acetaminophen . | P Valuea . |
|---|---|---|---|
| Duration of fever, d, median (IQR) | 2 (1–3) | 2 (1–3) | 1.00 |
| Duration of GCS score < 15, d, median (IQR) | 3 (1–5) | 2 (1–5) | .45 |
| Seizures | 130/185 (70) | 123/185 (67) | .43 |
| Focal neurological signs | 76/181 (42) | 79/175 (45) | .55 |
| Duration of survivors’ hospital stay, d, median (IQR) | 12 (10–17) | 14 (10–20) | .14 |
| Nonstudy treatments given | |||
| Supplementary oxygen | 76/186 (41) | 76/186 (41) | 1.00 |
| Blood transfusion | 44/186 (24) | 47/187 (25) | .74 |
| Anticonvulsive medication | 89/185 (48) | 87/186 (47) | .80 |
| Malaria treatment | 92/186 (50) | 90/187 (48) | .80 |
| Ibuprofen, during first 4 d | 71/185 (38) | 49/187 (26) | .01 |
| Additional antibiotic treatment (after first 7 d) | 70/184 (38) | 79/186 (43) | .39 |
| Tuberculosis treatment | 11/185 (6) | 15/187 (8) | .43 |
| Variable . | Bolus + Placebo . | Infusion + Acetaminophen . | P Valuea . |
|---|---|---|---|
| Duration of fever, d, median (IQR) | 2 (1–3) | 2 (1–3) | 1.00 |
| Duration of GCS score < 15, d, median (IQR) | 3 (1–5) | 2 (1–5) | .45 |
| Seizures | 130/185 (70) | 123/185 (67) | .43 |
| Focal neurological signs | 76/181 (42) | 79/175 (45) | .55 |
| Duration of survivors’ hospital stay, d, median (IQR) | 12 (10–17) | 14 (10–20) | .14 |
| Nonstudy treatments given | |||
| Supplementary oxygen | 76/186 (41) | 76/186 (41) | 1.00 |
| Blood transfusion | 44/186 (24) | 47/187 (25) | .74 |
| Anticonvulsive medication | 89/185 (48) | 87/186 (47) | .80 |
| Malaria treatment | 92/186 (50) | 90/187 (48) | .80 |
| Ibuprofen, during first 4 d | 71/185 (38) | 49/187 (26) | .01 |
| Additional antibiotic treatment (after first 7 d) | 70/184 (38) | 79/186 (43) | .39 |
| Tuberculosis treatment | 11/185 (6) | 15/187 (8) | .43 |
Data are displayed as no. of patients (%) unless otherwise indicated.
Abbreviations: GCS, Glasgow Coma Scale; IQR, interquartile range.
aObtained using χ 2 test or Mann-Whitney U test, as appropriate.
| Variable . | Bolus + Placebo . | Infusion + Acetaminophen . | P Valuea . |
|---|---|---|---|
| Duration of fever, d, median (IQR) | 2 (1–3) | 2 (1–3) | 1.00 |
| Duration of GCS score < 15, d, median (IQR) | 3 (1–5) | 2 (1–5) | .45 |
| Seizures | 130/185 (70) | 123/185 (67) | .43 |
| Focal neurological signs | 76/181 (42) | 79/175 (45) | .55 |
| Duration of survivors’ hospital stay, d, median (IQR) | 12 (10–17) | 14 (10–20) | .14 |
| Nonstudy treatments given | |||
| Supplementary oxygen | 76/186 (41) | 76/186 (41) | 1.00 |
| Blood transfusion | 44/186 (24) | 47/187 (25) | .74 |
| Anticonvulsive medication | 89/185 (48) | 87/186 (47) | .80 |
| Malaria treatment | 92/186 (50) | 90/187 (48) | .80 |
| Ibuprofen, during first 4 d | 71/185 (38) | 49/187 (26) | .01 |
| Additional antibiotic treatment (after first 7 d) | 70/184 (38) | 79/186 (43) | .39 |
| Tuberculosis treatment | 11/185 (6) | 15/187 (8) | .43 |
| Variable . | Bolus + Placebo . | Infusion + Acetaminophen . | P Valuea . |
|---|---|---|---|
| Duration of fever, d, median (IQR) | 2 (1–3) | 2 (1–3) | 1.00 |
| Duration of GCS score < 15, d, median (IQR) | 3 (1–5) | 2 (1–5) | .45 |
| Seizures | 130/185 (70) | 123/185 (67) | .43 |
| Focal neurological signs | 76/181 (42) | 79/175 (45) | .55 |
| Duration of survivors’ hospital stay, d, median (IQR) | 12 (10–17) | 14 (10–20) | .14 |
| Nonstudy treatments given | |||
| Supplementary oxygen | 76/186 (41) | 76/186 (41) | 1.00 |
| Blood transfusion | 44/186 (24) | 47/187 (25) | .74 |
| Anticonvulsive medication | 89/185 (48) | 87/186 (47) | .80 |
| Malaria treatment | 92/186 (50) | 90/187 (48) | .80 |
| Ibuprofen, during first 4 d | 71/185 (38) | 49/187 (26) | .01 |
| Additional antibiotic treatment (after first 7 d) | 70/184 (38) | 79/186 (43) | .39 |
| Tuberculosis treatment | 11/185 (6) | 15/187 (8) | .43 |
Data are displayed as no. of patients (%) unless otherwise indicated.
Abbreviations: GCS, Glasgow Coma Scale; IQR, interquartile range.
aObtained using χ 2 test or Mann-Whitney U test, as appropriate.
Potential adverse events of the treatments were registered as part of the normal follow-up. Furthermore, any abnormal signs of illness, not compatible with the diagnosis of meningitis, were registered in the follow-up sheet. In this regard, the groups did not differ.
Discussion
No benefit was found from using a prolonged 4-day infusion of cefotaxime combined with oral acetaminophen in this trial on childhood BM in Luanda, Angola. Mortality and neurological sequelae remained the same regardless of whether cefotaxime with or without oral acetaminophen was administered conventionally in boluses every 6 hours, or as continuous infusion for 4 days.
Our rationale for using continuous infusion of antibiotic in BM relies, in addition to the pharmacodynamic advantages, also on relieving the initial inflammatory burst triggered by the strongly bacteriolytic β-lactam in the central nervous system. The basis for using oral acetaminophen at the same time laid, on the other hand, in the large retrospective study in which it was significantly associated with decreased mortality in adult bacteremia [12]. In our previous trial in Angola [11], an initial 24-hour cefotaxime infusion with the simultaneous 48-hour oral acetaminophen reduced BM mortality in children for several days—this effect being finally lost at discharge from hospital. It was thus logical to assume that prolonging our modified treatment would lead to permanent mortality decrease. As disappointing as it was, this was not the case here: No benefit was achieved with such a treatment prolongation.
To find explanations for this discrepancy, we searched for differences between these 2 patient populations (Supplementary Table 1). In brief, participants in this study arrived to hospital more severely ill, were more frequently malnourished and dehydrated, and clearly differed in etiology. The role of Hib meningitis was dramatically reduced while the proportion of less common meningitis agents increased, and more participants were left with an unknown bacterial etiology. The participants of this study were also older and had received preadmission antibiotics more often, compared with the participants of our previous trial.
Our interpretation is that many children here had already passed the moment when the new treatment would have had a potential to help the patient [15]. Of special note is our earlier finding that in childhood BM, the presenting condition surpasses all other cofactors predicting death, being as a single covariant even more important than the causative agent as such [9]. The major changes in bacterial etiology might also contribute to the discrepancy between our 2 trials.
Some correlation to the adult studies, in which continuous or prolonged antibiotic infusions have also led to nonconsistent results, might here be justifiably drawn [16]. Slow, continuous infusion probably performs best in some patient subpopulations whose identification poses a challenge to future trials.
Successful Hib and pneumococcal vaccinations in Angola hindered our collection of the targeted minimum of 165 confirmed BM cases in both study groups. The large heterogeneous group, which was excluded from the per-protocol analysis, comprised children with diseases such as tuberculous meningitis, cerebral malaria, and rabies—and among them there probably were some cases of partially treated BM as well. A major limitation was also the lack of adequate audiological measurements in survivors, which prevented us from evaluating the treatment’s possible effect on hearing impairment.
All of these shortcomings no doubt lessened the value of our observations. This said, however, our experience is a truthful description of the circumstances prevailing today in many parts of sub-Saharan Africa. Acknowledging the frequent lack of diagnostic tools and the challenging differential diagnostics, our intention-to-treat population probably well represents children who are treated for BM in these settings. Therefore, in spite of the large dropout rate, we believe our main result remains valid and applicable for this kind of patient population.
Our study underlines the gloomy prognosis of childhood BM in Angola. A continuous antibiotic infusion and oral acetaminophen did not improve the outcome of these severely ill children, who often came late for treatment. Since the prehospital delay is unbearably long, more efforts should be put into the parents’ knowledge of the symptoms and signs of BM. In addition, better availability of healthcare would hasten adequate treatment. Large-scale vaccinations against the relevant agents are good servants when combating this devastating disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the external data and safety monitoring board for performing the interim safety analyses; Matti Kataja and Paula Bergman for skillful statistical consulting; and Orion Diagnostica for providing reagents for the measurements of C-reactive protein. The authors also thank our colleagues at Hospital Pediátrico David Bernardino for excellent collaboration and all of the involved children and their families in Luanda for participating in this trial.
Financial support. This work was supported by the Pediatric Research Foundation; the Päivikki and Sakari Sohlberg Foundation; the Sigrid Jusélius Foundation; Finska Läkaresällskapet; Stiftelsen Dorothea Olivia, Karl Walter och Jarl Walter Perkléns minne; and the Orion Research Foundation. T. P. reports grants from Lastentautien Tutkimussäätiö.
Potential conflicts of interest. T. P. has received research funding from Sanofi Pasteur (Lyon, France). All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




