-
PDF
- Split View
-
Views
-
Cite
Cite
Carlos A DiazGranados, Edith Langevin, Matthew Bonaparte, Saranya Sridhar, Tifany Machabert, Gustavo Dayan, Rémi Forrat, Stephen Savarino, CYD Tetravalent Dengue Vaccine Performance by Baseline Immune Profile (Monotypic/Multitypic) in Dengue-Seropositive Individuals, Clinical Infectious Diseases, Volume 72, Issue 10, 15 May 2021, Pages 1730–1737, https://doi.org/10.1093/cid/ciaa304
Close - Share Icon Share
Abstract
The immune profile of dengue-experienced individuals is a determinant of dengue reinfection severity risk. Individuals with a single prior dengue infection (monotypic) are at highest risk for severe disease, while individuals with ≥ 2 prior dengue infections (multitypic) are at lower risk. The tetravalent dengue vaccine (CYD-TDV) has shown efficacy in the prevention of dengue in individuals with prior dengue infection. We estimated efficacy in individuals with monotypic or multitypic immune profiles.
Participants enrolled in the immunogenicity subsets of 2 randomized placebo-controlled phase 3 studies (CYD14, NCT01373281; CYD15, NCT01374516) were classified as either monotypic or multitypic, based on measured baseline dengue plaque reduction neutralization test. Vaccine efficacy (VE) against symptomatic virologically confirmed dengue (VCD) was assessed over 25 months and against VCD hospitalization over 6 years.
Of 3927 participants in the immunogenicity subsets, 496 and 257 in the CYD-TDV and placebo groups, respectively, were classified as monotypic immune, and 1227 and 612, respectively, as multitypic immune. VE against symptomatic VCD was 77.4% (95% CI, 56.4%–88.2%) for monotypic and 89.2% (95% CI, 71.5%–95.9%) for multitypic profiles, with corresponding absolute risk reductions (ARRs) of 4.48% (95% CI, 2.32%–6.65%) for monotypics and 1.67% (95% CI, .89%–2.46%) for multitypics. VE against hospitalized VCD was 75.3% (95% CI, 42.7%–90.2%) in monotypics and 81.2% (95% CI, 21.7%–96.8%) in multitypics, with ARRs of 0.95% (95% CI, .37%–1.53%) for monotypics and 0.18% (95% CI, .02%–.34%) for multitypics.
CYD-TDV benefits individuals with monotypic and multitypic immune profiles. Larger public health benefit is expected to derive from the protection of individuals with a monotypic immune profile.
Dengue infection is caused by 4 related but antigenically distinct virus serotypes that often co-circulate. Long-term protective immunity is achieved to the infecting serotype, with short-term cross-protective immunity against subsequent infection by the other serotypes. It is generally accepted that a second infection with a different serotype is associated with increased risk of severe disease [1]. Postsecondary infections are believed to be mainly asymptomatic because severe disease is rarely observed [2, 3]. Despite lower disease rates, postsecondary infections may constitute a significant proportion of apparent infections, although there are currently limited data regarding this, and risk may vary according to virus [4]. There is some evidence that hospitalized postsecondary dengue infection cases have a similar risk of progressing to dengue hemorrhagic fever as those experiencing a second infection [2].
The recombinant, live, attenuated, tetravalent dengue vaccine (CYD-TDV, Dengvaxia; Sanofi Pasteur) has been licensed for the prevention of dengue in > 20 countries. Recent data have shown that CYD-TDV protects against hospitalized and severe dengue for at least 5 years in individuals with prior dengue infection (PDI) before vaccination, with evidence of increased risk in those without PDI [5]. Prevaccination screening for PDI is recommended by the World Health Organization (WHO) in countries considering vaccination as part of their dengue control program [1]. Limiting vaccination to those with evidence of PDI could help to maximize the vaccine risk-benefit profile.
While the benefit of the vaccine in preventing symptomatic, hospitalized, and severe dengue in those with PDI has been established, it is imperative to understand the performance of the vaccine according to the extent of the PDIs. Given the paradigm of increased risk of hospitalized/severe dengue in secondary infections (ie, individuals with PDI to a single serotype, dengue monotypic immune) compared to postsecondary infections (PDI to ≥ 2 serotypes, dengue multitypic immune), we analyzed data from the 2 CYD-TDV phase 3 trials to further characterize vaccine efficacy and risk of hospitalization in these populations.
METHODS
Study Design
These post hoc analyses were based on data from 2 phase 3 efficacy studies, CYD14 (ClinicalTrials.gov identifier NCT01373281) and CYD15 (NCT01374516), which were performed in parallel and were nearly identical in design [6–8]. CYD14 was conducted in 5 countries in Asia Pacific, in participants aged 2–14 years, and CYD15 was conducted in 5 countries in Latin America, in participants aged 9–16 years. Participants were randomized 2:1 to receive 3 injections, 6 months apart, of either CYD-TDV or placebo. The period from the first injection to month 25 is referred to as the “active phase,” during which active surveillance was used to detect symptomatic dengue, whether associated with hospitalization or not. Surveillance for dengue hospitalization occurred from enrollment to study end, thus spanning 72 months (6 years).
The trials were undertaken in compliance with good clinical practice guidelines and the principles of the Declaration of Helsinki. Ethics review committees approved the protocol, amendments, consent, and assent forms. Parents or legal guardians provided informed consent before participation, and written assent was obtained from older children, in compliance with the regulations of each country.
Study Participants
A subset of participants in both trials provided blood samples for the evaluation of dengue neutralizing antibodies at baseline and after each injection, based on WHO guidance at the time of study design [9]. Twenty percent of the planned sample size of CYD14 and 10% of CYD15 were stratified by age and study site and randomly assigned to the immunogenicity subsets, and randomized to receive either vaccine or placebo. The post hoc analyses presented here were restricted to participants within these immunogenicity subsets because the classification of participants by dengue immune profile as either “dengue monotypic” or “dengue multitypic” required the measurement of antibodies against each of the 4 dengue serotypes at baseline (before vaccination).
Definitions
We used plaque reduction neutralization test (PRNT90) as the main method for classifying the baseline immune profile. According to WHO guidelines on PRNT [10], PRNT90 titers are more useful than PRNT50 titers in dengue-endemic areas for epidemiological studies or for diagnostic purposes, by decreasing the background serum cross-reactivity among flaviviruses. PRNT90 is more specific than PRNT50 and therefore associated with less false-positive dengue classification and less cross-reactivity between serotypes [11]. This is particularly important for the evaluation of the number of previous serotype exposures in this study. For each serotype, the neutralizing titer was expressed as the highest reciprocal dilution of sera that reduced the infectivity of a challenge virus by 90%. Dengue monotypic immune was defined as PRNT90 titer ≥ 10 (1/dilution) against a single dengue serotype. Dengue multitypic immune was defined as PRNT90 titer ≥ 10 (1/dilution) against at least 2 dengue serotypes.
Due to the lower specificity of the plaque reduction neutralization test (PRNT50) vs PRNT90, participants classified as positive for a serotype based on PRNT50, but negative for that same serotype by the PRNT90, were considered dengue seronegative for that serotype for the purposes of the main assessments in this post hoc analysis. This helped to minimize false positives in the dengue monotypic immune group, and was supported by our observations that the “immunological behavior” of participants who are PRNT50 positive but PRNT90 negative is more akin to that of PRNT50 seronegative participants rather than to those who are PRNT90 monotypic immune (Supplementary Figure 1).
Additional sensitivity analyses were performed using alternative definitions based on PRNT50: dengue monotypic was defined as PRNT50 titer ≥ 40 for only 1 serotype (to minimize erroneous classification of dengue seronegatives as monotypic immunes), or at least a 6-fold higher titer between the dominant serotype and any other (to minimize the erroneous classification of monotypic immunes as multitypic immunes); dengue multitypic immune was defined as PRNT50 titer ≥ 40 for at least 2 serotypes and less than a 6-fold difference between the dominant serotype and any other [12].
Symptomatic virologically confirmed dengue (VCD) was defined as an acute febrile episode with temperature ≥ 38°C on at least 2 consecutive days, associated with virological confirmation of dengue. An episode was virologically confirmed if the acute blood sample collected at the time of illness tested positive with the dengue screen reverse-transcription polymerase chain reaction and/or positive dengue NS1 antigen enzyme-linked immunosorbent assay [6, 7]. Hospitalization for VCD was also assessed.
Immunogenicity Assessments
Immunogenicity was presented for both monotypic and multitypic immune participants and stratified by age group (9–16 years and 2–8 years) and treatment group (CYD-TDV and placebo). Geometric mean antibody titers (GMTs) for each dengue serotype as measured by PRNT50 at baseline and approximately 28 days after the third study injection are presented.
The PRNT50 and PRNT90 assays were performed at Sanofi Pasteur’s Global Clinical Immunology Laboratory (Swiftwater, Pennsylvania) with the PRNT50 following validated protocols [13]. While WHO guidance on PRNT states a preference of PRNT90 assay for diagnostic or epidemiological purposes, it recommends the PRNT50 assay for the assessment of dengue vaccine immune responses as it provides more accurate results from the linear portion of the titration curve [10].
Statistical Methods
The original efficacy studies were not powered for these post hoc assessments. The power for these analyses was fixed, as it was based on data from existing samples and clinical data. To maximize the power for vaccine efficacy assessment, we performed pooled analyses for all age groups included in the original trials (age 2–16 years at the time of vaccination). Given that, at the time of the writing of this report, the vaccine is currently indicated for individuals aged at least 9 years, these analyses were also performed in those aged 9–16 years. The analyses were stratified based on the baseline immune profile (dengue monotypic immune or dengue multitypic immune).
For the analysis of CYD-TDV efficacy against symptomatic VCD, cases during the active phase of the trial were included and density incidence was calculated as the number of participants with at least 1 symptomatic VCD episode during the active phase per 100 person-years at risk. For integrated (pooled across both trials) vaccine efficacy estimates and confidence intervals (CIs), Cox regression models were used. Absolute risk reduction (ARR) was estimated as the absolute difference in outcomes between the control and CYD-TDV groups and the CIs were calculated using a standard normal table for 95% CIs. The analysis set for estimation of efficacy against symptomatic VCD and for immunogenicity corresponded to participants in the immunogenicity subsets who received at least 1 study injection and who were dengue seropositive to 1 or more serotypes, with treatment assignment as randomized.
For analysis of the risk of hospitalized VCD, annualized incidence was estimated as 100 times the number of participants with at least 1 episode of hospitalized VCD, divided by the mean of number of participants present at the beginning of each year of follow-up and divided by 6 (because of the 6 years of follow-up). The relative risk reduction was 1 minus the relative risk where relative risk was estimated as the ratio of the annual incidence in the vaccine and the control groups, and CIs were calculated using the exact method described by Breslow and Day [14]. Absolute risk reduction was estimated for symptomatic VCD. The analysis set for the estimation of dengue hospitalization risk corresponded to participants in the immunogenicity subset safety analysis set (including any participant who received at least 1 study injection) who were dengue seropositive to 1 or more serotypes, with treatment assignment as per study injection received.
For immunogenicity assessments, geometric means of the individual titers (GMT) for each serotype was calculated for the different age strata. The 95% CIs for the GMTs were calculated using the normal approximation method. Assuming that log10 transformation of the titers followed a normal distribution, the mean and 95% CI were calculated on log10 (titers) utilizing the usual calculation for normal distribution; then, antilog transformations were applied to the results of calculations to compute GMTs and 95% CIs.
RESULTS
Study Population
Within the immunogenicity subsets, 2624 participants were randomized to the CYD-TDV group and 1303 participants to the placebo group. Of these, 496 and 257 participants in the CYD-TDV and placebo group, respectively, were classified as monotypic immune and, 1227 and 612, respectively, as multitypic immune; the rest were classified as dengue nonimmune by PRNT90 (Table 1). There was a higher proportion of multitypic participants in both studies, in both the 2–16 years and 9–16 years age groups. The proportion of multitypic participants was greater in the 9–16 years group compared to the 2–16 years group.
Baseline Dengue Status Overall as Measured by PRNT90 by Study and Across Both Age Subsets (Immunogenicity Full Analysis Set)
| Study and Age Group . | Baseline Dengue Statusa . | Vaccine Group . | Control . |
|---|---|---|---|
| . | . | no./No. (%) . | no./No. (%) . |
| CYD14 ± CYD15 | |||
| 2–16 y | Monotypic | 496/2624 (18.90) | 257/1303 (19.72) |
| Multitypic | 1227/2624 (46.76) | 612/1303 (46.97) | |
| Nonimmune | 886/2624 (33.77) | 421/1303 (32.31) | |
| 9–16 y | Monotypic | 353/1916 (18.42) | 175/954 (18.34) |
| Multitypic | 1086/1916 (56.68) | 542/954 (56.81) | |
| Nonimmune | 471/1916 (24.58) | 228/954 (23.90) | |
| CYD14 | |||
| 2–14 y | Monotypic | 272/1323 (20.56) | 155/660 (23.48) |
| Multitypic | 459/1323 (34.69) | 235/660 (35.61) | |
| Nonimmune | 579/1323 (43.76) | 263/660 (39.85) | |
| 9–14 y | Monotypic | 129/615 (20.98) | 73/311 (23.47) |
| Multitypic | 318/615 (51.71) | 165/311 (53.05) | |
| Nonimmune | 164/615 (26.67) | 70/311 (22.51) | |
| CYD15 | |||
| 9–16 y | Monotypic | 224/1301 (17.22) | 102/643 (15.86) |
| Multitypic | 768/1301 (59.03) | 377/643 (58.63) | |
| Nonimmune | 307/1301 (23.60) | 158/643 (24.57) |
| Study and Age Group . | Baseline Dengue Statusa . | Vaccine Group . | Control . |
|---|---|---|---|
| . | . | no./No. (%) . | no./No. (%) . |
| CYD14 ± CYD15 | |||
| 2–16 y | Monotypic | 496/2624 (18.90) | 257/1303 (19.72) |
| Multitypic | 1227/2624 (46.76) | 612/1303 (46.97) | |
| Nonimmune | 886/2624 (33.77) | 421/1303 (32.31) | |
| 9–16 y | Monotypic | 353/1916 (18.42) | 175/954 (18.34) |
| Multitypic | 1086/1916 (56.68) | 542/954 (56.81) | |
| Nonimmune | 471/1916 (24.58) | 228/954 (23.90) | |
| CYD14 | |||
| 2–14 y | Monotypic | 272/1323 (20.56) | 155/660 (23.48) |
| Multitypic | 459/1323 (34.69) | 235/660 (35.61) | |
| Nonimmune | 579/1323 (43.76) | 263/660 (39.85) | |
| 9–14 y | Monotypic | 129/615 (20.98) | 73/311 (23.47) |
| Multitypic | 318/615 (51.71) | 165/311 (53.05) | |
| Nonimmune | 164/615 (26.67) | 70/311 (22.51) | |
| CYD15 | |||
| 9–16 y | Monotypic | 224/1301 (17.22) | 102/643 (15.86) |
| Multitypic | 768/1301 (59.03) | 377/643 (58.63) | |
| Nonimmune | 307/1301 (23.60) | 158/643 (24.57) |
Data are shown as number of participants/number of participants in full analysis set (%). Subjects with undetermined baseline status (no titer greater than or equal to the lower limit of quantification and at least 1 missing titer) are excluded.
aMonotypic immune profile: participants with plaque reduction neutralization test (PRNT90) titers ≥ 10 (1/dilution) against only 1 dengue serotype at baseline. Multitypic immune profile: participants with PRNT90 titers ≥ 10 (1/dilution) against at least 2 dengue serotypes at baseline. Nonimmune profile: participants with PRNT90 titer < 10 (1/dilution) against all 4 dengue serotypes.
Baseline Dengue Status Overall as Measured by PRNT90 by Study and Across Both Age Subsets (Immunogenicity Full Analysis Set)
| Study and Age Group . | Baseline Dengue Statusa . | Vaccine Group . | Control . |
|---|---|---|---|
| . | . | no./No. (%) . | no./No. (%) . |
| CYD14 ± CYD15 | |||
| 2–16 y | Monotypic | 496/2624 (18.90) | 257/1303 (19.72) |
| Multitypic | 1227/2624 (46.76) | 612/1303 (46.97) | |
| Nonimmune | 886/2624 (33.77) | 421/1303 (32.31) | |
| 9–16 y | Monotypic | 353/1916 (18.42) | 175/954 (18.34) |
| Multitypic | 1086/1916 (56.68) | 542/954 (56.81) | |
| Nonimmune | 471/1916 (24.58) | 228/954 (23.90) | |
| CYD14 | |||
| 2–14 y | Monotypic | 272/1323 (20.56) | 155/660 (23.48) |
| Multitypic | 459/1323 (34.69) | 235/660 (35.61) | |
| Nonimmune | 579/1323 (43.76) | 263/660 (39.85) | |
| 9–14 y | Monotypic | 129/615 (20.98) | 73/311 (23.47) |
| Multitypic | 318/615 (51.71) | 165/311 (53.05) | |
| Nonimmune | 164/615 (26.67) | 70/311 (22.51) | |
| CYD15 | |||
| 9–16 y | Monotypic | 224/1301 (17.22) | 102/643 (15.86) |
| Multitypic | 768/1301 (59.03) | 377/643 (58.63) | |
| Nonimmune | 307/1301 (23.60) | 158/643 (24.57) |
| Study and Age Group . | Baseline Dengue Statusa . | Vaccine Group . | Control . |
|---|---|---|---|
| . | . | no./No. (%) . | no./No. (%) . |
| CYD14 ± CYD15 | |||
| 2–16 y | Monotypic | 496/2624 (18.90) | 257/1303 (19.72) |
| Multitypic | 1227/2624 (46.76) | 612/1303 (46.97) | |
| Nonimmune | 886/2624 (33.77) | 421/1303 (32.31) | |
| 9–16 y | Monotypic | 353/1916 (18.42) | 175/954 (18.34) |
| Multitypic | 1086/1916 (56.68) | 542/954 (56.81) | |
| Nonimmune | 471/1916 (24.58) | 228/954 (23.90) | |
| CYD14 | |||
| 2–14 y | Monotypic | 272/1323 (20.56) | 155/660 (23.48) |
| Multitypic | 459/1323 (34.69) | 235/660 (35.61) | |
| Nonimmune | 579/1323 (43.76) | 263/660 (39.85) | |
| 9–14 y | Monotypic | 129/615 (20.98) | 73/311 (23.47) |
| Multitypic | 318/615 (51.71) | 165/311 (53.05) | |
| Nonimmune | 164/615 (26.67) | 70/311 (22.51) | |
| CYD15 | |||
| 9–16 y | Monotypic | 224/1301 (17.22) | 102/643 (15.86) |
| Multitypic | 768/1301 (59.03) | 377/643 (58.63) | |
| Nonimmune | 307/1301 (23.60) | 158/643 (24.57) |
Data are shown as number of participants/number of participants in full analysis set (%). Subjects with undetermined baseline status (no titer greater than or equal to the lower limit of quantification and at least 1 missing titer) are excluded.
aMonotypic immune profile: participants with plaque reduction neutralization test (PRNT90) titers ≥ 10 (1/dilution) against only 1 dengue serotype at baseline. Multitypic immune profile: participants with PRNT90 titers ≥ 10 (1/dilution) against at least 2 dengue serotypes at baseline. Nonimmune profile: participants with PRNT90 titer < 10 (1/dilution) against all 4 dengue serotypes.
Dengue Risk in Monotypic Versus Multitypic Immune Profiles
The incidence of symptomatic VCD during the active phase in participants aged 2–16 years receiving placebo and classified as monotypic immune (5.8%) was approximately 3 times higher than both placebo recipients classified as multitypic immune (1.9%) and CYD-TDV recipients classified as monotypic immune (1.3%) (Table 2). The annualized incidence of hospitalized dengue during the 6 years of follow-up in the 2- to 16-year-old placebo recipients classified as monotypic immune (1.3%) was approximately 4–6 times higher than in placebo recipients classified as multitypic immune (0.2%) and CYD-TDV recipients classified as monotypic immune (0.3%) (Table 3).
Incidence of Symptomatic Dengue and Vaccine Performance in Monotypic and Multitypic Participants Aged 2–16 Years and 9–16 Years (CYD14 + CYD15 Immunosubsets)
| . | Incidence of Symptomatic VCD (0–25 mo) . | . | . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Vaccine Group . | Placebo . | . | . | ||||
| Groupa . | No. of Cases . | Person-years . | Density Incidence, % . | No. of Cases . | Person-years . | Density Incidence, % . | Absolute Risk Reduction, % (95% CI) . | Vaccine Efficacy,b % (95% CI) . |
| Participants aged 2–16 y | ||||||||
| Monotypic | 13 | 992 | 1.3 | 29 | 501 | 5.8 | 4.48 (2.32–6.65) | 77.4 (56.4–88.2) |
| Multitypic | 5 | 2472 | 0.2 | 23 | 1225 | 1.9 | 1.67 (.89–2.46) | 89.2 (71.5–95.9) |
| Participants aged 9–16 y | ||||||||
| Monotypic | 8 | 704 | 1.1 | 18 | 341 | 5.3 | 4.14 (1.65–6.64) | 78.7 (50.9–90.7) |
| Multitypic | 3 | 2187 | 0.1 | 19 | 1084 | 1.8 | 1.62 (.82–2.41) | 92.1 (73.4–97.7) |
| . | Incidence of Symptomatic VCD (0–25 mo) . | . | . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Vaccine Group . | Placebo . | . | . | ||||
| Groupa . | No. of Cases . | Person-years . | Density Incidence, % . | No. of Cases . | Person-years . | Density Incidence, % . | Absolute Risk Reduction, % (95% CI) . | Vaccine Efficacy,b % (95% CI) . |
| Participants aged 2–16 y | ||||||||
| Monotypic | 13 | 992 | 1.3 | 29 | 501 | 5.8 | 4.48 (2.32–6.65) | 77.4 (56.4–88.2) |
| Multitypic | 5 | 2472 | 0.2 | 23 | 1225 | 1.9 | 1.67 (.89–2.46) | 89.2 (71.5–95.9) |
| Participants aged 9–16 y | ||||||||
| Monotypic | 8 | 704 | 1.1 | 18 | 341 | 5.3 | 4.14 (1.65–6.64) | 78.7 (50.9–90.7) |
| Multitypic | 3 | 2187 | 0.1 | 19 | 1084 | 1.8 | 1.62 (.82–2.41) | 92.1 (73.4–97.7) |
Abbreviations: CI, confidence interval; VCD, virologically confirmed dengue.
aMonotypic immune profile: participants with plaque reduction neutralization test (PRNT90) titers ≥ 10 (1/dilution) against only 1 dengue serotype at baseline. Multitypic immune profile: participants with PRNT90 titers ≥ 10 (1/dilution) against at least 2 dengue serotypes at baseline.
bVaccine efficacy is equivalent to relative risk reduction.
Incidence of Symptomatic Dengue and Vaccine Performance in Monotypic and Multitypic Participants Aged 2–16 Years and 9–16 Years (CYD14 + CYD15 Immunosubsets)
| . | Incidence of Symptomatic VCD (0–25 mo) . | . | . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Vaccine Group . | Placebo . | . | . | ||||
| Groupa . | No. of Cases . | Person-years . | Density Incidence, % . | No. of Cases . | Person-years . | Density Incidence, % . | Absolute Risk Reduction, % (95% CI) . | Vaccine Efficacy,b % (95% CI) . |
| Participants aged 2–16 y | ||||||||
| Monotypic | 13 | 992 | 1.3 | 29 | 501 | 5.8 | 4.48 (2.32–6.65) | 77.4 (56.4–88.2) |
| Multitypic | 5 | 2472 | 0.2 | 23 | 1225 | 1.9 | 1.67 (.89–2.46) | 89.2 (71.5–95.9) |
| Participants aged 9–16 y | ||||||||
| Monotypic | 8 | 704 | 1.1 | 18 | 341 | 5.3 | 4.14 (1.65–6.64) | 78.7 (50.9–90.7) |
| Multitypic | 3 | 2187 | 0.1 | 19 | 1084 | 1.8 | 1.62 (.82–2.41) | 92.1 (73.4–97.7) |
| . | Incidence of Symptomatic VCD (0–25 mo) . | . | . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Vaccine Group . | Placebo . | . | . | ||||
| Groupa . | No. of Cases . | Person-years . | Density Incidence, % . | No. of Cases . | Person-years . | Density Incidence, % . | Absolute Risk Reduction, % (95% CI) . | Vaccine Efficacy,b % (95% CI) . |
| Participants aged 2–16 y | ||||||||
| Monotypic | 13 | 992 | 1.3 | 29 | 501 | 5.8 | 4.48 (2.32–6.65) | 77.4 (56.4–88.2) |
| Multitypic | 5 | 2472 | 0.2 | 23 | 1225 | 1.9 | 1.67 (.89–2.46) | 89.2 (71.5–95.9) |
| Participants aged 9–16 y | ||||||||
| Monotypic | 8 | 704 | 1.1 | 18 | 341 | 5.3 | 4.14 (1.65–6.64) | 78.7 (50.9–90.7) |
| Multitypic | 3 | 2187 | 0.1 | 19 | 1084 | 1.8 | 1.62 (.82–2.41) | 92.1 (73.4–97.7) |
Abbreviations: CI, confidence interval; VCD, virologically confirmed dengue.
aMonotypic immune profile: participants with plaque reduction neutralization test (PRNT90) titers ≥ 10 (1/dilution) against only 1 dengue serotype at baseline. Multitypic immune profile: participants with PRNT90 titers ≥ 10 (1/dilution) against at least 2 dengue serotypes at baseline.
bVaccine efficacy is equivalent to relative risk reduction.
Risk of Dengue Hospitalization in Monotypic and Multitypic Participants Aged 2–16 Years and Aged 9–16 Years (CYD14 + CYD15 Immunosubset)
| . | Annualized Risk of Dengue Hospitalizationb . | . | . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Vaccine Group . | Placebo . | . | . | ||||
| Groupa . | no. . | No. . | Annual Incidence . | no. . | No. . | Annual Incidence . | Absolute Risk Reduction, % (95% CI) . | Relative Risk Reduction,c % (95% CI) . |
| Participants aged 2–16 y | ||||||||
| Monotypic | 9 | 481 | 0.3 | 19 | 251 | 1.3 | 0.95 (.37–1.53) | 75.3 (42.7–90.2) |
| Multitypic | 3 | 1183 | < 0.1 | 8 | 593 | 0.2 | 0.18 (.02–.34) | 81.2 (21.7–96.8) |
| Participants aged 9–16 y | ||||||||
| Monotypic | 5 | 338 | 0.2 | 11 | 169 | 1.1 | 0.84 (.18–1.50) | 77.3 (29.2–93.8) |
| Multitypic | 2 | 1042 | < 0.1 | 4 | 524 | 0.1 | 0.1 (−.04 to .23) | 74.8 (−75.5 to 97.7) |
| . | Annualized Risk of Dengue Hospitalizationb . | . | . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Vaccine Group . | Placebo . | . | . | ||||
| Groupa . | no. . | No. . | Annual Incidence . | no. . | No. . | Annual Incidence . | Absolute Risk Reduction, % (95% CI) . | Relative Risk Reduction,c % (95% CI) . |
| Participants aged 2–16 y | ||||||||
| Monotypic | 9 | 481 | 0.3 | 19 | 251 | 1.3 | 0.95 (.37–1.53) | 75.3 (42.7–90.2) |
| Multitypic | 3 | 1183 | < 0.1 | 8 | 593 | 0.2 | 0.18 (.02–.34) | 81.2 (21.7–96.8) |
| Participants aged 9–16 y | ||||||||
| Monotypic | 5 | 338 | 0.2 | 11 | 169 | 1.1 | 0.84 (.18–1.50) | 77.3 (29.2–93.8) |
| Multitypic | 2 | 1042 | < 0.1 | 4 | 524 | 0.1 | 0.1 (−.04 to .23) | 74.8 (−75.5 to 97.7) |
Data are shown as number of participants (no.)/number of participants in full analysis set (No.) unless otherwise indicated.
Abbreviation: CI, confidence interval.
aMonotypic immune profile: participants with plaque reduction neutralization test (PRNT90) titers ≥ 10 (1/dilution) against only 1 dengue serotype at baseline. Multitypic immune profile: participants with PRNT90 titers ≥ 10 (1/dilution) against at least 2 dengue serotypes at baseline.
bThis was based on the cumulative risk over 6 years of follow-up.
cRelative risk reduction is equivalent to vaccine efficacy.
Risk of Dengue Hospitalization in Monotypic and Multitypic Participants Aged 2–16 Years and Aged 9–16 Years (CYD14 + CYD15 Immunosubset)
| . | Annualized Risk of Dengue Hospitalizationb . | . | . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Vaccine Group . | Placebo . | . | . | ||||
| Groupa . | no. . | No. . | Annual Incidence . | no. . | No. . | Annual Incidence . | Absolute Risk Reduction, % (95% CI) . | Relative Risk Reduction,c % (95% CI) . |
| Participants aged 2–16 y | ||||||||
| Monotypic | 9 | 481 | 0.3 | 19 | 251 | 1.3 | 0.95 (.37–1.53) | 75.3 (42.7–90.2) |
| Multitypic | 3 | 1183 | < 0.1 | 8 | 593 | 0.2 | 0.18 (.02–.34) | 81.2 (21.7–96.8) |
| Participants aged 9–16 y | ||||||||
| Monotypic | 5 | 338 | 0.2 | 11 | 169 | 1.1 | 0.84 (.18–1.50) | 77.3 (29.2–93.8) |
| Multitypic | 2 | 1042 | < 0.1 | 4 | 524 | 0.1 | 0.1 (−.04 to .23) | 74.8 (−75.5 to 97.7) |
| . | Annualized Risk of Dengue Hospitalizationb . | . | . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Vaccine Group . | Placebo . | . | . | ||||
| Groupa . | no. . | No. . | Annual Incidence . | no. . | No. . | Annual Incidence . | Absolute Risk Reduction, % (95% CI) . | Relative Risk Reduction,c % (95% CI) . |
| Participants aged 2–16 y | ||||||||
| Monotypic | 9 | 481 | 0.3 | 19 | 251 | 1.3 | 0.95 (.37–1.53) | 75.3 (42.7–90.2) |
| Multitypic | 3 | 1183 | < 0.1 | 8 | 593 | 0.2 | 0.18 (.02–.34) | 81.2 (21.7–96.8) |
| Participants aged 9–16 y | ||||||||
| Monotypic | 5 | 338 | 0.2 | 11 | 169 | 1.1 | 0.84 (.18–1.50) | 77.3 (29.2–93.8) |
| Multitypic | 2 | 1042 | < 0.1 | 4 | 524 | 0.1 | 0.1 (−.04 to .23) | 74.8 (−75.5 to 97.7) |
Data are shown as number of participants (no.)/number of participants in full analysis set (No.) unless otherwise indicated.
Abbreviation: CI, confidence interval.
aMonotypic immune profile: participants with plaque reduction neutralization test (PRNT90) titers ≥ 10 (1/dilution) against only 1 dengue serotype at baseline. Multitypic immune profile: participants with PRNT90 titers ≥ 10 (1/dilution) against at least 2 dengue serotypes at baseline.
bThis was based on the cumulative risk over 6 years of follow-up.
cRelative risk reduction is equivalent to vaccine efficacy.
Vaccine Efficacy Against Symptomatic Dengue During the Active Phase
In participants aged 2–16 years, the incidence of symptomatic VCD during the active phase was lower in the CYD-TDV group than the placebo group for both monotypic and multitypic immune participants (Table 2). Vaccine efficacy point estimates were 77.4% and 89.2% in monotypic and multitypic participants, respectively, with ARRs of 4.48% (95% CI, 2.32%–6.65%) and 1.67% (95% CI, .89%–2.46%), respectively (Table 2). Estimates by serotype suggest contribution to the overall efficacy from protection against the 4 serotypes for both monotypic and multitypic immune profiles (Supplementary Table 1).
Similarly, in participants aged 9–16 years, the incidence of symptomatic VCD during the active phase was lower in the CYD-TDV group than the placebo group for both monotypic and multitypic participants (Table 2). Vaccine efficacy point estimates were 78.7% and 92.1% in monotypic and multitypic participants, respectively, with ARRs of 4.14% (95% CI, 1.65%–6.64%) and 1.62% (95% CI, .82%–2.41%), respectively (Table 2).
In sensitivity analyses using the alternative definition for monotypic and multitypic immune participants based on PRNT50, the results were consistent with those using the main definition (Supplementary Table 2).
Dengue Hospitalization Risk During Long-term Follow-up
In participants aged 2–16 years, the annualized incidence of hospitalized VCD was lower in the CYD-TDV group than in the placebo group for both monotypic and multitypic immune participants (Table 3). Risk in the CYD-TDV group relative to the placebo group was reduced by 75% and 81% in monotypic and multitypic participants, respectively. Absolute annualized risk reductions were 0.95% (95% CI, .37%–1.53%) in monotypic participants and 0.18% (95% CI, .02%–.34%) in multitypic participants. Although imprecise, estimates by serotype suggest protection against all serotypes in those with monotypic immune profiles (Supplementary Table 3). Similarly, while data by serotype in multitypic immunes are largely imprecise, some contribution to protection by serotypes 1, 2, and 3 is suggested but no pattern can be discerned against hospitalized dengue caused by serotype 4 as there were no cases in this subset.
In participants aged 9–16 years, the precision of the estimates for hospitalized VCD was decreased due to the smaller number of events. However, the annualized incidence of hospitalized VCD was lower in the CYD-TDV group than in the placebo group for both monotypic and multitypic participants, and the risk for CYD-TDV compared to placebo was reduced by 77% and 75% in monotypic and multitypic participants, respectively (Table 3). Absolute annualized risk reductions were 0.84% (95% CI, .18%–1.50%) in the monotypic group and 0.1% (95% CI, −.04% to .23%) in the multitypic group.
In sensitivity analyses using the alternative definition for monotypic and multitypic immune participants based on PRNT50, results were consistent across all endpoints (Supplementary Table 4).
Immunogenicity
Neutralizing antibody titers prevaccination were higher for all 4 serotypes in individuals with a baseline multitypic immune profile than in those with a baseline monotypic immune profile, for both those aged 9–16 years and 2–8 years (Figures 1 and 2). The magnitude of the increase in antibody titers from baseline to postvaccination was larger in those with a monotypic immune profile at baseline, but titers postvaccination remained higher in those with a multitypic immune profile.
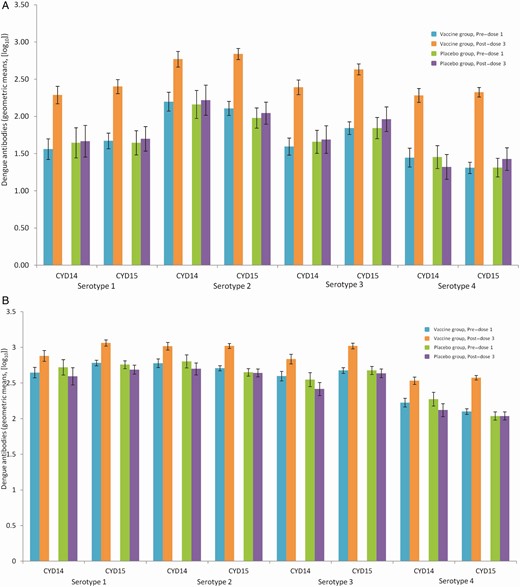
Geometric means of dengue plaque reduction neutralization test (PRNT50) antibody at baseline and post–dose 3 by baseline immune profile in monotypic (A) and multitypic (B) participants 9–16 years of age (CYD14 + CYD15, full analysis set). Monotypic immune profile: participants with plaque reduction neutralization test (PRNT90) titers ≥ 10 (1/dilution) against only 1 dengue serotype at baseline. Multitypic immune profile: participants with PRNT90 titers ≥ 10 (1/dilution) against at least 2 dengue serotypes at baseline.
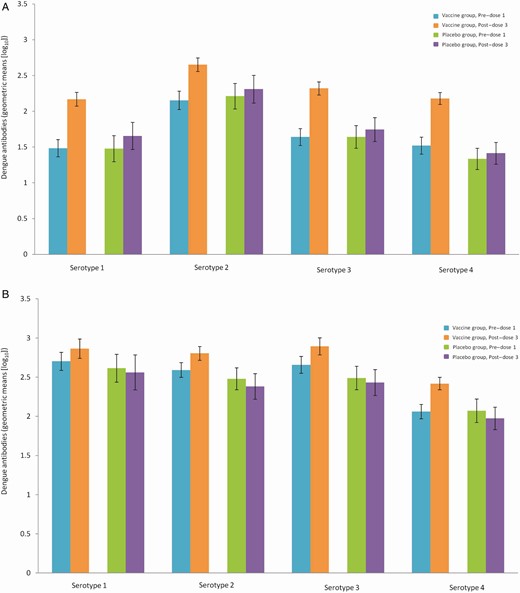
Geometric means of dengue plaque reduction neutralization test (PRNT50) antibody (1/dilution) at baseline and post–dose 3 by baseline immune profile in monotypic (A) and multitypic (B) dengue-seropositive participants 2–8 years of age (CYD14, full analysis set). Monotypic immune profile: participants with plaque reduction neutralization test (PRNT90) titers ≥ 10 (1/dilution) against only 1 dengue serotype at baseline. Multitypic immune profile: participants with PRNT90 titers ≥ 10 (1/dilution) against at least 2 dengue serotypes at baseline.
DISCUSSION
In this study, a higher proportion of multitypic participants was reported across both studies and age groups, which was expected given the high dengue endemicity and co-circulation of the 4 serotypes in the study regions [15]. We observed differences in incidences of both symptomatic and hospitalized dengue between monotypic and multitypic immune participants in the placebo group. This is consistent with the dengue paradigm according to which the risk of symptomatic and severe dengue is increased upon infection with a second dengue serotype (ie, second dengue infection, occurring in individuals with a monotypic immune profile) [16], whereas postsecondary dengue infections (ie, dengue infection occurring in individuals with > 1 dengue serotype exposure or a multitypic immune profile) tend to be asymptomatic or minimally symptomatic [17, 18]. Although the incidence of symptomatic VCD and hospitalizations for VCD were lower in multitypic immune participants in our study, the risk of these outcomes still remained, highlighting the need for continued dengue prevention in this population. Previous studies have reported that the frequency of dengue admissions caused by a third or fourth dengue infection was extremely low (0.1%–0.8%), but once admitted, the risk for dengue hemorrhagic fever relative to dengue fever was similar for those experiencing third or fourth dengue infections over those experiencing a second dengue infection [2]. Therefore, while most value from prevention is expected to come from prevention of second dengue infections, there is still individual and public health value in preventing postsecondary infections.
Not surprisingly, baseline neutralizing antibody titers were higher in individuals with a baseline multitypic immune profile, which was in agreement with other findings [19]. Importantly, the CYD-TDV vaccine elicited robust immune responses in both monotypic and multitypic participants.
Our data suggest that CYD-TDV is associated with significant protection against symptomatic dengue and hospitalized dengue in individuals with a monotypic immune profile. The magnitude of vaccine effect is consistent with that recently reported overall for dengue-seropositive individuals [5]. Our data show that individuals with a multitypic immune profile also benefit from CYD-TDV, even if their risks of symptomatic dengue and dengue hospitalization are substantially lower than for those with a monotypic immune profile. While the relative risk reduction of hospitalized dengue in those with monotypic immune profile is of similar magnitude to that observed in those with a multitypic immune profile (75% vs 81%), the absolute risk difference is expectedly of higher magnitude in the monotypic immunes (1.3% vs 0.2%). This translates into higher overall public health benefit expected from vaccination in those who are monotypic immune, as these individuals are shifted from an “at risk” population to a protected population. However, as there is no commercially available test that can accurately distinguish the number or type of previous dengue infections, vaccination is expected to target those who are dengue seropositive [20], regardless of whether they have a monotypic or multitypic immune profile. The fact that we observed protection in both immune profile groups is reassuring given expected benefits deriving from CYD-TDV vaccination in the overall dengue-seropositive population. Additionally, we may be underestimating the vaccine benefit given that individuals who are not vaccinated are likely to have a progressive decrease in their own risk of dengue as they become naturally exposed to the virus (resulting in lower risk of subsequent dengue outcomes).
Our study has several limitations. The classification of monotypic and multitypic participants used here is not standardized, and our database does not allow us to validate these definitions. Moreover, disparate definitions have been used in the literature to define monotypic and multitypic immune profiles [12, 17, 21–24]. However, given the high specificity of the PRNT90 assay [10], it is expected that our classification minimized inclusion of false dengue-seropositive individuals in our analysis (important for preventing contamination of monotypic immune estimates with true dengue seronegatives), as well as the cross-reactivity between serotypes (important for preventing misclassification of monotypic immunes as multitypic immune). When using an alternative definition for monotypic and multitypic immunity, consistency of the estimates of protection from CYD-TDV provide reassurance on the robustness of our findings. Additionally, this post hoc analysis had limited statistical power, particularly for subgroup analyses (age strata, by study, by specific serotype, or by number of serotypes). The study size was fixed and determined by the design and objectives of the original clinical trials [6, 7]. Finally, hospitalization for dengue was used as a proxy for severe disease as the occurrence of severe dengue within the immunogenicity subsets was too infrequent to allow for meaningful assessment.
In conclusion, CYD-TDV is likely to benefit seropositive individuals both with a single prior dengue serotype infection and those with previous exposure to 2 or more dengue serotypes; larger public health benefit is expected to derive from the protection of those with a single prior dengue infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. A. D. G., E. L., M. B., and S. Sr. all contributed to the study design. E. L., M. B., T. M., G. D., and R. F. contributed to data acquisition. C. A. D. G., E. L., M. B., S. Sr., T. M., and S. Sa. all contributed to data analysis or interpretation of results. C. A. D. G. drafted the publication and all authors had input into critical revision and review.
Acknowledgments. The authors thank the participants included in the studies, the investigators of CYD14 and CYD15, coordinators, and study teams. Editorial assistance with the preparation of the manuscript was provided by Sophieanne Wastling, inScience Communications, Springer Healthcare Ltd, UK, and was funded by Sanofi Pasteur. The authors also thank Jean-Sébastien Persico for editorial assistance and manuscript coordination on behalf of Sanofi Pasteur.
Financial support. This study was funded by Sanofi Pasteur.
Potential conflicts of interest. The authors are employees of Sanofi Pasteur. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




