-
PDF
- Split View
-
Views
-
Cite
Cite
Ruth N. Moro, Andrey S. Borisov, Jussi Saukkonen, Awal Khan, Timothy R. Sterling, M. Elsa Villarino, Nigel A. Scott, Nong Shang, Amy Kerrigan, Stefan V. Goldberg, Factors Associated With Noncompletion of Latent Tuberculosis Infection Treatment: Experience From the PREVENT TB Trial in the United States and Canada, Clinical Infectious Diseases, Volume 62, Issue 11, 1 June 2016, Pages 1390–1400, https://doi.org/10.1093/cid/ciw126
Close - Share Icon Share
Abstract
Background. Overall rates of noncompletion of treatment (NCT) for latent tuberculosis infection (LTBI) in the PREVENT TB trial were 18% for 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg) (3HP-DOT) and 31% for 9 months of daily self-administered isoniazid (maximum dose, 300 mg; 9H-SAT). NCT for LTBI reduces its effectiveness. The study objective was to assess factors associated with NCT for LTBI among adult participants enrolled at US and Canadian sites of the PREVENT TB trial.
Methods. This was a post hoc exploratory analysis of the randomized, open-label PREVENT TB trial. Factors were analyzed by univariate and multivariate logistic regression (with enrollment site as a random effect).
Results. From 6232 participants analyzed, 1406 (22.6%) did not complete LTBI treatment (317 NCT attributed to an adverse event [NCT-AE] and 1089 NCT attributed to reasons other than an adverse event [NCT-O]). The proportion of NCT-AE was similar with both regimens (3HP-DOT = 6.4% vs 9H-SAT = 5.9%; P = .23); NCT-O was higher among participants enrolled in 9H-SAT (9H-SAT = 24.5% vs 3HP-DOT = 12.7%; P = .02). Among those in the NCT-AE group, being non-Hispanic and receiving 3HP-DOT, having cirrhosis and receiving 9H-SAT, alcohol consumption among men, and use of concomitant medication were associated with NCT-AE. Among those in the NCT-O group, receiving 9H-SAT, missing ≥1 early visit, men receiving 9H-SAT, men with a history of incarceration, alcohol abuse, use ever of intravenous drugs, younger age receiving 9H-SAT, and smoking were associated with NCT-O.
Conclusions. Factors associated with NCT, such as missing a clinic visit early during treatment, might help identify persons for whom tailored interventions could improve completion of LTBI treatment.
Clinical Trials Registration. NCT00023452.
(See the Editorial Commentary by Garcia and Lopez on pages 1401–2.)
Treatment of latent tuberculosis infection (LTBI) can prevent progression to tuberculosis and plays an important role in the US tuberculosis elimination strategy [1]. However, LTBI treatment completion remains a challenge.
The most commonly used treatment for LTBI has been daily self-administered isoniazid, which has a 93% efficacy rate among patients who complete a 12-month treatment regimen [2]. However, protection against tuberculosis development decreases to 69% if the regimen is taken for only 6 months [3]. The noncompletion rate in US tuberculosis programs for a 9- or 6-month isoniazid regimen has been reported to range from 47% to 53% [4–6] and from 45% to 63%, respectively [4, 7]. A short regimen of 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg) (3HP-DOT), evaluated by the TB Trials Consortium (TBTC) in the PREVENT TB trial, demonstrated a higher treatment completion rate. That trial reported overall noncompletion of treatment (NCT) rates of 31% for the 9 months of daily self-administered isoniazid regimen (maximum dose, 300 mg; 9H-SAT) and 18% for the combination regimen, which is a significant difference (P < .001) [8].
Discontinuation of treatment can be associated with clinical, social, behavioral, or demographic factors. In contrast, certain treatment regimens and clinic characteristics have been associated with increased treatment completion rates, including shorter regimens, regimens with fewer side effects, clinic features that facilitate patient visits, and clinic processes that optimize provider–patient rapport [6]. However, NCT rates might be higher in programs or practice than in clinical trial settings.
In this study we examined factors associated with NCT for LTBI among adult participants in the PREVENT TB trial who were at high risk for developing tuberculosis. The results of this analysis suggest strategies for improving LTBI treatment completion and tuberculosis control program practices in North America.
METHODS
We performed a post hoc analysis of the PREVENT TB trial, which is a phase 3, open-label, randomized trial of LTBI treatment. Participants infected with Mycobacteria tuberculosis and at high risk for tuberculosis were enrolled from June 2001 to February 2008 at 28 TBTC sites [8]. This analysis included only persons enrolled at North American sites because clinical practice is less variable in the United States and Canada than across all sites including Brazil and Spain (Figure 1). All participants provided written informed consent. Institutional review boards at the Centers for Disease Control and Prevention (CDC) and at participating clinical sites approved the study protocol.
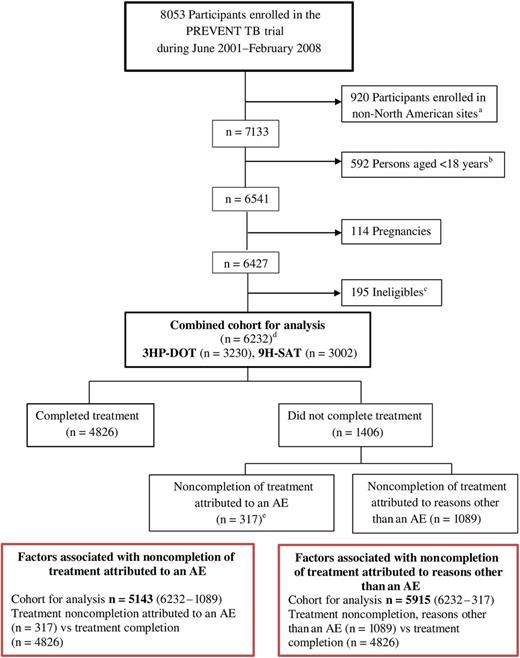
Flow chart of participants evaluated for factors associated with noncompletion of latent tuberculosis infection (LTBI) treatment in North America (PREVENT TB trial). This figure shows the number of participants who were enrolled in the trial, the groups that were excluded from the analysis, and the remaining cohort that was evaluated for factors associated with noncompletion of latent Mycobacterium tuberculosis infection treatment. Exclusion criteria include the following: current confirmed tuberculosis; suspected tuberculosis; tuberculosis resistant to isoniazid or rifampin in the source patient; history of treatment for >14 consecutive days with rifamycin or >30 consecutive days with isoniazid during the previous 2 years; documented history of completing adequate treatment for active tuberculosis or latent M. tuberculosis infection in a human immunodeficiency virus (HIV)–seronegative person; history of sensitivity/intolerance to isoniazid or rifamycin; serum aspartate aminotransferase (AST) >5 times the upper limit of normal if AST was determined; pregnant or lactating females; persons currently receiving or planning to receive HIV-1 therapy ≤90 days after enrollment; or weight <10.0 kg. aThis analysis includes enrollment sites from the United States and Canada. Enrollment sites from Brazil and Spain were excluded; bThis analysis considers adults aged ≥18 years only; cPREVENT TB trial reasons for ineligibility include source patient’s infection resistant to isoniazid/rifampin (50%) and source patient being culture negative (31%), or other (19%); dReasons for LTBI treatment (not mutually exclusive) include the following: contact with infectious tuberculosis patient (n = 4156), tuberculin skin test converter (n = 2205), HIV-positive (n = 140), or fibrosis (n = 181); eNoncompletion of treatment attributed to an adverse event (AE) was designated if participants discontinued treatment after experiencing an adverse drug reaction associated with either 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg) (3HP-DOT) or 9 months of daily self-administered isoniazid (maximum dose, 300 mg; 9H-SAT) that was considered definitely, possibly, or probably related to the study drug(s) by the site investigators (n = 311); if participants discontinued the regimen because of the physician’s preference and had a related AE of grade 2 or higher ≤3 days after their last regimen dose; or if the participant experienced tuberculosis or died ≤7 days after the last study dose.
Participants receiving 3HP-DOT had scheduled visits for clinical evaluation at weeks 4, 8, and 12; participants receiving 9H-SAT had 9 monthly scheduled visits during treatment. At each visit, we collected data regarding weight, adverse reactions, new diagnoses or hospitalizations, symptoms of tuberculosis or of methadone withdrawal, and concomitant medication use. For both regimens, we collected reports of adverse events (AEs) that occurred within 60 days of the last study drug dose. Adherence to the assigned regimen was evaluated at each visit by observed therapy records for 3HP-DOT and by pill count and self-report for 9H-SAT. Site consent forms were reviewed to determine local monetary compensation practices. Participants aged <18 years were excluded from analysis because treatment completion by children might reflect parental behaviors. Factors associated with NCT were assessed as attributed to an AE (NCT-AE) or to reasons other than an AE (NCT-O). Factors possibly associated with NCT were organized as demographic (age, sex, race, ethnicity, country of origin, education, unemployment, homelessness, history of incarceration, or need for an interpreter), clinical (body mass index, human immunodeficiency virus [HIV] status, concomitant medication reported at enrollment and during therapy, indication for LTBI treatment, cirrhosis [by self-report], or methadone treatment), social (alcohol consumption [definition follows], intravenous drug use ever, or current smoking at the time of enrollment), behavioral (missing an early visit [definition follows]), regimen assigned, or enrollment site. Noncompletion was defined as not taking at least 11 of 12 doses in 10–16 weeks for 3HP-DOT or at least 240 of 270 doses in 35–52 weeks for 9H-SAT. NCT at 6 months for daily isoniazid (6H) was also calculated within the 9H-SAT regimen. NCT of 6H was defined as not taking ≥162 of 180 doses in 23–36 weeks.
Participants who permanently discontinued were categorized as NCT attributed to an adverse event (NCT-AE) if they discontinued after experiencing an adverse drug reaction associated with either 3HP-DOT or 9H-SAT that was considered definitely, possibly, or probably related to the study drug(s) by the site investigators; they discontinued the regimen because of physician preference and had a related AE of grade 2 or higher ≤3 days after their last regimen dose; or they developed tuberculosis or died ≤7 days after the last regimen dose. Study participants were considered to have missed an early visit in the 3HP-DOT regimen if they missed ≥1 of the first 3 observed doses, followed by receiving an observed dose at any time during the treatment period, or in the 9H-SAT regimen if they missed ≥1 of the first 3 monthly visits, followed by a monthly visit not missed at any time during the treatment period. Alcohol use was defined by an affirmative response to a question asking whether the participant ever drank alcoholic beverages. Alcohol abuse was defined by a score of 2 or more on the Cut down, Annoyed, Guilty, and Eye-opener questionnaire [9]. Smoking was ascertained based on a patient's statement of whether smoking was current at time of enrollment. Alternate treatment was not evaluated for persons who discontinued study treatment.
NCT proportions were calculated for all factors outlined. Because this was an exploratory analysis, we did not adjust for multiple comparisons. Age was categorized as above or below the median age of the study population (38 years). Odds ratios (ORs), 95% confidence intervals (CIs), and P values were calculated for potential risk factors for NCT. All statistically significant (P ≤ .05) factors in the univariate analysis plus regimen, age, and sex were included in the multivariate model. Collinearity was evaluated. Interaction terms included in the multivariate model were selected on the basis of published scientific literature and expert opinion. Statistical interactions were evaluated to determine if a factor had a different effect on the outcome at different values of another factor. We used a generalized linear mixed model, with enrollment site considered as a random effect [10]. We performed backward elimination manually to select statistically significant interaction terms and single factors with P < .05. We used Pearson correlation coefficient and a linear regression model to evaluate the relationship between the maximum number of doses taken and the proportion of NCT and between the proportions of enrollment by site and noninitiation of treatment. Kaplan–Meier curves were constructed to show treatment discontinuation across the duration of the trial. We compared these curves by using the Wilcoxon test, which takes into consideration the different regimen durations.
Data analysis was conducted with SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Of 8053 participants enrolled in the PREVENT TB trial, 1821 were excluded from our analysis: 920 from sites outside North America, 592 aged <18 years, 114 who became pregnant, and 195 determined ineligible after enrollment. Of the remaining 6232 (combined cohort for analysis), 1406 (22.6%) did not complete treatment. Characteristics among persons who did not complete treatment, categorized as either NCT-AE (n = 317) or NCT-O (n = 1089), were compared with those of participants who completed treatment (n = 4826; Figure 1). Collinearity was not identified in either the NCT-AE group or the NCT-O group.
NCT was statistically greater for 9H-SAT (9H-SAT, 28% vs 3HP-DOT, 18%; P < .001). Although the proportion of NCT-AE was similar in both regimens (9H-SAT, 5.9% vs 3HP-DOT, 6.4%; P = .23), the NCT-O proportion was higher for 9H-SAT (9H-SAT, 24.5% vs 3HP-DOT, 12.7%; P = .02; Supplementary Table 1).
For NCT-AE among 5143 adults (Figure 1), 317 met the case definition for NCT-AE. Age below the median of 38 years, being black or Asian, and lower education level were associated with low odds (<1) of NCT-AE; use of concomitant medication was associated with increased odds of NCT-AE (1.86). The random effect of site was statistically significant, indicating that the odds of NCT-AE varied across sites, even after adjusting for other factors. Evaluation for statistically significant interaction terms in the model revealed that the estimated odds of NCT-AE in the 3HP-DOT regimen were 3 times higher among non-Hispanics compared with Hispanics (P < .001); the odds of NCT-AE in the 9H-SAT regimen were approximately 3 times higher among participants who reported having cirrhosis at enrollment compared with those who did not (P = .001); and the odds of NCT-AE among men who reported use or abuse of alcohol were 1.6 or 2.2 times higher (P = .03 or P = .01) compared with men who denied intake of alcohol (Table 1). The most frequent cause of NCT-AE in the 3HP-DOT regimen was influenza-like syndrome; in the 9H-SAT regimen, it was hepatotoxicity (Supplementary Table 2A and 2B).
Univariate and Multivariate Analysis of Factors Associated With Noncompletion of Latent Tuberculosis Infection Treatment Attributed to an Adverse Event (n = 317) Compared With Those Who Completed Treatment (n = 4826)
| Characteristic . | Noncompletion of Treatment Attributed to an Adverse Event (NCT-AE) (n = 5143 [317 + 4826]) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Univariate . | Multivariate . | |||||
| NCT (%) . | OR . | 95% CI . | P Value . | Adjusted OR . | 95% CI . | P Value . | |
| Regimen | |||||||
| 3HP-DOT (n = 2844) Ref. | 6.4 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9H-SAT (n = 2299) | 5.9 | 1.1 | .87–1.38 | .43 | 1.73 | 1.01–2.95 | .23 |
| Age (y; median, 38) | |||||||
| <38 (n = 2534) | 4.5 | 0.56 | .44–.71 | <.001 | 0.61 | .47–.79 | <.001 |
| ≥38 (n = 2609) Ref. | 7.8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Sex | |||||||
| Female (n = 2282) Ref. | 7.7 | Ref. | Ref. | Ref. | |||
| Male (n = 2861) | 5.0 | 0.63 | .50–.79 | <.001 | |||
| Race | |||||||
| White (n = 2854) Ref. | 6.9 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black (n = 1315) | 4.1 | 0.58 | .43–.79 | .001 | 0.33 | .23–.47 | <.001 |
| Asian (n = 765) | 6.0 | 0.87 | .62–1.21 | .40 | 0.52 | .35–.78 | .001 |
| Othera (n = 209) | 10.1 | 1.52 | .94–2.43 | .09 | 0.88 | .51–1.53 | .65 |
| Ethnicity | |||||||
| Non-Hispanic (n = 3013) Ref. | 7.5 | Ref. | Ref. | Ref. | |||
| Hispanic (n = 2130) | 4.2 | 0.54 | .42–.70 | <.001 | |||
| HIV status | |||||||
| Negative (n = 2518) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Positive (n = 121) | 4.1 | 0.66 | .27–1.64 | .37 | |||
| Unknown (n = 2504) | 6.3 | 1.03 | .82–1.30 | .78 | |||
| Country of origin | |||||||
| Non-US (n = 3133) Ref. | 5.5 | Ref. | Ref. | Ref. | |||
| United States (n = 2010) | 7.2 | 1.34 | 1.07–1.68 | .01 | |||
| Body mass index | |||||||
| Underweight (n = 85) | 4.7 | 0.67 | .24–1.86 | .44 | |||
| Normal (n = 1464) Ref. | 6.9 | Ref. | Ref. | Ref. | |||
| Overweight (n = 1827) | 6.5 | 0.94 | .71–1.24 | .66 | |||
| Obese (n = 1767) | 5.3 | 0.75 | .56–1.0 | .05 | |||
| Education | |||||||
| ≤8th grade (n = 931) | 3.8 | 0.39 | .26–.59 | <.001 | 0.55 | .35–.87 | .01 |
| 8th grade through some college (n = 3207) | 6.0 | 0.64 | .49–.83 | .001 | 0.90 | .67–1.21 | .49 |
| ≥College degree (n = 1005) Ref. | 9.1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Incarcerationb | |||||||
| No (n = 4860) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 283) | 7.8 | 1.31 | .83–2.05 | .25 | |||
| Unemployed | |||||||
| No (n = 4534) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 609) | 6.4 | 1.05 | .74–1.48 | .79 | |||
| Homelessc | |||||||
| No (n = 4760) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 383) | 6.3 | 1.02 | .66–1.57 | .93 | |||
| Alcohol consumptiond | |||||||
| No (n = 2406) Ref. | 5.4 | Ref. | Ref. | Ref. | |||
| Use (n = 2382) | 6.6 | 1.24 | .97–1.57 | .08 | |||
| Abuse (n = 355) | 9.0 | 1.75 | 1.17–2.60 | .01 | |||
| Intravenous drug use ever | |||||||
| No (n = 4942) Ref. | 6.0 | Ref. | Ref. | Ref. | |||
| Yes (n = 201) | 10.0 | 1.73 | 1.07–2.78 | .02 | |||
| Cirrhosis, by self-report | |||||||
| No (n = 4949) Ref. | 6.0 | Ref. | Ref. | Ref. | |||
| Yes (n = 194) | 10.8 | 1.91 | 1.20–3.05 | .01 | |||
| Current smokere | |||||||
| No (n = 3642) Ref. | 5.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 1501) | 7.1 | 1.26 | .99–1.60 | .07 | |||
| Methadone treatment | |||||||
| No (n = 5035) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 108) | 8.3 | 1.40 | .70–2.79 | .34 | |||
| Enrollment sitef | .01 | .02 | |||||
| Concomitant medications | |||||||
| No (n = 2566) Ref. | 3.6 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 2577) | 8.7 | 2.53 | 1.98–3.25 | <.001 | 1.86 | 1.42–2.44 | <.001 |
| LTBI for contact tuberculosis | |||||||
| No (n = 1769) Ref. | 7.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 3374) | 5.7 | 0.79 | .63–1.0 | .05 | |||
| LTBI for tuberculin skin test converter | |||||||
| No (n = 3292) Ref. | 5.7 | Ref. | Ref. | Ref. | |||
| Yes (n = 1851) | 6.9 | 1.22 | .97–1.54 | .09 | |||
| LTBI for fibrosis | |||||||
| No (n = 4985) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 158) | 7.0 | 1.14 | .61–2.13 | .67 | |||
| LTBI for HIV infection | |||||||
| No (n = 5026) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 117) | 4.3 | 0.68 | .27–1.66 | .39 | |||
| Having missed an early visitg | |||||||
| No (n = 4936) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 207) | 4.8 | 0.77 | .40–1.46 | .42 | |||
| Interaction terms | |||||||
| Ethnicity × regimen | |||||||
| 3HP-DOT: Non-Hispanic vs Hispanic | 3.0 | 1.93–4.69 | <.001 | ||||
| 9H-SAT: Non-Hispanic vs Hispanic | 1.44 | .92–2.26 | .11 | ||||
| Cirrhosis, by self-report × regimen | |||||||
| 3HP-DOT: Cirrhosis: yes vs no | 0.80 | .35–1.83 | .60 | ||||
| 9H-SAT-: Cirrhosis: yes vs no | 2.93 | 1.52–5.65 | .001 | ||||
| Alcohol × sex | |||||||
| Male | |||||||
| Users vs no alcohol | 1.64 | 1.05–2.57 | .03 | ||||
| Abusers vs no alcohol | 2.15 | 1.19–3.89 | .01 | ||||
| Female | |||||||
| Users vs no alcohol | 0.84 | .59–1.19 | .32 | ||||
| Abusers vs no alcohol | 0.70 | .26–1.92 | .49 | ||||
| Characteristic . | Noncompletion of Treatment Attributed to an Adverse Event (NCT-AE) (n = 5143 [317 + 4826]) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Univariate . | Multivariate . | |||||
| NCT (%) . | OR . | 95% CI . | P Value . | Adjusted OR . | 95% CI . | P Value . | |
| Regimen | |||||||
| 3HP-DOT (n = 2844) Ref. | 6.4 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9H-SAT (n = 2299) | 5.9 | 1.1 | .87–1.38 | .43 | 1.73 | 1.01–2.95 | .23 |
| Age (y; median, 38) | |||||||
| <38 (n = 2534) | 4.5 | 0.56 | .44–.71 | <.001 | 0.61 | .47–.79 | <.001 |
| ≥38 (n = 2609) Ref. | 7.8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Sex | |||||||
| Female (n = 2282) Ref. | 7.7 | Ref. | Ref. | Ref. | |||
| Male (n = 2861) | 5.0 | 0.63 | .50–.79 | <.001 | |||
| Race | |||||||
| White (n = 2854) Ref. | 6.9 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black (n = 1315) | 4.1 | 0.58 | .43–.79 | .001 | 0.33 | .23–.47 | <.001 |
| Asian (n = 765) | 6.0 | 0.87 | .62–1.21 | .40 | 0.52 | .35–.78 | .001 |
| Othera (n = 209) | 10.1 | 1.52 | .94–2.43 | .09 | 0.88 | .51–1.53 | .65 |
| Ethnicity | |||||||
| Non-Hispanic (n = 3013) Ref. | 7.5 | Ref. | Ref. | Ref. | |||
| Hispanic (n = 2130) | 4.2 | 0.54 | .42–.70 | <.001 | |||
| HIV status | |||||||
| Negative (n = 2518) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Positive (n = 121) | 4.1 | 0.66 | .27–1.64 | .37 | |||
| Unknown (n = 2504) | 6.3 | 1.03 | .82–1.30 | .78 | |||
| Country of origin | |||||||
| Non-US (n = 3133) Ref. | 5.5 | Ref. | Ref. | Ref. | |||
| United States (n = 2010) | 7.2 | 1.34 | 1.07–1.68 | .01 | |||
| Body mass index | |||||||
| Underweight (n = 85) | 4.7 | 0.67 | .24–1.86 | .44 | |||
| Normal (n = 1464) Ref. | 6.9 | Ref. | Ref. | Ref. | |||
| Overweight (n = 1827) | 6.5 | 0.94 | .71–1.24 | .66 | |||
| Obese (n = 1767) | 5.3 | 0.75 | .56–1.0 | .05 | |||
| Education | |||||||
| ≤8th grade (n = 931) | 3.8 | 0.39 | .26–.59 | <.001 | 0.55 | .35–.87 | .01 |
| 8th grade through some college (n = 3207) | 6.0 | 0.64 | .49–.83 | .001 | 0.90 | .67–1.21 | .49 |
| ≥College degree (n = 1005) Ref. | 9.1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Incarcerationb | |||||||
| No (n = 4860) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 283) | 7.8 | 1.31 | .83–2.05 | .25 | |||
| Unemployed | |||||||
| No (n = 4534) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 609) | 6.4 | 1.05 | .74–1.48 | .79 | |||
| Homelessc | |||||||
| No (n = 4760) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 383) | 6.3 | 1.02 | .66–1.57 | .93 | |||
| Alcohol consumptiond | |||||||
| No (n = 2406) Ref. | 5.4 | Ref. | Ref. | Ref. | |||
| Use (n = 2382) | 6.6 | 1.24 | .97–1.57 | .08 | |||
| Abuse (n = 355) | 9.0 | 1.75 | 1.17–2.60 | .01 | |||
| Intravenous drug use ever | |||||||
| No (n = 4942) Ref. | 6.0 | Ref. | Ref. | Ref. | |||
| Yes (n = 201) | 10.0 | 1.73 | 1.07–2.78 | .02 | |||
| Cirrhosis, by self-report | |||||||
| No (n = 4949) Ref. | 6.0 | Ref. | Ref. | Ref. | |||
| Yes (n = 194) | 10.8 | 1.91 | 1.20–3.05 | .01 | |||
| Current smokere | |||||||
| No (n = 3642) Ref. | 5.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 1501) | 7.1 | 1.26 | .99–1.60 | .07 | |||
| Methadone treatment | |||||||
| No (n = 5035) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 108) | 8.3 | 1.40 | .70–2.79 | .34 | |||
| Enrollment sitef | .01 | .02 | |||||
| Concomitant medications | |||||||
| No (n = 2566) Ref. | 3.6 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 2577) | 8.7 | 2.53 | 1.98–3.25 | <.001 | 1.86 | 1.42–2.44 | <.001 |
| LTBI for contact tuberculosis | |||||||
| No (n = 1769) Ref. | 7.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 3374) | 5.7 | 0.79 | .63–1.0 | .05 | |||
| LTBI for tuberculin skin test converter | |||||||
| No (n = 3292) Ref. | 5.7 | Ref. | Ref. | Ref. | |||
| Yes (n = 1851) | 6.9 | 1.22 | .97–1.54 | .09 | |||
| LTBI for fibrosis | |||||||
| No (n = 4985) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 158) | 7.0 | 1.14 | .61–2.13 | .67 | |||
| LTBI for HIV infection | |||||||
| No (n = 5026) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 117) | 4.3 | 0.68 | .27–1.66 | .39 | |||
| Having missed an early visitg | |||||||
| No (n = 4936) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 207) | 4.8 | 0.77 | .40–1.46 | .42 | |||
| Interaction terms | |||||||
| Ethnicity × regimen | |||||||
| 3HP-DOT: Non-Hispanic vs Hispanic | 3.0 | 1.93–4.69 | <.001 | ||||
| 9H-SAT: Non-Hispanic vs Hispanic | 1.44 | .92–2.26 | .11 | ||||
| Cirrhosis, by self-report × regimen | |||||||
| 3HP-DOT: Cirrhosis: yes vs no | 0.80 | .35–1.83 | .60 | ||||
| 9H-SAT-: Cirrhosis: yes vs no | 2.93 | 1.52–5.65 | .001 | ||||
| Alcohol × sex | |||||||
| Male | |||||||
| Users vs no alcohol | 1.64 | 1.05–2.57 | .03 | ||||
| Abusers vs no alcohol | 2.15 | 1.19–3.89 | .01 | ||||
| Female | |||||||
| Users vs no alcohol | 0.84 | .59–1.19 | .32 | ||||
| Abusers vs no alcohol | 0.70 | .26–1.92 | .49 | ||||
Regimen factor is also part of certain interaction terms. Blank cells indicate not applicable or not tested. Use of a less stringent entry criterion (P ≤ .2) produced the same final model.
Abbreviations: 3HP-DOT, 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg); 9H-SAT, 9 months of daily self-administered isoniazid (maximum dose, 300 mg); CI, confidence interval; HIV, human immunodeficiency virus; LTBI, latent tuberculosis infection; NCT, noncompletion of treatment; OR, odds ratio; Ref., reference.
a Includes North American Indian and other participants in the United States and Canada.
b History of living in a correctional institution for ≥1 month before enrollment.
c History of homelessness or living in a shelter or single-room occupancy for ≥6 months before enrollment.
d Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ≥2 on the Cut down, Annoyed, Guilty, and Eye-opener questionnaire [9].
e Smoking was ascertained on the basis of the patient's statement of whether smoking was current at time of enrollment.
f Enrollment site was analyzed as the random effect variable of a generalized linear mixed model. Two-tailed P value was calculated on the basis of the z score (estimate/standard error).
g Missing ≥1 of the first 3 directly observed therapy (DOT) sessions for the 3HP regimen or ≥1 of the 3 monthly clinic visits for the 9H regimen, followed by a DOT or a monthly visit, respectively. The variable includes those who did not receive any study dose (n = 148). In the univariate analysis, the need for an interpreter among non-US- or non-Canadian-born participants (n = 3133 [use = 1355; no use = 1778]) resulted in statistical significance (OR, 0.55; 95% CI, .40–.77; P = .001) among participants who did not complete treatment attributed to an adverse event.
Univariate and Multivariate Analysis of Factors Associated With Noncompletion of Latent Tuberculosis Infection Treatment Attributed to an Adverse Event (n = 317) Compared With Those Who Completed Treatment (n = 4826)
| Characteristic . | Noncompletion of Treatment Attributed to an Adverse Event (NCT-AE) (n = 5143 [317 + 4826]) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Univariate . | Multivariate . | |||||
| NCT (%) . | OR . | 95% CI . | P Value . | Adjusted OR . | 95% CI . | P Value . | |
| Regimen | |||||||
| 3HP-DOT (n = 2844) Ref. | 6.4 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9H-SAT (n = 2299) | 5.9 | 1.1 | .87–1.38 | .43 | 1.73 | 1.01–2.95 | .23 |
| Age (y; median, 38) | |||||||
| <38 (n = 2534) | 4.5 | 0.56 | .44–.71 | <.001 | 0.61 | .47–.79 | <.001 |
| ≥38 (n = 2609) Ref. | 7.8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Sex | |||||||
| Female (n = 2282) Ref. | 7.7 | Ref. | Ref. | Ref. | |||
| Male (n = 2861) | 5.0 | 0.63 | .50–.79 | <.001 | |||
| Race | |||||||
| White (n = 2854) Ref. | 6.9 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black (n = 1315) | 4.1 | 0.58 | .43–.79 | .001 | 0.33 | .23–.47 | <.001 |
| Asian (n = 765) | 6.0 | 0.87 | .62–1.21 | .40 | 0.52 | .35–.78 | .001 |
| Othera (n = 209) | 10.1 | 1.52 | .94–2.43 | .09 | 0.88 | .51–1.53 | .65 |
| Ethnicity | |||||||
| Non-Hispanic (n = 3013) Ref. | 7.5 | Ref. | Ref. | Ref. | |||
| Hispanic (n = 2130) | 4.2 | 0.54 | .42–.70 | <.001 | |||
| HIV status | |||||||
| Negative (n = 2518) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Positive (n = 121) | 4.1 | 0.66 | .27–1.64 | .37 | |||
| Unknown (n = 2504) | 6.3 | 1.03 | .82–1.30 | .78 | |||
| Country of origin | |||||||
| Non-US (n = 3133) Ref. | 5.5 | Ref. | Ref. | Ref. | |||
| United States (n = 2010) | 7.2 | 1.34 | 1.07–1.68 | .01 | |||
| Body mass index | |||||||
| Underweight (n = 85) | 4.7 | 0.67 | .24–1.86 | .44 | |||
| Normal (n = 1464) Ref. | 6.9 | Ref. | Ref. | Ref. | |||
| Overweight (n = 1827) | 6.5 | 0.94 | .71–1.24 | .66 | |||
| Obese (n = 1767) | 5.3 | 0.75 | .56–1.0 | .05 | |||
| Education | |||||||
| ≤8th grade (n = 931) | 3.8 | 0.39 | .26–.59 | <.001 | 0.55 | .35–.87 | .01 |
| 8th grade through some college (n = 3207) | 6.0 | 0.64 | .49–.83 | .001 | 0.90 | .67–1.21 | .49 |
| ≥College degree (n = 1005) Ref. | 9.1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Incarcerationb | |||||||
| No (n = 4860) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 283) | 7.8 | 1.31 | .83–2.05 | .25 | |||
| Unemployed | |||||||
| No (n = 4534) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 609) | 6.4 | 1.05 | .74–1.48 | .79 | |||
| Homelessc | |||||||
| No (n = 4760) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 383) | 6.3 | 1.02 | .66–1.57 | .93 | |||
| Alcohol consumptiond | |||||||
| No (n = 2406) Ref. | 5.4 | Ref. | Ref. | Ref. | |||
| Use (n = 2382) | 6.6 | 1.24 | .97–1.57 | .08 | |||
| Abuse (n = 355) | 9.0 | 1.75 | 1.17–2.60 | .01 | |||
| Intravenous drug use ever | |||||||
| No (n = 4942) Ref. | 6.0 | Ref. | Ref. | Ref. | |||
| Yes (n = 201) | 10.0 | 1.73 | 1.07–2.78 | .02 | |||
| Cirrhosis, by self-report | |||||||
| No (n = 4949) Ref. | 6.0 | Ref. | Ref. | Ref. | |||
| Yes (n = 194) | 10.8 | 1.91 | 1.20–3.05 | .01 | |||
| Current smokere | |||||||
| No (n = 3642) Ref. | 5.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 1501) | 7.1 | 1.26 | .99–1.60 | .07 | |||
| Methadone treatment | |||||||
| No (n = 5035) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 108) | 8.3 | 1.40 | .70–2.79 | .34 | |||
| Enrollment sitef | .01 | .02 | |||||
| Concomitant medications | |||||||
| No (n = 2566) Ref. | 3.6 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 2577) | 8.7 | 2.53 | 1.98–3.25 | <.001 | 1.86 | 1.42–2.44 | <.001 |
| LTBI for contact tuberculosis | |||||||
| No (n = 1769) Ref. | 7.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 3374) | 5.7 | 0.79 | .63–1.0 | .05 | |||
| LTBI for tuberculin skin test converter | |||||||
| No (n = 3292) Ref. | 5.7 | Ref. | Ref. | Ref. | |||
| Yes (n = 1851) | 6.9 | 1.22 | .97–1.54 | .09 | |||
| LTBI for fibrosis | |||||||
| No (n = 4985) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 158) | 7.0 | 1.14 | .61–2.13 | .67 | |||
| LTBI for HIV infection | |||||||
| No (n = 5026) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 117) | 4.3 | 0.68 | .27–1.66 | .39 | |||
| Having missed an early visitg | |||||||
| No (n = 4936) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 207) | 4.8 | 0.77 | .40–1.46 | .42 | |||
| Interaction terms | |||||||
| Ethnicity × regimen | |||||||
| 3HP-DOT: Non-Hispanic vs Hispanic | 3.0 | 1.93–4.69 | <.001 | ||||
| 9H-SAT: Non-Hispanic vs Hispanic | 1.44 | .92–2.26 | .11 | ||||
| Cirrhosis, by self-report × regimen | |||||||
| 3HP-DOT: Cirrhosis: yes vs no | 0.80 | .35–1.83 | .60 | ||||
| 9H-SAT-: Cirrhosis: yes vs no | 2.93 | 1.52–5.65 | .001 | ||||
| Alcohol × sex | |||||||
| Male | |||||||
| Users vs no alcohol | 1.64 | 1.05–2.57 | .03 | ||||
| Abusers vs no alcohol | 2.15 | 1.19–3.89 | .01 | ||||
| Female | |||||||
| Users vs no alcohol | 0.84 | .59–1.19 | .32 | ||||
| Abusers vs no alcohol | 0.70 | .26–1.92 | .49 | ||||
| Characteristic . | Noncompletion of Treatment Attributed to an Adverse Event (NCT-AE) (n = 5143 [317 + 4826]) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Univariate . | Multivariate . | |||||
| NCT (%) . | OR . | 95% CI . | P Value . | Adjusted OR . | 95% CI . | P Value . | |
| Regimen | |||||||
| 3HP-DOT (n = 2844) Ref. | 6.4 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9H-SAT (n = 2299) | 5.9 | 1.1 | .87–1.38 | .43 | 1.73 | 1.01–2.95 | .23 |
| Age (y; median, 38) | |||||||
| <38 (n = 2534) | 4.5 | 0.56 | .44–.71 | <.001 | 0.61 | .47–.79 | <.001 |
| ≥38 (n = 2609) Ref. | 7.8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Sex | |||||||
| Female (n = 2282) Ref. | 7.7 | Ref. | Ref. | Ref. | |||
| Male (n = 2861) | 5.0 | 0.63 | .50–.79 | <.001 | |||
| Race | |||||||
| White (n = 2854) Ref. | 6.9 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black (n = 1315) | 4.1 | 0.58 | .43–.79 | .001 | 0.33 | .23–.47 | <.001 |
| Asian (n = 765) | 6.0 | 0.87 | .62–1.21 | .40 | 0.52 | .35–.78 | .001 |
| Othera (n = 209) | 10.1 | 1.52 | .94–2.43 | .09 | 0.88 | .51–1.53 | .65 |
| Ethnicity | |||||||
| Non-Hispanic (n = 3013) Ref. | 7.5 | Ref. | Ref. | Ref. | |||
| Hispanic (n = 2130) | 4.2 | 0.54 | .42–.70 | <.001 | |||
| HIV status | |||||||
| Negative (n = 2518) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Positive (n = 121) | 4.1 | 0.66 | .27–1.64 | .37 | |||
| Unknown (n = 2504) | 6.3 | 1.03 | .82–1.30 | .78 | |||
| Country of origin | |||||||
| Non-US (n = 3133) Ref. | 5.5 | Ref. | Ref. | Ref. | |||
| United States (n = 2010) | 7.2 | 1.34 | 1.07–1.68 | .01 | |||
| Body mass index | |||||||
| Underweight (n = 85) | 4.7 | 0.67 | .24–1.86 | .44 | |||
| Normal (n = 1464) Ref. | 6.9 | Ref. | Ref. | Ref. | |||
| Overweight (n = 1827) | 6.5 | 0.94 | .71–1.24 | .66 | |||
| Obese (n = 1767) | 5.3 | 0.75 | .56–1.0 | .05 | |||
| Education | |||||||
| ≤8th grade (n = 931) | 3.8 | 0.39 | .26–.59 | <.001 | 0.55 | .35–.87 | .01 |
| 8th grade through some college (n = 3207) | 6.0 | 0.64 | .49–.83 | .001 | 0.90 | .67–1.21 | .49 |
| ≥College degree (n = 1005) Ref. | 9.1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Incarcerationb | |||||||
| No (n = 4860) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 283) | 7.8 | 1.31 | .83–2.05 | .25 | |||
| Unemployed | |||||||
| No (n = 4534) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 609) | 6.4 | 1.05 | .74–1.48 | .79 | |||
| Homelessc | |||||||
| No (n = 4760) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 383) | 6.3 | 1.02 | .66–1.57 | .93 | |||
| Alcohol consumptiond | |||||||
| No (n = 2406) Ref. | 5.4 | Ref. | Ref. | Ref. | |||
| Use (n = 2382) | 6.6 | 1.24 | .97–1.57 | .08 | |||
| Abuse (n = 355) | 9.0 | 1.75 | 1.17–2.60 | .01 | |||
| Intravenous drug use ever | |||||||
| No (n = 4942) Ref. | 6.0 | Ref. | Ref. | Ref. | |||
| Yes (n = 201) | 10.0 | 1.73 | 1.07–2.78 | .02 | |||
| Cirrhosis, by self-report | |||||||
| No (n = 4949) Ref. | 6.0 | Ref. | Ref. | Ref. | |||
| Yes (n = 194) | 10.8 | 1.91 | 1.20–3.05 | .01 | |||
| Current smokere | |||||||
| No (n = 3642) Ref. | 5.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 1501) | 7.1 | 1.26 | .99–1.60 | .07 | |||
| Methadone treatment | |||||||
| No (n = 5035) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 108) | 8.3 | 1.40 | .70–2.79 | .34 | |||
| Enrollment sitef | .01 | .02 | |||||
| Concomitant medications | |||||||
| No (n = 2566) Ref. | 3.6 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 2577) | 8.7 | 2.53 | 1.98–3.25 | <.001 | 1.86 | 1.42–2.44 | <.001 |
| LTBI for contact tuberculosis | |||||||
| No (n = 1769) Ref. | 7.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 3374) | 5.7 | 0.79 | .63–1.0 | .05 | |||
| LTBI for tuberculin skin test converter | |||||||
| No (n = 3292) Ref. | 5.7 | Ref. | Ref. | Ref. | |||
| Yes (n = 1851) | 6.9 | 1.22 | .97–1.54 | .09 | |||
| LTBI for fibrosis | |||||||
| No (n = 4985) Ref. | 6.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 158) | 7.0 | 1.14 | .61–2.13 | .67 | |||
| LTBI for HIV infection | |||||||
| No (n = 5026) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 117) | 4.3 | 0.68 | .27–1.66 | .39 | |||
| Having missed an early visitg | |||||||
| No (n = 4936) Ref. | 6.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 207) | 4.8 | 0.77 | .40–1.46 | .42 | |||
| Interaction terms | |||||||
| Ethnicity × regimen | |||||||
| 3HP-DOT: Non-Hispanic vs Hispanic | 3.0 | 1.93–4.69 | <.001 | ||||
| 9H-SAT: Non-Hispanic vs Hispanic | 1.44 | .92–2.26 | .11 | ||||
| Cirrhosis, by self-report × regimen | |||||||
| 3HP-DOT: Cirrhosis: yes vs no | 0.80 | .35–1.83 | .60 | ||||
| 9H-SAT-: Cirrhosis: yes vs no | 2.93 | 1.52–5.65 | .001 | ||||
| Alcohol × sex | |||||||
| Male | |||||||
| Users vs no alcohol | 1.64 | 1.05–2.57 | .03 | ||||
| Abusers vs no alcohol | 2.15 | 1.19–3.89 | .01 | ||||
| Female | |||||||
| Users vs no alcohol | 0.84 | .59–1.19 | .32 | ||||
| Abusers vs no alcohol | 0.70 | .26–1.92 | .49 | ||||
Regimen factor is also part of certain interaction terms. Blank cells indicate not applicable or not tested. Use of a less stringent entry criterion (P ≤ .2) produced the same final model.
Abbreviations: 3HP-DOT, 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg); 9H-SAT, 9 months of daily self-administered isoniazid (maximum dose, 300 mg); CI, confidence interval; HIV, human immunodeficiency virus; LTBI, latent tuberculosis infection; NCT, noncompletion of treatment; OR, odds ratio; Ref., reference.
a Includes North American Indian and other participants in the United States and Canada.
b History of living in a correctional institution for ≥1 month before enrollment.
c History of homelessness or living in a shelter or single-room occupancy for ≥6 months before enrollment.
d Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ≥2 on the Cut down, Annoyed, Guilty, and Eye-opener questionnaire [9].
e Smoking was ascertained on the basis of the patient's statement of whether smoking was current at time of enrollment.
f Enrollment site was analyzed as the random effect variable of a generalized linear mixed model. Two-tailed P value was calculated on the basis of the z score (estimate/standard error).
g Missing ≥1 of the first 3 directly observed therapy (DOT) sessions for the 3HP regimen or ≥1 of the 3 monthly clinic visits for the 9H regimen, followed by a DOT or a monthly visit, respectively. The variable includes those who did not receive any study dose (n = 148). In the univariate analysis, the need for an interpreter among non-US- or non-Canadian-born participants (n = 3133 [use = 1355; no use = 1778]) resulted in statistical significance (OR, 0.55; 95% CI, .40–.77; P = .001) among participants who did not complete treatment attributed to an adverse event.
For NCT-O among 5915 adults (Figure 1), 1089 discontinued treatment for reasons other than an AE. We determined that the odds of NCT-O were 3 times higher among participants receiving 9H-SAT compared with 3HP-DOT, after controlling for other factors (P = .02). In the same model, being Asian was associated with increased odds of completion, whereas alcohol abuse, use ever of intravenous drugs, and current smoking at enrollment were independently associated with NCT-O. The random effect of site was statistically significant, indicating that the odds of NCT-O varied across sites, even after adjusting for other factors. The odds of NCT-O among participants receiving 9H-SAT were 4.8 times higher among those who missed an early visit compared with those who did not (P < .001) and were 1.7 times higher among those receiving 3HP-DOT (P = .05). In the 9H-SAT regimen, the odds of NCT-O were 2 times higher among men compared with women (P < .01). In the 9H-SAT regimen, the odds of NCT-O were 36% higher among participants aged <38 years compared with older participants (P = .001). Among men, the odds of NCT-O were almost 92% higher among participants with a history of incarceration compared with those without incarceration history (P < .001; Table 2).
Univariate and Multivariate Analysis of Factors Associated With Noncompletion of Latent Tuberculosis Infection Treatment Attributed to Reasons Other Than an Adverse Event (n = 1089) Compared With Those Who Completed Treatment (n = 4826)
| Characteristic . | Noncompletion of Treatment, Reasons Other Than an Adverse Event (NCT-O) (n = 5915 [1089 + 4826]) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Univariate . | Multivariate . | |||||
| NCT (%) . | OR . | 95% CI . | P Value . | Adjusted OR . | 95% CI . | P Value . | |
| Regimen | |||||||
| 3HP-DOT (n = 3048) Ref. | 12.7 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9H-SAT (n = 2867) | 24.5 | 2.24 | 1.95–2.57 | <.001 | 3.17 | 2.33–4.31 | .02 |
| Age (y; median, 38) | |||||||
| < 38 (n = 2989) | 19.0 | 1.09 | .95–1.24 | .21 | |||
| ≥38 (n = 2926) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Sex | |||||||
| Female (n = 2563) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Male (n = 3352) | 18.9 | 1.08 | .94–1.23 | .28 | |||
| Race | |||||||
| White (n = 3273) Ref. | 18.8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black (n = 1575) | 19.9 | 1.08 | .93–1.25 | .34 | 1.04 | .87–1.24 | .70 |
| Asian (n = 834) | 13.8 | 0.69 | .56–.86 | .001 | 0.69 | .54–.88 | .003 |
| Othera (n = 233) | 19.3 | 1.04 | .74–1.45 | .84 | 1.03 | .70–1.51 | .89 |
| Ethnicity | |||||||
| Non-Hispanic (n = 3403) Ref. | 18.1 | Ref. | Ref. | Ref. | |||
| Hispanic (n = 2512) | 18.8 | 1.04 | .91–1.19 | .52 | |||
| HIV status | |||||||
| Negative (n = 2932) Ref. | 19.4 | Ref. | Ref. | Ref. | |||
| Positive (n = 143) | 18.9 | 0.97 | .63–1.49 | .88 | |||
| Unknown (n = 2840) | 17.4 | 0.88 | .77–1.00 | .05 | |||
| Country of origin | |||||||
| Non-US (n = 3570) Ref. | 17.1 | Ref. | Ref. | Ref. | |||
| United States (n = 2345) | 20.5 | 1.25 | 1.10–1.43 | .001 | |||
| Body mass index | |||||||
| Underweight (n = 100) | 19.0 | 1.0 | .60–1.67 | .99 | |||
| Normal (n = 1684) Ref. | 19.1 | Ref. | Ref. | Ref. | |||
| Overweight (n = 2102) | 18.7 | 0.98 | .83–1.15 | .80 | |||
| Obese (n = 2029) | 17.5 | 0.90 | .76–1.06 | .22 | |||
| Education | |||||||
| ≤8th grade (n = 1093) | 18.0 | 1.31 | 1.04–1.65 | .02 | |||
| 8th grade through some college (n = 3755) | 19.7 | 1.46 | 1.21–1.77 | <.001 | |||
| ≥College degree (n = 1067) Ref. | 14.3 | Ref. | Ref. | Ref. | |||
| Incarcerationb | |||||||
| No (n = 5544) Ref. | 17.7 | Ref. | Ref. | Ref. | |||
| Yes (n = 371) | 29.7 | 1.97 | 1.56–2.48 | <.001 | |||
| Unemployed | |||||||
| No (n = 5179) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 736) | 22.6 | 1.34 | 1.11–1.62 | .002 | |||
| Homelessc | |||||||
| No (n = 5434) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 481) | 25.4 | 1.57 | 1.26–1.95 | <.001 | |||
| Alcohol consumptiond | |||||||
| No (n = 2722) Ref. | 16.4 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Use (n = 2742) | 18.8 | 1.19 | 1.03–1.36 | .02 | 1.08 | .92–1.28 | .33 |
| Abuse (n = 451) | 28.4 | 2.03 | 1.61–2.55 | <.001 | 1.73 | 1.30–2.30 | <.001 |
| Intravenous drug use ever | |||||||
| No (n = 5668) Ref. | 18.1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 247) | 26.7 | 1.66 | 1.24–2.21 | .001 | 1.44 | 1.03–2.03 | .04 |
| Cirrhosis, by self-report | |||||||
| No (n = 5684) Ref. | 18.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 231) | 25.1 | 1.51 | 1.12–2.05 | .01 | |||
| Current smokere | |||||||
| No (n = 4112) Ref. | 16.5 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 1803) | 22.7 | 1.48 | 1.29–1.70 | <.001 | 1.26 | 1.07–1.49 | .01 |
| Methadone treatment | |||||||
| No (n = 5787) Ref. | 18.3 | Ref. | Ref. | Ref. | |||
| Yes (n = 128) | 22.7 | 1.31 | .86–1.99 | .21 | |||
| Enrollment sitef | .01 | .01 | |||||
| Having missed an early visitg | |||||||
| No (n = 5521) Ref. | 16.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 394) | 50.0 | 5.19 | 4.21–6.40 | <.001 | |||
| Interaction terms | |||||||
| Sex × regimen | |||||||
| 3HP-DOT: Males vs females | 1.48 | .86–2.56 | .15 | ||||
| 9H-SAT: Males vs females | 2.13 | 1.25–3.64 | .01 | ||||
| Age (y) × regimen | |||||||
| 3HP-DOT: <38 vs ≥38 | 1.01 | .81–1.26 | .96 | ||||
| 9H-SAT: <38 vs ≥38 | 1.36 | 1.13–1.64 | .001 | ||||
| Having missed an early visit × regimen | |||||||
| 3HP-DOT: Missing early visit, yes vs no | 1.72 | .99–2.99 | .05 | ||||
| 9H-SAT: Missing early visit, yes vs no | 4.82 | 3.74–6.21 | <.001 | ||||
| Incarceration × sex | |||||||
| Males: Incarcerated, yes vs no | 1.92 | 1.44–2.57 | <.001 | ||||
| Females: Incarcerated, yes vs no | 0.44 | .16–1.21 | .11 | ||||
| Characteristic . | Noncompletion of Treatment, Reasons Other Than an Adverse Event (NCT-O) (n = 5915 [1089 + 4826]) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Univariate . | Multivariate . | |||||
| NCT (%) . | OR . | 95% CI . | P Value . | Adjusted OR . | 95% CI . | P Value . | |
| Regimen | |||||||
| 3HP-DOT (n = 3048) Ref. | 12.7 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9H-SAT (n = 2867) | 24.5 | 2.24 | 1.95–2.57 | <.001 | 3.17 | 2.33–4.31 | .02 |
| Age (y; median, 38) | |||||||
| < 38 (n = 2989) | 19.0 | 1.09 | .95–1.24 | .21 | |||
| ≥38 (n = 2926) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Sex | |||||||
| Female (n = 2563) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Male (n = 3352) | 18.9 | 1.08 | .94–1.23 | .28 | |||
| Race | |||||||
| White (n = 3273) Ref. | 18.8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black (n = 1575) | 19.9 | 1.08 | .93–1.25 | .34 | 1.04 | .87–1.24 | .70 |
| Asian (n = 834) | 13.8 | 0.69 | .56–.86 | .001 | 0.69 | .54–.88 | .003 |
| Othera (n = 233) | 19.3 | 1.04 | .74–1.45 | .84 | 1.03 | .70–1.51 | .89 |
| Ethnicity | |||||||
| Non-Hispanic (n = 3403) Ref. | 18.1 | Ref. | Ref. | Ref. | |||
| Hispanic (n = 2512) | 18.8 | 1.04 | .91–1.19 | .52 | |||
| HIV status | |||||||
| Negative (n = 2932) Ref. | 19.4 | Ref. | Ref. | Ref. | |||
| Positive (n = 143) | 18.9 | 0.97 | .63–1.49 | .88 | |||
| Unknown (n = 2840) | 17.4 | 0.88 | .77–1.00 | .05 | |||
| Country of origin | |||||||
| Non-US (n = 3570) Ref. | 17.1 | Ref. | Ref. | Ref. | |||
| United States (n = 2345) | 20.5 | 1.25 | 1.10–1.43 | .001 | |||
| Body mass index | |||||||
| Underweight (n = 100) | 19.0 | 1.0 | .60–1.67 | .99 | |||
| Normal (n = 1684) Ref. | 19.1 | Ref. | Ref. | Ref. | |||
| Overweight (n = 2102) | 18.7 | 0.98 | .83–1.15 | .80 | |||
| Obese (n = 2029) | 17.5 | 0.90 | .76–1.06 | .22 | |||
| Education | |||||||
| ≤8th grade (n = 1093) | 18.0 | 1.31 | 1.04–1.65 | .02 | |||
| 8th grade through some college (n = 3755) | 19.7 | 1.46 | 1.21–1.77 | <.001 | |||
| ≥College degree (n = 1067) Ref. | 14.3 | Ref. | Ref. | Ref. | |||
| Incarcerationb | |||||||
| No (n = 5544) Ref. | 17.7 | Ref. | Ref. | Ref. | |||
| Yes (n = 371) | 29.7 | 1.97 | 1.56–2.48 | <.001 | |||
| Unemployed | |||||||
| No (n = 5179) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 736) | 22.6 | 1.34 | 1.11–1.62 | .002 | |||
| Homelessc | |||||||
| No (n = 5434) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 481) | 25.4 | 1.57 | 1.26–1.95 | <.001 | |||
| Alcohol consumptiond | |||||||
| No (n = 2722) Ref. | 16.4 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Use (n = 2742) | 18.8 | 1.19 | 1.03–1.36 | .02 | 1.08 | .92–1.28 | .33 |
| Abuse (n = 451) | 28.4 | 2.03 | 1.61–2.55 | <.001 | 1.73 | 1.30–2.30 | <.001 |
| Intravenous drug use ever | |||||||
| No (n = 5668) Ref. | 18.1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 247) | 26.7 | 1.66 | 1.24–2.21 | .001 | 1.44 | 1.03–2.03 | .04 |
| Cirrhosis, by self-report | |||||||
| No (n = 5684) Ref. | 18.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 231) | 25.1 | 1.51 | 1.12–2.05 | .01 | |||
| Current smokere | |||||||
| No (n = 4112) Ref. | 16.5 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 1803) | 22.7 | 1.48 | 1.29–1.70 | <.001 | 1.26 | 1.07–1.49 | .01 |
| Methadone treatment | |||||||
| No (n = 5787) Ref. | 18.3 | Ref. | Ref. | Ref. | |||
| Yes (n = 128) | 22.7 | 1.31 | .86–1.99 | .21 | |||
| Enrollment sitef | .01 | .01 | |||||
| Having missed an early visitg | |||||||
| No (n = 5521) Ref. | 16.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 394) | 50.0 | 5.19 | 4.21–6.40 | <.001 | |||
| Interaction terms | |||||||
| Sex × regimen | |||||||
| 3HP-DOT: Males vs females | 1.48 | .86–2.56 | .15 | ||||
| 9H-SAT: Males vs females | 2.13 | 1.25–3.64 | .01 | ||||
| Age (y) × regimen | |||||||
| 3HP-DOT: <38 vs ≥38 | 1.01 | .81–1.26 | .96 | ||||
| 9H-SAT: <38 vs ≥38 | 1.36 | 1.13–1.64 | .001 | ||||
| Having missed an early visit × regimen | |||||||
| 3HP-DOT: Missing early visit, yes vs no | 1.72 | .99–2.99 | .05 | ||||
| 9H-SAT: Missing early visit, yes vs no | 4.82 | 3.74–6.21 | <.001 | ||||
| Incarceration × sex | |||||||
| Males: Incarcerated, yes vs no | 1.92 | 1.44–2.57 | <.001 | ||||
| Females: Incarcerated, yes vs no | 0.44 | .16–1.21 | .11 | ||||
Regimen factor is also part of certain interaction terms. Blank cells indicate not applicable or not tested. Use of a less stringent entry criterion (P ≤ .2) did not produce a substantively different final model.
Abbreviations: 3HP-DOT, 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg); 9H-SAT, 9 months of daily self-administered isoniazid (maximum dose, 300 mg); CI, confidence interval; HIV, human immunodeficiency virus; NCT, noncompletion of treatment; OR, odds ratio; Ref., reference.
a Includes North American Indian and other participants in the United States and Canada.
b History of living in a correctional institution for ≥1 month before enrollment.
c History of homelessness or living in a shelter or single-room occupancy for ≥6 months before enrollment.
d Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ≥2 on the Cut down, Annoyed, Guilty, and Eye-opener questionnaire [9].
e Smoking was ascertained on the basis of the patient's statement of whether smoking was current at time of enrollment.
f Enrollment site was analyzed as the random effect variable of a generalized linear mixed model. Two-tailed P value was calculated on the basis of the z score (estimate/standard error).
g Missing ≥1 of the first 3 directly observed therapy (DOT) sessions for the 3HP regimen or ≥1 of the 3 monthly clinic visits for the 9H regimen, followed by a DOT or a monthly visit, respectively. The variable includes those who did not receive any study dose (n = 148). In the univariate analysis, need for an interpreter among non-US- or non-Canadian-born participants (n = 3570 [use = 1556; no use = 2014]) resulted in statistical non-significance (OR, 0.90; 95% CI, .76–1.08; P = .26) among participants who did not complete treatment attributed to reasons other than an adverse event.
Univariate and Multivariate Analysis of Factors Associated With Noncompletion of Latent Tuberculosis Infection Treatment Attributed to Reasons Other Than an Adverse Event (n = 1089) Compared With Those Who Completed Treatment (n = 4826)
| Characteristic . | Noncompletion of Treatment, Reasons Other Than an Adverse Event (NCT-O) (n = 5915 [1089 + 4826]) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Univariate . | Multivariate . | |||||
| NCT (%) . | OR . | 95% CI . | P Value . | Adjusted OR . | 95% CI . | P Value . | |
| Regimen | |||||||
| 3HP-DOT (n = 3048) Ref. | 12.7 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9H-SAT (n = 2867) | 24.5 | 2.24 | 1.95–2.57 | <.001 | 3.17 | 2.33–4.31 | .02 |
| Age (y; median, 38) | |||||||
| < 38 (n = 2989) | 19.0 | 1.09 | .95–1.24 | .21 | |||
| ≥38 (n = 2926) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Sex | |||||||
| Female (n = 2563) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Male (n = 3352) | 18.9 | 1.08 | .94–1.23 | .28 | |||
| Race | |||||||
| White (n = 3273) Ref. | 18.8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black (n = 1575) | 19.9 | 1.08 | .93–1.25 | .34 | 1.04 | .87–1.24 | .70 |
| Asian (n = 834) | 13.8 | 0.69 | .56–.86 | .001 | 0.69 | .54–.88 | .003 |
| Othera (n = 233) | 19.3 | 1.04 | .74–1.45 | .84 | 1.03 | .70–1.51 | .89 |
| Ethnicity | |||||||
| Non-Hispanic (n = 3403) Ref. | 18.1 | Ref. | Ref. | Ref. | |||
| Hispanic (n = 2512) | 18.8 | 1.04 | .91–1.19 | .52 | |||
| HIV status | |||||||
| Negative (n = 2932) Ref. | 19.4 | Ref. | Ref. | Ref. | |||
| Positive (n = 143) | 18.9 | 0.97 | .63–1.49 | .88 | |||
| Unknown (n = 2840) | 17.4 | 0.88 | .77–1.00 | .05 | |||
| Country of origin | |||||||
| Non-US (n = 3570) Ref. | 17.1 | Ref. | Ref. | Ref. | |||
| United States (n = 2345) | 20.5 | 1.25 | 1.10–1.43 | .001 | |||
| Body mass index | |||||||
| Underweight (n = 100) | 19.0 | 1.0 | .60–1.67 | .99 | |||
| Normal (n = 1684) Ref. | 19.1 | Ref. | Ref. | Ref. | |||
| Overweight (n = 2102) | 18.7 | 0.98 | .83–1.15 | .80 | |||
| Obese (n = 2029) | 17.5 | 0.90 | .76–1.06 | .22 | |||
| Education | |||||||
| ≤8th grade (n = 1093) | 18.0 | 1.31 | 1.04–1.65 | .02 | |||
| 8th grade through some college (n = 3755) | 19.7 | 1.46 | 1.21–1.77 | <.001 | |||
| ≥College degree (n = 1067) Ref. | 14.3 | Ref. | Ref. | Ref. | |||
| Incarcerationb | |||||||
| No (n = 5544) Ref. | 17.7 | Ref. | Ref. | Ref. | |||
| Yes (n = 371) | 29.7 | 1.97 | 1.56–2.48 | <.001 | |||
| Unemployed | |||||||
| No (n = 5179) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 736) | 22.6 | 1.34 | 1.11–1.62 | .002 | |||
| Homelessc | |||||||
| No (n = 5434) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 481) | 25.4 | 1.57 | 1.26–1.95 | <.001 | |||
| Alcohol consumptiond | |||||||
| No (n = 2722) Ref. | 16.4 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Use (n = 2742) | 18.8 | 1.19 | 1.03–1.36 | .02 | 1.08 | .92–1.28 | .33 |
| Abuse (n = 451) | 28.4 | 2.03 | 1.61–2.55 | <.001 | 1.73 | 1.30–2.30 | <.001 |
| Intravenous drug use ever | |||||||
| No (n = 5668) Ref. | 18.1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 247) | 26.7 | 1.66 | 1.24–2.21 | .001 | 1.44 | 1.03–2.03 | .04 |
| Cirrhosis, by self-report | |||||||
| No (n = 5684) Ref. | 18.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 231) | 25.1 | 1.51 | 1.12–2.05 | .01 | |||
| Current smokere | |||||||
| No (n = 4112) Ref. | 16.5 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 1803) | 22.7 | 1.48 | 1.29–1.70 | <.001 | 1.26 | 1.07–1.49 | .01 |
| Methadone treatment | |||||||
| No (n = 5787) Ref. | 18.3 | Ref. | Ref. | Ref. | |||
| Yes (n = 128) | 22.7 | 1.31 | .86–1.99 | .21 | |||
| Enrollment sitef | .01 | .01 | |||||
| Having missed an early visitg | |||||||
| No (n = 5521) Ref. | 16.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 394) | 50.0 | 5.19 | 4.21–6.40 | <.001 | |||
| Interaction terms | |||||||
| Sex × regimen | |||||||
| 3HP-DOT: Males vs females | 1.48 | .86–2.56 | .15 | ||||
| 9H-SAT: Males vs females | 2.13 | 1.25–3.64 | .01 | ||||
| Age (y) × regimen | |||||||
| 3HP-DOT: <38 vs ≥38 | 1.01 | .81–1.26 | .96 | ||||
| 9H-SAT: <38 vs ≥38 | 1.36 | 1.13–1.64 | .001 | ||||
| Having missed an early visit × regimen | |||||||
| 3HP-DOT: Missing early visit, yes vs no | 1.72 | .99–2.99 | .05 | ||||
| 9H-SAT: Missing early visit, yes vs no | 4.82 | 3.74–6.21 | <.001 | ||||
| Incarceration × sex | |||||||
| Males: Incarcerated, yes vs no | 1.92 | 1.44–2.57 | <.001 | ||||
| Females: Incarcerated, yes vs no | 0.44 | .16–1.21 | .11 | ||||
| Characteristic . | Noncompletion of Treatment, Reasons Other Than an Adverse Event (NCT-O) (n = 5915 [1089 + 4826]) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Univariate . | Multivariate . | |||||
| NCT (%) . | OR . | 95% CI . | P Value . | Adjusted OR . | 95% CI . | P Value . | |
| Regimen | |||||||
| 3HP-DOT (n = 3048) Ref. | 12.7 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9H-SAT (n = 2867) | 24.5 | 2.24 | 1.95–2.57 | <.001 | 3.17 | 2.33–4.31 | .02 |
| Age (y; median, 38) | |||||||
| < 38 (n = 2989) | 19.0 | 1.09 | .95–1.24 | .21 | |||
| ≥38 (n = 2926) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Sex | |||||||
| Female (n = 2563) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Male (n = 3352) | 18.9 | 1.08 | .94–1.23 | .28 | |||
| Race | |||||||
| White (n = 3273) Ref. | 18.8 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black (n = 1575) | 19.9 | 1.08 | .93–1.25 | .34 | 1.04 | .87–1.24 | .70 |
| Asian (n = 834) | 13.8 | 0.69 | .56–.86 | .001 | 0.69 | .54–.88 | .003 |
| Othera (n = 233) | 19.3 | 1.04 | .74–1.45 | .84 | 1.03 | .70–1.51 | .89 |
| Ethnicity | |||||||
| Non-Hispanic (n = 3403) Ref. | 18.1 | Ref. | Ref. | Ref. | |||
| Hispanic (n = 2512) | 18.8 | 1.04 | .91–1.19 | .52 | |||
| HIV status | |||||||
| Negative (n = 2932) Ref. | 19.4 | Ref. | Ref. | Ref. | |||
| Positive (n = 143) | 18.9 | 0.97 | .63–1.49 | .88 | |||
| Unknown (n = 2840) | 17.4 | 0.88 | .77–1.00 | .05 | |||
| Country of origin | |||||||
| Non-US (n = 3570) Ref. | 17.1 | Ref. | Ref. | Ref. | |||
| United States (n = 2345) | 20.5 | 1.25 | 1.10–1.43 | .001 | |||
| Body mass index | |||||||
| Underweight (n = 100) | 19.0 | 1.0 | .60–1.67 | .99 | |||
| Normal (n = 1684) Ref. | 19.1 | Ref. | Ref. | Ref. | |||
| Overweight (n = 2102) | 18.7 | 0.98 | .83–1.15 | .80 | |||
| Obese (n = 2029) | 17.5 | 0.90 | .76–1.06 | .22 | |||
| Education | |||||||
| ≤8th grade (n = 1093) | 18.0 | 1.31 | 1.04–1.65 | .02 | |||
| 8th grade through some college (n = 3755) | 19.7 | 1.46 | 1.21–1.77 | <.001 | |||
| ≥College degree (n = 1067) Ref. | 14.3 | Ref. | Ref. | Ref. | |||
| Incarcerationb | |||||||
| No (n = 5544) Ref. | 17.7 | Ref. | Ref. | Ref. | |||
| Yes (n = 371) | 29.7 | 1.97 | 1.56–2.48 | <.001 | |||
| Unemployed | |||||||
| No (n = 5179) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 736) | 22.6 | 1.34 | 1.11–1.62 | .002 | |||
| Homelessc | |||||||
| No (n = 5434) Ref. | 17.8 | Ref. | Ref. | Ref. | |||
| Yes (n = 481) | 25.4 | 1.57 | 1.26–1.95 | <.001 | |||
| Alcohol consumptiond | |||||||
| No (n = 2722) Ref. | 16.4 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Use (n = 2742) | 18.8 | 1.19 | 1.03–1.36 | .02 | 1.08 | .92–1.28 | .33 |
| Abuse (n = 451) | 28.4 | 2.03 | 1.61–2.55 | <.001 | 1.73 | 1.30–2.30 | <.001 |
| Intravenous drug use ever | |||||||
| No (n = 5668) Ref. | 18.1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 247) | 26.7 | 1.66 | 1.24–2.21 | .001 | 1.44 | 1.03–2.03 | .04 |
| Cirrhosis, by self-report | |||||||
| No (n = 5684) Ref. | 18.1 | Ref. | Ref. | Ref. | |||
| Yes (n = 231) | 25.1 | 1.51 | 1.12–2.05 | .01 | |||
| Current smokere | |||||||
| No (n = 4112) Ref. | 16.5 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes (n = 1803) | 22.7 | 1.48 | 1.29–1.70 | <.001 | 1.26 | 1.07–1.49 | .01 |
| Methadone treatment | |||||||
| No (n = 5787) Ref. | 18.3 | Ref. | Ref. | Ref. | |||
| Yes (n = 128) | 22.7 | 1.31 | .86–1.99 | .21 | |||
| Enrollment sitef | .01 | .01 | |||||
| Having missed an early visitg | |||||||
| No (n = 5521) Ref. | 16.2 | Ref. | Ref. | Ref. | |||
| Yes (n = 394) | 50.0 | 5.19 | 4.21–6.40 | <.001 | |||
| Interaction terms | |||||||
| Sex × regimen | |||||||
| 3HP-DOT: Males vs females | 1.48 | .86–2.56 | .15 | ||||
| 9H-SAT: Males vs females | 2.13 | 1.25–3.64 | .01 | ||||
| Age (y) × regimen | |||||||
| 3HP-DOT: <38 vs ≥38 | 1.01 | .81–1.26 | .96 | ||||
| 9H-SAT: <38 vs ≥38 | 1.36 | 1.13–1.64 | .001 | ||||
| Having missed an early visit × regimen | |||||||
| 3HP-DOT: Missing early visit, yes vs no | 1.72 | .99–2.99 | .05 | ||||
| 9H-SAT: Missing early visit, yes vs no | 4.82 | 3.74–6.21 | <.001 | ||||
| Incarceration × sex | |||||||
| Males: Incarcerated, yes vs no | 1.92 | 1.44–2.57 | <.001 | ||||
| Females: Incarcerated, yes vs no | 0.44 | .16–1.21 | .11 | ||||
Regimen factor is also part of certain interaction terms. Blank cells indicate not applicable or not tested. Use of a less stringent entry criterion (P ≤ .2) did not produce a substantively different final model.
Abbreviations: 3HP-DOT, 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg); 9H-SAT, 9 months of daily self-administered isoniazid (maximum dose, 300 mg); CI, confidence interval; HIV, human immunodeficiency virus; NCT, noncompletion of treatment; OR, odds ratio; Ref., reference.
a Includes North American Indian and other participants in the United States and Canada.
b History of living in a correctional institution for ≥1 month before enrollment.
c History of homelessness or living in a shelter or single-room occupancy for ≥6 months before enrollment.
d Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ≥2 on the Cut down, Annoyed, Guilty, and Eye-opener questionnaire [9].
e Smoking was ascertained on the basis of the patient's statement of whether smoking was current at time of enrollment.
f Enrollment site was analyzed as the random effect variable of a generalized linear mixed model. Two-tailed P value was calculated on the basis of the z score (estimate/standard error).
g Missing ≥1 of the first 3 directly observed therapy (DOT) sessions for the 3HP regimen or ≥1 of the 3 monthly clinic visits for the 9H regimen, followed by a DOT or a monthly visit, respectively. The variable includes those who did not receive any study dose (n = 148). In the univariate analysis, need for an interpreter among non-US- or non-Canadian-born participants (n = 3570 [use = 1556; no use = 2014]) resulted in statistical non-significance (OR, 0.90; 95% CI, .76–1.08; P = .26) among participants who did not complete treatment attributed to reasons other than an adverse event.
Among the 6232 participants for whom data were analyzed, treatment was not initiated for 71/3230 (2.2%) assigned to receive 3HP-DOT and for 77/3002 (2.6%) assigned to receive 9H-SAT. The most common reason for not receiving any doses in the 3HP-DOT regimen was participant refusal (1.24%), whereas the most common reason in the 9H-SAT regimen was loss of contact with the participant for >3 months after enrollment and randomization (0.87%). No statistically significant correlation existed between the proportions of enrollment by site and noninitiation of LTBI treatment (Pearson correlation coefficient = 0.21; P = .29; r2 = 0.046). Among those who received ≥1 dose (ie, initiators), the most common reasons for NCT were loss of contact for >3 months (in the 9H-SAT regimen [8.3%] only), followed by refusal to continue (5.0% in the 9H-SAT and 4.4% in the 3HP-DOT), and AE (5.6% in the 3HP-DOT and 4.5% in the 9H-SAT regimen; Table 3). The combined NCT-AE plus NCT-O proportion decreased with an increase in the number of 3HP-DOT doses taken (Pearson correlation coefficient = −0.93; P < .001; r2 = 0.86; Figure 2), whereas the combined proportion of NCT in the 9H-SAT regimen decreased substantially after the first 30-dose interval (from 4.1% to 2.3%) and remained constant thereafter (Pearson correlation coefficient = −0.68; P = .03; r2 = 0.46; Figure 3; Supplementary Table 3). The proportions of NCT by enrollment site ranged from 0% to 33% (median, 22.5%). The proportion of NCT and the number of participants enrolled by site are shown in Supplementary Table 4.
Reasons for Not Receiving Any 3 Months of Directly Observed Once-weekly Rifapentine (Maximum Dose, 900 mg) Plus Isoniazid (Maximum Dose, 900 mg) or 9 Months of Daily Self-administered Isoniazid (Maximum Dose, 300 mg) Doses and Reasons for Noncompletion of Latent Tuberculosis Infection Treatment, by Regimen Among Participants Who Received ≥1 Study Dose
| Reason . | Reasons for Not Receiving Any Study Dose . | Reasons for Noncompletion of Treatment After ≥1 Dose . | ||
|---|---|---|---|---|
| 3HP-DOT n = 3230 No. (%) . | 9H-SAT n = 3002 No. (%) . | 3HP-DOT n = 3230 No. (%) . | 9H-SAT n = 3002 No. (%) . | |
| Discontinuation attributed to an adverse event | … | … | 182 (5.6) | 135 (4.5) |
| Lost ≥3 mo before first dose | 7 (0.22) | 26 (0.87) | … | … |
| Lost ≥3 mo after the first dose during treatment | … | … | 49 (1.5) | 250 (8.3) |
| Participant refusal | 40 (1.24) | 20 (0.67) | 143 (4.4) | 150 (5.0) |
| Participant noncompliant with schedule | … | … | 19 (0.6) | 39 (1.3) |
| Participant withdrew informed consent | 22 (0.68) | 22 (0.73) | 24 (0.7) | 39 (1.3) |
| Treatment cancelled by physician | … | 2 (0.07) | 14 (0.4) | 38 (1.27) |
| Other reason | 2 (0.06)a | 7 (0.23)a | 48 (1.5)b | 109 (3.7)b |
| Missing information | … | … | 18 (0.6) | 1 (0.03) |
| Total (n = 1406) | 71 (2.2) | 77 (2.6) | 497 (15.4) | 761 (25.3) |
| Reason . | Reasons for Not Receiving Any Study Dose . | Reasons for Noncompletion of Treatment After ≥1 Dose . | ||
|---|---|---|---|---|
| 3HP-DOT n = 3230 No. (%) . | 9H-SAT n = 3002 No. (%) . | 3HP-DOT n = 3230 No. (%) . | 9H-SAT n = 3002 No. (%) . | |
| Discontinuation attributed to an adverse event | … | … | 182 (5.6) | 135 (4.5) |
| Lost ≥3 mo before first dose | 7 (0.22) | 26 (0.87) | … | … |
| Lost ≥3 mo after the first dose during treatment | … | … | 49 (1.5) | 250 (8.3) |
| Participant refusal | 40 (1.24) | 20 (0.67) | 143 (4.4) | 150 (5.0) |
| Participant noncompliant with schedule | … | … | 19 (0.6) | 39 (1.3) |
| Participant withdrew informed consent | 22 (0.68) | 22 (0.73) | 24 (0.7) | 39 (1.3) |
| Treatment cancelled by physician | … | 2 (0.07) | 14 (0.4) | 38 (1.27) |
| Other reason | 2 (0.06)a | 7 (0.23)a | 48 (1.5)b | 109 (3.7)b |
| Missing information | … | … | 18 (0.6) | 1 (0.03) |
| Total (n = 1406) | 71 (2.2) | 77 (2.6) | 497 (15.4) | 761 (25.3) |
Abbreviations: 3HP-DOT, 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg); 9H-SAT, 9 months of daily self-administered isoniazid (maximum dose, 300 mg).
a 3HP-DOT: moved out of state or to another country (n = 2). 9H-SAT: incarcerated (n = 5); moved to another county (n = 1); legal problems (n = 1).
b 3HP-DOT: moved out of state or to another country (n = 11), incarcerated (n = 10), unknown (n = 27). 9H-SAT: moved out of state or to another country (n = 32); incarcerated (n = 10); error in dosages (n = 4); terminal illness (n = 2); unknown (n = 61).
Reasons for Not Receiving Any 3 Months of Directly Observed Once-weekly Rifapentine (Maximum Dose, 900 mg) Plus Isoniazid (Maximum Dose, 900 mg) or 9 Months of Daily Self-administered Isoniazid (Maximum Dose, 300 mg) Doses and Reasons for Noncompletion of Latent Tuberculosis Infection Treatment, by Regimen Among Participants Who Received ≥1 Study Dose
| Reason . | Reasons for Not Receiving Any Study Dose . | Reasons for Noncompletion of Treatment After ≥1 Dose . | ||
|---|---|---|---|---|
| 3HP-DOT n = 3230 No. (%) . | 9H-SAT n = 3002 No. (%) . | 3HP-DOT n = 3230 No. (%) . | 9H-SAT n = 3002 No. (%) . | |
| Discontinuation attributed to an adverse event | … | … | 182 (5.6) | 135 (4.5) |
| Lost ≥3 mo before first dose | 7 (0.22) | 26 (0.87) | … | … |
| Lost ≥3 mo after the first dose during treatment | … | … | 49 (1.5) | 250 (8.3) |
| Participant refusal | 40 (1.24) | 20 (0.67) | 143 (4.4) | 150 (5.0) |
| Participant noncompliant with schedule | … | … | 19 (0.6) | 39 (1.3) |
| Participant withdrew informed consent | 22 (0.68) | 22 (0.73) | 24 (0.7) | 39 (1.3) |
| Treatment cancelled by physician | … | 2 (0.07) | 14 (0.4) | 38 (1.27) |
| Other reason | 2 (0.06)a | 7 (0.23)a | 48 (1.5)b | 109 (3.7)b |
| Missing information | … | … | 18 (0.6) | 1 (0.03) |
| Total (n = 1406) | 71 (2.2) | 77 (2.6) | 497 (15.4) | 761 (25.3) |
| Reason . | Reasons for Not Receiving Any Study Dose . | Reasons for Noncompletion of Treatment After ≥1 Dose . | ||
|---|---|---|---|---|
| 3HP-DOT n = 3230 No. (%) . | 9H-SAT n = 3002 No. (%) . | 3HP-DOT n = 3230 No. (%) . | 9H-SAT n = 3002 No. (%) . | |
| Discontinuation attributed to an adverse event | … | … | 182 (5.6) | 135 (4.5) |
| Lost ≥3 mo before first dose | 7 (0.22) | 26 (0.87) | … | … |
| Lost ≥3 mo after the first dose during treatment | … | … | 49 (1.5) | 250 (8.3) |
| Participant refusal | 40 (1.24) | 20 (0.67) | 143 (4.4) | 150 (5.0) |
| Participant noncompliant with schedule | … | … | 19 (0.6) | 39 (1.3) |
| Participant withdrew informed consent | 22 (0.68) | 22 (0.73) | 24 (0.7) | 39 (1.3) |
| Treatment cancelled by physician | … | 2 (0.07) | 14 (0.4) | 38 (1.27) |
| Other reason | 2 (0.06)a | 7 (0.23)a | 48 (1.5)b | 109 (3.7)b |
| Missing information | … | … | 18 (0.6) | 1 (0.03) |
| Total (n = 1406) | 71 (2.2) | 77 (2.6) | 497 (15.4) | 761 (25.3) |
Abbreviations: 3HP-DOT, 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg); 9H-SAT, 9 months of daily self-administered isoniazid (maximum dose, 300 mg).
a 3HP-DOT: moved out of state or to another country (n = 2). 9H-SAT: incarcerated (n = 5); moved to another county (n = 1); legal problems (n = 1).
b 3HP-DOT: moved out of state or to another country (n = 11), incarcerated (n = 10), unknown (n = 27). 9H-SAT: moved out of state or to another country (n = 32); incarcerated (n = 10); error in dosages (n = 4); terminal illness (n = 2); unknown (n = 61).
![Percentage of participants assigned to receive 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg) (3HP-DOT) who did not complete latent tuberculosis infection (LTBI) treatment attributed to an adverse event (AE) and attributed to reasons other than an AE, by maximum number of doses taken (n = 3230). This figure shows that the combined proportion of participants who did not complete LTBI treatment attributed to an AE plus the proportion of those who did not complete treatment for reasons other than an AE decreased with an increase in the number of 3HP-DOT doses taken. Pearson correlation coefficient in a linear regression model = −0.93; P < .001; r2 = 0.86. a11 doses in <10 weeks (3 [0.1%]), 11 doses in >16 weeks (7 [0.2%]); b12 doses in <10 weeks (21 [0.7%]), 12 doses in >16 weeks (27 [0.8%]). Abbreviations: NCT-AE, non-completion of LTBI treatment attributed to an adverse event; NCT-O, non-completion of LTBI treatment attributed to reasons other than an adverse event.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/62/11/10.1093_cid_ciw126/1/m_ciw12602.jpeg?Expires=1749873209&Signature=AnXVfUtB1ESIntksPY7q-aa~mxuAAgFAuxwdLs7fFYBS4T08Ytj5bbojTICTe7KvvU05B6Esy~P5yA4LsJSoqR~Fs62Vt-DVF5J7gIpCMUjd2mCKLRqNbNuKEMdDx2NRJllG~GhYcnTYzdhc9Qd0-em4asJuXDreq~9XZ1HRXJPXeoTaHxqL4xTpChxE20t38cvolWOM~NNB4kx2E2qC-vFbwkH5jv-zhWVq7idu91E0RRKVCFaqX1HMlgclizyOzYVtMniUlKYY75bmDEkcyQS17hE4GAfgpN6wXj-wGIZCht7XHTWUBmNHVQSn8XaH4naCSBYZ2mqQDYSMqjlPbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Percentage of participants assigned to receive 3 months of directly observed once-weekly rifapentine (maximum dose, 900 mg) plus isoniazid (maximum dose, 900 mg) (3HP-DOT) who did not complete latent tuberculosis infection (LTBI) treatment attributed to an adverse event (AE) and attributed to reasons other than an AE, by maximum number of doses taken (n = 3230). This figure shows that the combined proportion of participants who did not complete LTBI treatment attributed to an AE plus the proportion of those who did not complete treatment for reasons other than an AE decreased with an increase in the number of 3HP-DOT doses taken. Pearson correlation coefficient in a linear regression model = −0.93; P < .001; r2 = 0.86. a11 doses in <10 weeks (3 [0.1%]), 11 doses in >16 weeks (7 [0.2%]); b12 doses in <10 weeks (21 [0.7%]), 12 doses in >16 weeks (27 [0.8%]). Abbreviations: NCT-AE, non-completion of LTBI treatment attributed to an adverse event; NCT-O, non-completion of LTBI treatment attributed to reasons other than an adverse event.
![Percentage of participants assigned to receive 9 months of daily self-administered isoniazid (maximum dose, 300 mg) (9H-SAT) who did not complete latent tuberculosis infection treatment (LTBI) attributed to an adverse event (AE) and attributed to reasons other than an AE, by doses taken (n = 3002). This figure shows how the combined proportion of participants who did not complete LTBI treatment attributed to an AE plus the proportion of those who did not complete treatment for reasons other than an AE in the 9H-SAT regimen decreased after the first 30-dose interval and remained constant thereafter. 9H-SAT: ≥240 doses in >52 weeks (60 [2.0%]). Pearson correlation coefficient in a linear regression model = −0.68; P = .03; r2 = 0.46. Abbreviations: NCT-AE, non-completion of LTBI treatment attributed to an adverse event; NCT-O, non-completion of LTBI treatment attributed to reasons other than an adverse event.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/62/11/10.1093_cid_ciw126/1/m_ciw12603.jpeg?Expires=1749873209&Signature=HbX2gNVwbLFD0sJu6BxpSTR1emLHcl~cJXXaXol-VIh-AehjoksmW4qMh0ISs4pnuabMgXm1g1-ERNssolUZP56u8IRXyBm64v0O4kmOjtEMLFc142X4YWTc3qpfEaeVXe~JumuV3knfabw9YuOaDbTwnuRjQF3YeIiwq3mzc8Zv~khLT6ukZHNN0~Kb4oAGkh6fcdwdb7KoA~wBgqN7UfkvEKJeFJKVxf9tGmXVKS0tc3q-BXSBTgGA8J4fkI20KI27IXHFORv37UjVwruiOZ~uVoV2coFvUr9jGZHfohJcAIYZ4eA14B19shhHw5CnoHC5rX7JEHHAg6qUwCmJDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Percentage of participants assigned to receive 9 months of daily self-administered isoniazid (maximum dose, 300 mg) (9H-SAT) who did not complete latent tuberculosis infection treatment (LTBI) attributed to an adverse event (AE) and attributed to reasons other than an AE, by doses taken (n = 3002). This figure shows how the combined proportion of participants who did not complete LTBI treatment attributed to an AE plus the proportion of those who did not complete treatment for reasons other than an AE in the 9H-SAT regimen decreased after the first 30-dose interval and remained constant thereafter. 9H-SAT: ≥240 doses in >52 weeks (60 [2.0%]). Pearson correlation coefficient in a linear regression model = −0.68; P = .03; r2 = 0.46. Abbreviations: NCT-AE, non-completion of LTBI treatment attributed to an adverse event; NCT-O, non-completion of LTBI treatment attributed to reasons other than an adverse event.
Additional analysis of NCT-AE and NCT-O by year of enrollment did not reveal a clear trend when evaluated overall or stratified by regimen (Supplementary Figures 1 and 2). Among the 3002 participants assigned to receive 9 months of isoniazid, 702 (23.5%) did not receive ≥162 doses in 23–36 weeks; however, 30 of these 702 (4.3%) continued treatment and were able to complete 9 months by taking 240 of 270 doses in 35–52 weeks of 9H-SAT. Among those who met the definition of 6-month treatment completion (2300/3002 = 76.6%), 7.2% failed to complete 9 months of treatment (Supplementary Figure 3). A Kaplan–Meier curve of NCT followed for ≤90 weeks and stratified by regimen indicated a significant difference between the regimens (Wilcoxon rank sum test, P < .001; Supplementary Figure 4). Most sites provided compensation to study participants, but methods and amounts were too variable for meaningful analysis.
DISCUSSION
Our analysis of factors associated with NCT was conducted among a large sample (n = 6232) of adult trial participants. The most important finding was that among persons who discontinued for reasons other than an AE, those who missed an early visit and returned at least once later were more likely to discontinue the treatment regimen; this is consistent with results from Menzies et al who reported that adherence during the first month can be an early predictor of LTBI treatment completion [11]. The majority of discontinuations occurred early (Supplementary Table 5). NCT-AE was not associated with missing an early visit; however, NCT-O was strongly associated with missing an early visit, and this association was notably stronger for 9H-SAT (Supplementary Table 6). Our finding identifies an accessible group for targeted intervention to increase adherence. An early missed visit might be considered a “sentinel event,” indicating high risk of noncompletion, triggering additional efforts to sustain adherence. Such efforts might include contact shortly before and after each visit (eg, by telephone or with text messages), efforts to identify individual barriers to adherence, and efforts to provide individually significant incentives or enablers (eg, assistance with transportation, food coupons, cash, or shorter clinic wait times).
Overall, 6% of participants did not complete treatment because of an AE. Analysis of NCT-O demonstrated a lower noncompletion proportion for the shorter 3HP-DOT regimen (12.7%) compared with the standard 9H-SAT (24.5%; OR, 3.2; P = .02), suggesting that use of a shorter DOT regimen led to greater treatment completion. This finding is consistent with findings from other studies that evaluated the effect of treatment duration on adherence [4–6, 12–17].
Additional factors associated with NCT differed between NCT-AE and NCT-O (Tables 1 and 2). Male sex and younger age were associated with NCT for 9H-SAT but not for 3HP-DOT, and only for NCT-O. Younger age was associated with a lower proportion of NCT-AE, which might reflect greater tolerance of LTBI drugs among younger participants. In previous studies, the association of age with NCT had not been reported consistently [5, 6, 18–21]. The odds of NCT-O among current smokers were 26% higher than among nonsmokers (P = .01). Recent evidence suggests that associations between tobacco exposure and tuberculosis infection and disease are attributable not only to social factors associated with tuberculosis (eg, poverty, malnutrition, and crowding) but also to direct impairment of local immunity against tuberculosis [22]. Both sets of associations support the importance of LTBI treatment completion for this group. We also found consistent trends of association between the level of alcohol intake and NCT, either attributed or not attributed to an AE, in both the univariate and multivariate analyses. Prior studies have reported a similar association, mainly with isoniazid-based LTBI [19, 23–25]. Additionally, self-reported cirrhosis was associated with NCT in 9H-SAT but not in 3HP-DOT, further supporting the value of the combined regimen. Our data concur with data from at least 1 prior report of lower NCT among persons of Hispanic ethnicity; the reasons for these findings are not apparent [19].
Our study reveals that the odds of NCT-O were 92% higher among men with any history of incarceration compared with men without such history (P < .001). New incarceration was the reported discontinuation reason for 25 participants, of whom 18 had reported a previous history of incarceration. This might indicate risk for new incarceration in a participant who reports previous incarceration; in that setting, use of a shorter regimen has evident advantage. It has also been reported that the NCT rate remains high after inmates are released from jail while on therapy [26–28]. Recently, the Federal Bureau of Prisons adopted 3HP-DOT as its standard LTBI regimen, after a pilot study reported 93% treatment completion with 3HP-DOT [29]. Use of shorter regimens might increase rates of completion among this challenging group.
Others have reported that site characteristics (eg, inconvenient clinic hours, long waiting times, unsympathetic clinic staff, lack of physician communication skills, strained physician–patient relationship, lack of incentives or enablers, and high travel-related expenses) can have a major impact on medication adherence [30–32]. Such characteristics might be responsible for some of the variability among sites in NCT (Supplementary Table 4). One limitation of this study was that site-specific characteristics and practice were not evaluated in detail. Also, differential ascertainment for AEs such as hepatotoxicity might have influenced clinical decisions regarding treatment discontinuation.
Factors evident at enrollment or at early visits are associated with completion vs noncompletion, suggesting interventions that might be usefully targeted. Social and demographic findings significantly associated with NCT (eg, smoking, alcohol use and abuse, intravenous drug use, history of incarceration, homelessness, and unemployment) could assist in identifying persons to be targeted with efforts to improve adherence. Characteristics associated with participants who discontinued treatment attributed to an AE (eg, use of concomitant medications, alcohol consumption, and reported cirrhosis at enrollment), although not modifiable, might identify patients needing closer monitoring.
Important differences may exist among clinics and persons participating in a clinical trial compared with those in purely programmatic settings. Clinics that participate in clinical trials are typically competitively selected, high-performance sites. Persons who participate in a lengthy follow-up trial might differ from those who decline to participate. However, some shared factors have been consistently identified in both research and nonresearch settings [19, 23, 24], suggesting that our findings are relevant to programmatic settings. Factors associated with NCT merit further evaluation. These findings might identify persons for whom tailored interventions could improve completion of LTBI treatment.
Notes
Acknowledgments. Andrew Vernon, MD, MHS (Centers for Disease Control and Prevention [CDC]), provided important guidance in final analysis and manuscript preparation. Charles Heilig, PhD (CDC), Andrew Hill, PhD (CDC), and Michael Chen, PhD (CDC), provided statistical consultation. Deirdre Sheehan (CDC) provided data management assistance. Pei-Jean Feng, MPH (CDC), collaborated as a manuscript reviewer. The study investigators and coordinators gratefully acknowledge all study participants. Ruth N. Moro, MD, MPH, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions. Authors’ contributions were as follows: conception or design of the work (R. N. M., A. S. B., J. S., S. V. G.), statistical analysis and interpretation of data (R. N. M., A. S. B., A. K., T. R. S., N. S.), drafting the manuscript (R. N. M., A. S. B., J. S., S. V. G.), critical review (R. N. M., A. S. B., J. S., T. R. S., S. V. G., A. K., M. E. V., N. S., N. A. S., A. K.), accountability for all aspects of the work (R. N. M., S. V. G.).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by the CDC. Sanofi US (Bridgewater, New Jersey) donated the rifapentine used in this study and donated more than $2.5 million to the CDC Foundation to supplement available US federal funding for rifapentine research. Details regarding use of these funds are available upon request. Sanofi US did not participate in the study design; data collection, analysis, or interpretation; writing the manuscript; or the decision to submit this manuscript for publication. R. N. M. and N. A. S. are employed by the CDC Foundation, which receives funds for rifapentine research from Sanofi US. In addition to Sanofi US contributions, no other disclosures have been reported by the authors.
Potential conflicts of interest. R. N. M. and N. A. S. are employed by the CDC Foundation, which receives funds for rifapentine research from Sanofi. T. R. S. is a member of data safety monitoring board for a clinical trial sponsored by Otsuka. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 18th International Union Against Tuberculosis and Lung Disease–North American Region, Boston, MA, 27 February–1 March 2014. Poster and oral presentation; 2015 National Tuberculosis Conference, Atlanta, GA, 9–11 June 2015. Poster presentation.




