-
PDF
- Split View
-
Views
-
Cite
Cite
John E. Mazuski, Leanne B. Gasink, Jon Armstrong, Helen Broadhurst, Greg G. Stone, Douglas Rank, Lily Llorens, Paul Newell, Jan Pachl, Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-abdominal Infection: Results From a Randomized, Controlled, Double-Blind, Phase 3 Program, Clinical Infectious Diseases, Volume 62, Issue 11, 1 June 2016, Pages 1380–1389, https://doi.org/10.1093/cid/ciw133
Close - Share Icon Share
Abstract
Background. When combined with ceftazidime, the novel non–β-lactam β-lactamase inhibitor avibactam provides a carbapenem alternative against multidrug-resistant infections. Efficacy and safety of ceftazidime-avibactam plus metronidazole were compared with meropenem in 1066 men and women with complicated intra-abdominal infections from 2 identical, randomized, double-blind phase 3 studies (NCT01499290 and NCT01500239).
Methods. The primary end point was clinical cure at test-of-cure visit 28–35 days after randomization, assessed by noninferiority of ceftazidime-avibactam plus metronidazole to meropenem in the microbiologically modified intention-to-treat (mMITT) population (in accordance with US Food and Drug Administration guidance), and the modified intention-to-treat and clinically evaluable populations (European Medicines Agency guidance). Noninferiority was considered met if the lower limit of the 95% confidence interval for between-group difference was greater than the prespecified noninferiority margin of −12.5%.
Results. Ceftazidime-avibactam plus metronidazole was noninferior to meropenem across all primary analysis populations. Clinical cure rates with ceftazidime-avibactam plus metronidazole and meropenem, respectively, were as follows: mMITT population, 81.6% and 85.1% (between-group difference, −3.5%; 95% confidence interval −8.64 to 1.58); modified intention-to-treat, 82.5% and 84.9% (−2.4%; −6.90 to 2.10); and clinically evaluable, 91.7% and 92.5% (−0.8%; −4.61 to 2.89). The clinical cure rate with ceftazidime-avibactam plus metronidazole for ceftazidime-resistant infections was comparable to that with meropenem (mMITT population, 83.0% and 85.9%, respectively) and similar to the regimen's own efficacy against ceftazidime-susceptible infections (82.0%). Adverse events were similar between groups.
Conclusions. Ceftazidime-avibactam plus metronidazole was noninferior to meropenem in the treatment of complicated intra-abdominal infections. Efficacy was similar against infections caused by ceftazidime-susceptible and ceftazidime-resistant pathogens. The safety profile of ceftazidime-avibactam plus metronidazole was consistent with that previously observed with ceftazidime alone.
Clinical Trials Registration. NCT01499290 and NCT01500239.
Complicated intra-abdominal infection (cIAI) generally results from perforation or necrosis of the gastrointestinal tract and release of bacteria into the peritoneal and retroperitoneal space [1]. Many pathogens implicated in these infections have developed resistance to standard antibiotics, thereby limiting treatment options. Moreover, the evolution of carbapenem-resistant Enterobacteriaceae is a growing concern [2, 3]. Thus, there is an urgent requirement for carbapenem-sparing therapies [4, 5].
Avibactam is a novel, first-in-class, non–β-lactam β-lactamase inhibitor that restores the in vitro activity of the established extended-spectrum antipseudomonal cephalosporin, ceftazidime, against Ambler class A, class C, and some class D β-lactamase–producing pathogens [6–9]. In phase 2 trials, ceftazidime-avibactam was effective and well tolerated in patients with cIAI (in combination with metronidazole) and complicated urinary tract infections [10, 11]. Based on these data, ceftazidime-avibactam was approved by the US Food and Drug Administration (FDA) for the treatment of cIAI (in combination with metronidazole) and complicated urinary tract infections (including pyelonephritis), in adults with limited or no alternative treatment options [12]. Here, we describe the results from 2 identical phase 3 trials combined to evaluate the efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in hospitalized adults with cIAI.
METHODS
Study Design and Participants
Data from 2 identical, prospective, randomized, multicenter, double-dummy, double-blind, comparative, global studies (NCT01499290 [RECLAIM 1] and NCT01500239 [RECLAIM 2]) were combined into a single inferential database with prespecified agreement from the US FDA and the European Medicines Agency. The studies were conducted from March 2012 to April 2014 at 136 centers in 30 countries (Supplementary Appendix).
Eligible participants were hospitalized male and female patients aged 18–90 years (18–65 years in India) with cIAI diagnosis requiring surgical intervention or percutaneous drainage within 24 hours before or after randomization. The diagnosis of cIAI was defined in accordance with the criteria outlined in Table 1. Key exclusion criteria included diagnosis of traumatic bowel perforation managed operatively within 12 hours; perforation of gastroduodenal ulcers managed operatively within 24 hours; intra-abdominal processes in which the primary cause was unlikely to be infectious; abdominal wall abscess, bowel obstruction, or ischemic bowel without perforation; simple cholecystitis or gangrenous cholecystitis without rupture; simple appendicitis; acute suppurative cholangitis; and infected necrotizing pancreatitis or pancreatic abscess (further details in Supplementary Appendix).
| Enrollment Timing . | Diagnostic Inclusion Criteria . |
|---|---|
| Intraoperative or postoperative enrollment with visual confirmation of an intra-abdominal infection associated with peritonitis with diagnosis of ≥1 of the listed criteria during surgical intervention | Cholecystitis with gangrenous rupture or perforation or progression of the infection beyond the gallbladder wall; diverticular disease with perforation or abscess;appendiceal perforation or periappendiceal abscess; acute gastric or duodenal perforations operated on >24 h after perforation; traumatic intestinal perforation operated on >12 h after perforation; secondary peritonitis, not including spontaneous bacterial peritonitis associated with cirrhosis and chronic ascites; intra-abdominal abscess, including of liver or spleen, provided that there was extension beyond the organ with evidence of intraperitoneal involvement |
| Preoperative enrollment with confirmation of infection by surgical intervention within 24 h of entry, when the listed clinical criteria were met | Requirement for surgical intervention, defined per protocol as open laparotomy, percutaneous drainage of an abscess, or laparoscopic surgery; evidence of systemic inflammatory indicators; Physical findings consistent with intra-abdominal infection; supportive radiological imaging findings of intra-abdominal infection; specimens from the surgical intervention sent for culture |
| Enrollment Timing . | Diagnostic Inclusion Criteria . |
|---|---|
| Intraoperative or postoperative enrollment with visual confirmation of an intra-abdominal infection associated with peritonitis with diagnosis of ≥1 of the listed criteria during surgical intervention | Cholecystitis with gangrenous rupture or perforation or progression of the infection beyond the gallbladder wall; diverticular disease with perforation or abscess;appendiceal perforation or periappendiceal abscess; acute gastric or duodenal perforations operated on >24 h after perforation; traumatic intestinal perforation operated on >12 h after perforation; secondary peritonitis, not including spontaneous bacterial peritonitis associated with cirrhosis and chronic ascites; intra-abdominal abscess, including of liver or spleen, provided that there was extension beyond the organ with evidence of intraperitoneal involvement |
| Preoperative enrollment with confirmation of infection by surgical intervention within 24 h of entry, when the listed clinical criteria were met | Requirement for surgical intervention, defined per protocol as open laparotomy, percutaneous drainage of an abscess, or laparoscopic surgery; evidence of systemic inflammatory indicators; Physical findings consistent with intra-abdominal infection; supportive radiological imaging findings of intra-abdominal infection; specimens from the surgical intervention sent for culture |
| Enrollment Timing . | Diagnostic Inclusion Criteria . |
|---|---|
| Intraoperative or postoperative enrollment with visual confirmation of an intra-abdominal infection associated with peritonitis with diagnosis of ≥1 of the listed criteria during surgical intervention | Cholecystitis with gangrenous rupture or perforation or progression of the infection beyond the gallbladder wall; diverticular disease with perforation or abscess;appendiceal perforation or periappendiceal abscess; acute gastric or duodenal perforations operated on >24 h after perforation; traumatic intestinal perforation operated on >12 h after perforation; secondary peritonitis, not including spontaneous bacterial peritonitis associated with cirrhosis and chronic ascites; intra-abdominal abscess, including of liver or spleen, provided that there was extension beyond the organ with evidence of intraperitoneal involvement |
| Preoperative enrollment with confirmation of infection by surgical intervention within 24 h of entry, when the listed clinical criteria were met | Requirement for surgical intervention, defined per protocol as open laparotomy, percutaneous drainage of an abscess, or laparoscopic surgery; evidence of systemic inflammatory indicators; Physical findings consistent with intra-abdominal infection; supportive radiological imaging findings of intra-abdominal infection; specimens from the surgical intervention sent for culture |
| Enrollment Timing . | Diagnostic Inclusion Criteria . |
|---|---|
| Intraoperative or postoperative enrollment with visual confirmation of an intra-abdominal infection associated with peritonitis with diagnosis of ≥1 of the listed criteria during surgical intervention | Cholecystitis with gangrenous rupture or perforation or progression of the infection beyond the gallbladder wall; diverticular disease with perforation or abscess;appendiceal perforation or periappendiceal abscess; acute gastric or duodenal perforations operated on >24 h after perforation; traumatic intestinal perforation operated on >12 h after perforation; secondary peritonitis, not including spontaneous bacterial peritonitis associated with cirrhosis and chronic ascites; intra-abdominal abscess, including of liver or spleen, provided that there was extension beyond the organ with evidence of intraperitoneal involvement |
| Preoperative enrollment with confirmation of infection by surgical intervention within 24 h of entry, when the listed clinical criteria were met | Requirement for surgical intervention, defined per protocol as open laparotomy, percutaneous drainage of an abscess, or laparoscopic surgery; evidence of systemic inflammatory indicators; Physical findings consistent with intra-abdominal infection; supportive radiological imaging findings of intra-abdominal infection; specimens from the surgical intervention sent for culture |
The study was undertaken in accordance with good clinical practice guidelines and adhered to the Declaration of Helsinki. All patients or their legal representatives provided informed written consent before screening. For each participating center, protocols (including all amendments) and informed consent documents were approved by an independent ethics committee and/or institutional review board.
Randomization and Masking
Patients were randomly allocated 1:1 according to a central randomization schedule, using a block size of 4, to receive either ceftazidime-avibactam (2000 mg of ceftazidime and 500 mg of avibactam as a 2-hour intravenous infusion every 8 hours), followed by metronidazole (500 mg as a 60-minute intravenous infusion every 8 hours); or meropenem (1000 mg as a 30-minute intravenous infusion every 8 hours). A double-dummy design was used, with patients receiving matching placebo as appropriate. All study treatments were administered for 5–14 days.
Patients with moderate renal impairment at baseline (estimated Cockcroft-Gault–calculated creatinine clearance [CrCl], >30 to ≤50 mL/min) received a reduced dose of ceftazidime-avibactam (1000 mg of ceftazidime and 250 mg of avibactam in a 2-hour intravenous infusion every 12 hours), or meropenem 1000 mg, 30-minute infusion every 12 hours was given. No adjustment for renal impairment was necessary for metronidazole.
After ≥5 full days of intravenous study therapy, treatment could be discontinued if patients showed clinical improvement. If Enterococcus species or methicillin-resistant Staphylococcus aureus was suspected or isolated, open-label vancomycin, linezolid, or daptomycin could be added to either regimen, according to the investigator's discretion. Antifungal therapy was not permitted. Study protocols were designed to minimize potential for prior use of other antibiotics that may confound assessment of the treatment effect (see Supplementary Appendix). Patients receiving systemic antibacterial therapy during the 72-hour period before study entry were enrolled only if they had a new infection (with ≤24 hours of therapy and ≤1 dose administered postoperatively ≤6 hours after the procedure), or were considered to have failed previous treatment (with ≥72 hours of therapy of inadequate therapy and requirement for operative intervention). Studies were stratified by baseline disease severity, based on the Acute Physiology and Chronic Health Evaluation (APACHE) II score (≤10 or >10 to ≤30), and by region (North America and Western Europe, Eastern Europe, and the rest of the world).
Procedures
Cultures were obtained for all patients, either from blood samples taken at baseline or intraoperatively from the intra-abdominal infection site. Susceptibility testing against study drugs for all isolates was performed by the local laboratory and confirmed by the central reference laboratory. Bacteria not expected to respond to either study drug (ie, Stenotrophomonas spp. and Acinetobacter spp.) were not considered eligible baseline pathogens. Clinical responses were determined by investigators at the end-of-treatment, test-of-cure and late-follow-up visits as cure, failure, or indeterminate. Clinical cure was defined as complete resolution or significant improvement of signs and symptoms of the index infection such that no further antibacterial therapy, drainage, or surgical intervention was necessary. Patients receiving continued antibacterial therapy for methicillin-resistant S. aureus or Enterococcus spp. could still have a response definition of cure. Clinical failure was defined as any of the following: death related to cIAI, persisting or recurring abdominal infection, postsurgical wound infection requiring additional antibiotics, ongoing cIAI symptoms requiring additional antibiotics, or any previously met criteria for failure.
Adequacy of source control, important in determining the success of clinical outcome when managing cIAI [13], was assessed by an independent surgical review panel comprising 3 surgeons and an interventional radiologist blinded to study therapy. Patients whose outcome was assessed as clinical failure by the investigator were reviewed by the surgical review panel, and those with inadequate source control were reclassified as indeterminate. Patients whose outcome was assessed as clinical cure and who underwent an additional procedure were also reviewed to determine whether their outcome should be reclassified as indeterminate or failure. Outcomes for patients in the microbiologically modified intention-to-treat (mMITT) and modified intention-to-treat (MITT) populations were also defined as indeterminate if they were lost to follow-up or if a death occurred in which cIAI was noncontributory.
Primary End Point
The primary end point of clinical cure at the test-of-cure visit 28–35 days after randomization was assessed by noninferiority of ceftazidime-avibactam plus metronidazole to meropenem. To adhere to guidance from different regulatory agencies, different primary analysis populations were required: the mMITT population, as requested by the FDA, constituting patients who met clinical disease criteria and had ≥1 pathogen identified at baseline, and the MITT and clinically evaluable at the time of the test-of-cure visit populations, as requested by the European Medicines Agency, constituting patients who received study drug and met the clinical disease criteria (MITT) and those in the MITT population with no protocol deviations affecting the efficacy of the study drug (clinically evaluable at test-of-cure visit). Analysis populations are described fully in the Supplementary Appendix.
Secondary End Points
Key secondary end points included clinical response at end-of-treatment (up to 24 hours after last infusion) and late-follow-up (42–49 days after randomization) visits; microbiological response at end-of-treatment, test-of-cure, and late-follow-up visits; and evaluation of the efficacy of ceftazidime-avibactam plus metronidazole or meropenem against ceftazidime-resistant pathogens (based on Clinical and Laboratory Standards Institute break point–defined resistant and intermediate categories for ceftazidime, ie, minimum inhibitory concentration [MIC] ≥8 mg/L against Enterobacteriaceae and ≥16 mg/L against Pseudomonas aeruginosa). Safety data were assessed from time of consent up to and including late-follow-up visits in the safety population (randomized patients who had received ≥1 dose of study drug). Progression of disease or signs and symptoms of disease were not reported as adverse events (AEs) unless they were more severe in intensity or more frequent than expected for the patient's condition.
Statistical Analysis
The primary end point of noninferiority was considered met by the sponsor if the lower limit of the 95% confidence interval (CI) for the between-group difference was above least −12.5%, although it was recognized that the FDA required a lower limit of above −10% for the mMITT population. The 2 combined studies were sized to provide 90% power for a 10% noninferiority margin and thus 95% power for a 12.5% noninferiority margin. Assuming both treatments had an underlying cure rate of 70% in the mMITT population and 80% of patients randomized were included in the mMITT population, this required 553 patients to be randomized per treatment group. Sample size was calculated using nQuery software (version 7; Statistical Solutions) using the Newcombe–Wilson score method (uncorrected) [14].
Descriptive summaries are provided, where appropriate, for primary and secondary variables, with between-group difference and 2-sided 95% CIs produced using the unstratified Miettinen and Nurminen method [15]. All statistical analyses were performed using SAS software (version 9.1 or higher; SAS Institute).
RESULTS
Patient Disposition and Baseline Characteristics
Of 1149 patients enrolled, 1066 were randomized. Of these, 529 received ≥1 dose of ceftazidime-avibactam plus metronidazole, and 529 ≥1 dose of meropenem. Overall, 113 patients discontinued treatment, 56 in the ceftazidime-avibactam plus metronidazole group and 57 in the meropenem group (Figure 1). There were no clinically significant differences in key baseline demographics between treatment groups (Table 2). The mean (standard deviation) duration of treatment with study drugs was 8.0 (3.3) days in the ceftazidime-avibactam plus metronidazole group and 8.3 (3.1) days in the meropenem group.
| Parameter . | Patients, No. (%)a . | |
|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 520) . | Meropenem (n = 523) . | |
| Age, mean (SD), y | 49.8 (17.5) | 50.3 (18.3) |
| Sex | ||
| Male | 326 (62.7) | 332 (63.5) |
| Raceb | ||
| White | 403 (77.5) | 396 (75.7) |
| Black or African American | 5 (1.0) | 2 (0.4) |
| Asian | 78 (15.0) | 89 (17.0) |
| Other | 27 (5.2) | 30 (5.7) |
| Body mass index, mean (SD), kg/m2 | 26.3 (5.1) | 26.2 (5.0) |
| APACHE II scorec | ||
| ≤10 | 437 (84.0) | 434 (83.0) |
| >10 to ≤30 | 78 (15.0) | 80 (15.3) |
| >30d | 1 (0.2) | 0 |
| Primary diagnosis | ||
| Cholecystitis | 87 (16.7) | 77 (14.7) |
| Diverticular disease | 35 (6.7) | 52 (9.9) |
| Appendicial perforation or periappendicial abscess | 218 (41.9) | 213 (40.7) |
| Acute gastric and duodenal perforations | 96 (18.5) | 99 (18.9) |
| Traumatic perforations | 9 (1.7) | 8 (1.5) |
| Secondary peritonitis | 36 (6.9) | 33 (6.3) |
| Intra-abdominal abscess | 39 (7.5) | 41 (7.8) |
| Single abscess | 32 (6.2) | 35 (6.7) |
| Prior treatment failure | 29 (5.6) | 31 (5.9) |
| Prior systemic antimicrobial therapy in the 72 h before randomization | 324 (62.3) | 325 (62.1) |
| Duration ≤24 h | 295 (91.0) | 294 (90.5) |
| Duration >24 to <72 h | 5 (1.5) | 4 (1.2) |
| Duration ≥72 h | 24 (7.4) | 27 (8.3) |
| Infection type | ||
| Monomicrobial | 209 (40.2) | 205 (39.2) |
| Polymicrobial | 208 (40.0) | 209 (40.0) |
| No study qualifying pathogen identified | 103 (19.8) | 109 (20.8) |
| Bacteremia | 22 (4.2) | 14 (2.7) |
| CrCl, mean (SD), mL/mine | 101.0 (42.2) | 102.4 (40.9) |
| Renal status | ||
| Normal renal function/mild impairment (CrCl, 50 mL/min) | 476 (91.5) | 478 (91.4) |
| Moderate impairment (CrCl, >30 to ≤50 mL/min) | 41 (7.9) | 43 (8.2) |
| Missingf | 3 (0.6) | 2 (0.4) |
| Parameter . | Patients, No. (%)a . | |
|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 520) . | Meropenem (n = 523) . | |
| Age, mean (SD), y | 49.8 (17.5) | 50.3 (18.3) |
| Sex | ||
| Male | 326 (62.7) | 332 (63.5) |
| Raceb | ||
| White | 403 (77.5) | 396 (75.7) |
| Black or African American | 5 (1.0) | 2 (0.4) |
| Asian | 78 (15.0) | 89 (17.0) |
| Other | 27 (5.2) | 30 (5.7) |
| Body mass index, mean (SD), kg/m2 | 26.3 (5.1) | 26.2 (5.0) |
| APACHE II scorec | ||
| ≤10 | 437 (84.0) | 434 (83.0) |
| >10 to ≤30 | 78 (15.0) | 80 (15.3) |
| >30d | 1 (0.2) | 0 |
| Primary diagnosis | ||
| Cholecystitis | 87 (16.7) | 77 (14.7) |
| Diverticular disease | 35 (6.7) | 52 (9.9) |
| Appendicial perforation or periappendicial abscess | 218 (41.9) | 213 (40.7) |
| Acute gastric and duodenal perforations | 96 (18.5) | 99 (18.9) |
| Traumatic perforations | 9 (1.7) | 8 (1.5) |
| Secondary peritonitis | 36 (6.9) | 33 (6.3) |
| Intra-abdominal abscess | 39 (7.5) | 41 (7.8) |
| Single abscess | 32 (6.2) | 35 (6.7) |
| Prior treatment failure | 29 (5.6) | 31 (5.9) |
| Prior systemic antimicrobial therapy in the 72 h before randomization | 324 (62.3) | 325 (62.1) |
| Duration ≤24 h | 295 (91.0) | 294 (90.5) |
| Duration >24 to <72 h | 5 (1.5) | 4 (1.2) |
| Duration ≥72 h | 24 (7.4) | 27 (8.3) |
| Infection type | ||
| Monomicrobial | 209 (40.2) | 205 (39.2) |
| Polymicrobial | 208 (40.0) | 209 (40.0) |
| No study qualifying pathogen identified | 103 (19.8) | 109 (20.8) |
| Bacteremia | 22 (4.2) | 14 (2.7) |
| CrCl, mean (SD), mL/mine | 101.0 (42.2) | 102.4 (40.9) |
| Renal status | ||
| Normal renal function/mild impairment (CrCl, 50 mL/min) | 476 (91.5) | 478 (91.4) |
| Moderate impairment (CrCl, >30 to ≤50 mL/min) | 41 (7.9) | 43 (8.2) |
| Missingf | 3 (0.6) | 2 (0.4) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CrCl, creatinine clearance; SD, standard deviation.
a Data represent No. (%) unless otherwise noted.
b Race was self-reported and data were missing from 2 patients (0.4%) in the ceftazidime-avibactam plus metronidazole group.
c The APACHE II score was calculated programmatically using data obtained at the site. Score details were missing for 4 (0.8%) patients in the ceftazidime-avibactam plus metronidazole group and 9 (1.7%) in the meropenem group.
d One patient with an APACHE II score >30 was entered, in violation of the inclusion/exclusion criteria.
e Data were available for 518 patients from the ceftazidime-avibactam plus metronidazole group and 522 from the meropenem group.
f Details of renal status were missing from 3 patients (0.6%) in the ceftazidime-avibactam plus metronidazole group and 2 (0.4%) in the meropenem group. Two patients had baseline CrCl <31 mL/min, classified as a missing renal status; these patients were not included in renal status subgroup analysis.
| Parameter . | Patients, No. (%)a . | |
|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 520) . | Meropenem (n = 523) . | |
| Age, mean (SD), y | 49.8 (17.5) | 50.3 (18.3) |
| Sex | ||
| Male | 326 (62.7) | 332 (63.5) |
| Raceb | ||
| White | 403 (77.5) | 396 (75.7) |
| Black or African American | 5 (1.0) | 2 (0.4) |
| Asian | 78 (15.0) | 89 (17.0) |
| Other | 27 (5.2) | 30 (5.7) |
| Body mass index, mean (SD), kg/m2 | 26.3 (5.1) | 26.2 (5.0) |
| APACHE II scorec | ||
| ≤10 | 437 (84.0) | 434 (83.0) |
| >10 to ≤30 | 78 (15.0) | 80 (15.3) |
| >30d | 1 (0.2) | 0 |
| Primary diagnosis | ||
| Cholecystitis | 87 (16.7) | 77 (14.7) |
| Diverticular disease | 35 (6.7) | 52 (9.9) |
| Appendicial perforation or periappendicial abscess | 218 (41.9) | 213 (40.7) |
| Acute gastric and duodenal perforations | 96 (18.5) | 99 (18.9) |
| Traumatic perforations | 9 (1.7) | 8 (1.5) |
| Secondary peritonitis | 36 (6.9) | 33 (6.3) |
| Intra-abdominal abscess | 39 (7.5) | 41 (7.8) |
| Single abscess | 32 (6.2) | 35 (6.7) |
| Prior treatment failure | 29 (5.6) | 31 (5.9) |
| Prior systemic antimicrobial therapy in the 72 h before randomization | 324 (62.3) | 325 (62.1) |
| Duration ≤24 h | 295 (91.0) | 294 (90.5) |
| Duration >24 to <72 h | 5 (1.5) | 4 (1.2) |
| Duration ≥72 h | 24 (7.4) | 27 (8.3) |
| Infection type | ||
| Monomicrobial | 209 (40.2) | 205 (39.2) |
| Polymicrobial | 208 (40.0) | 209 (40.0) |
| No study qualifying pathogen identified | 103 (19.8) | 109 (20.8) |
| Bacteremia | 22 (4.2) | 14 (2.7) |
| CrCl, mean (SD), mL/mine | 101.0 (42.2) | 102.4 (40.9) |
| Renal status | ||
| Normal renal function/mild impairment (CrCl, 50 mL/min) | 476 (91.5) | 478 (91.4) |
| Moderate impairment (CrCl, >30 to ≤50 mL/min) | 41 (7.9) | 43 (8.2) |
| Missingf | 3 (0.6) | 2 (0.4) |
| Parameter . | Patients, No. (%)a . | |
|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 520) . | Meropenem (n = 523) . | |
| Age, mean (SD), y | 49.8 (17.5) | 50.3 (18.3) |
| Sex | ||
| Male | 326 (62.7) | 332 (63.5) |
| Raceb | ||
| White | 403 (77.5) | 396 (75.7) |
| Black or African American | 5 (1.0) | 2 (0.4) |
| Asian | 78 (15.0) | 89 (17.0) |
| Other | 27 (5.2) | 30 (5.7) |
| Body mass index, mean (SD), kg/m2 | 26.3 (5.1) | 26.2 (5.0) |
| APACHE II scorec | ||
| ≤10 | 437 (84.0) | 434 (83.0) |
| >10 to ≤30 | 78 (15.0) | 80 (15.3) |
| >30d | 1 (0.2) | 0 |
| Primary diagnosis | ||
| Cholecystitis | 87 (16.7) | 77 (14.7) |
| Diverticular disease | 35 (6.7) | 52 (9.9) |
| Appendicial perforation or periappendicial abscess | 218 (41.9) | 213 (40.7) |
| Acute gastric and duodenal perforations | 96 (18.5) | 99 (18.9) |
| Traumatic perforations | 9 (1.7) | 8 (1.5) |
| Secondary peritonitis | 36 (6.9) | 33 (6.3) |
| Intra-abdominal abscess | 39 (7.5) | 41 (7.8) |
| Single abscess | 32 (6.2) | 35 (6.7) |
| Prior treatment failure | 29 (5.6) | 31 (5.9) |
| Prior systemic antimicrobial therapy in the 72 h before randomization | 324 (62.3) | 325 (62.1) |
| Duration ≤24 h | 295 (91.0) | 294 (90.5) |
| Duration >24 to <72 h | 5 (1.5) | 4 (1.2) |
| Duration ≥72 h | 24 (7.4) | 27 (8.3) |
| Infection type | ||
| Monomicrobial | 209 (40.2) | 205 (39.2) |
| Polymicrobial | 208 (40.0) | 209 (40.0) |
| No study qualifying pathogen identified | 103 (19.8) | 109 (20.8) |
| Bacteremia | 22 (4.2) | 14 (2.7) |
| CrCl, mean (SD), mL/mine | 101.0 (42.2) | 102.4 (40.9) |
| Renal status | ||
| Normal renal function/mild impairment (CrCl, 50 mL/min) | 476 (91.5) | 478 (91.4) |
| Moderate impairment (CrCl, >30 to ≤50 mL/min) | 41 (7.9) | 43 (8.2) |
| Missingf | 3 (0.6) | 2 (0.4) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CrCl, creatinine clearance; SD, standard deviation.
a Data represent No. (%) unless otherwise noted.
b Race was self-reported and data were missing from 2 patients (0.4%) in the ceftazidime-avibactam plus metronidazole group.
c The APACHE II score was calculated programmatically using data obtained at the site. Score details were missing for 4 (0.8%) patients in the ceftazidime-avibactam plus metronidazole group and 9 (1.7%) in the meropenem group.
d One patient with an APACHE II score >30 was entered, in violation of the inclusion/exclusion criteria.
e Data were available for 518 patients from the ceftazidime-avibactam plus metronidazole group and 522 from the meropenem group.
f Details of renal status were missing from 3 patients (0.6%) in the ceftazidime-avibactam plus metronidazole group and 2 (0.4%) in the meropenem group. Two patients had baseline CrCl <31 mL/min, classified as a missing renal status; these patients were not included in renal status subgroup analysis.
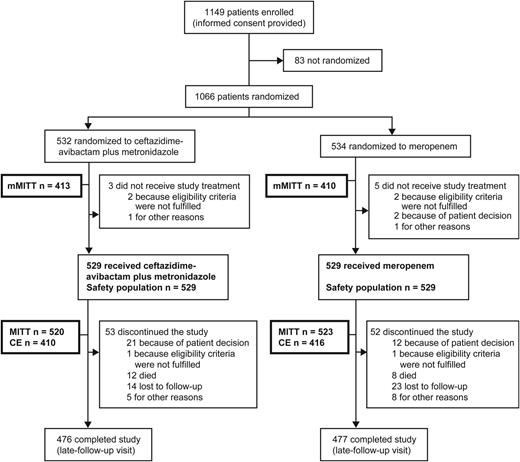
Patient flow. The detailed reasons for exclusion from each population are summarized in Supplementary Table 1 of the Supplementary appendix. Patients who discontinued the study include those stopped study treatment before receiving the minimum 5 days of treatment for any reason or whose treatment was stopped at any time and for any reason before the complicated intra-abdominal infection was considered resolved or cured, with a nonstudy antibiotic required to complete treatment. Abbreviations: CE, clinically evaluable; MITT, modified intention-to-treat; mMITT, microbiologically MITT.
Baseline pathogens isolated from the blood and the intra-abdominal site in the mMITT population (n = 823) were typical of those found in patients with cIAI and similar between treatment groups. Overall, 111 patients (13.5%) had a ceftazidime-resistant aerobic Gram-negative pathogen (ceftazidime-avibactam plus metronidazole, n = 47; meropenem, n = 64), the majority of which were Escherichia coli or Klebsiella pneumoniae. The ceftazidime-avibactam MIC was left-shifted compared with ceftazidime alone in these isolates, with almost all isolates having a ceftazidime-avibactam MIC ≤8 mg/L. Of the ceftazidime-resistant Enterobacteriaceae isolates identified in the mMITT population, the MIC90 for ceftazidime-avibactam was 2 mg/L. Approximately 80% of patients with ceftazidime-resistant pathogens had an extended-spectrum β-lactamase (ESBL)–positive infection, and approximately 3% a metallo-β-lactamase–positive infection.
Of all Gram-negative pathogens isolated at baseline, 9 were potentially nonsusceptible to ceftazidime-avibactam (ceftazidime-resistant Enterobacteriaceae for 2 patients in each treatment group, Comamonas testosteroni for 1 in the ceftazidime-avibactam plus metronidazole group, and ceftazidime-resistant P. aeruginosa for 4 in the meropenem group), with MIC values ≥16 mg/L (based on ceftazidime break points because ceftazidime-avibactam break points were not defined at the time of the studies). Eight were nonsusceptible to meropenem (Enterobacteriaceae for 3 in the ceftazidime-avibactam plus metronidazole group and 2 in the meropenem group, Burkholderia cepacia for 1 in the ceftazidime-avibactam plus metronidazole group and P. aeruginosa for 1 in each group), all with MIC values ≥4 mg/L (meropenem break points: Enterobacteriaceae, MIC ≥2 mg/L; P. aeruginosa, ≥4 mg/L) except for 1 case of E. coli categorized as resistant to meropenem from local laboratory disk zone diameter results.
The proportions of patients with anaerobic pathogens were similar between groups (134 [32.4%] for ceftazidime-avibactam plus metronidazole and 126 [30.7%] for meropenem). Gram-positive aerobes were observed in 38% of patients in each treatment group, most frequently from the Streptococcus anginosus group in 133 patients (16.2%). In the MITT population, Gram-positive infections were treated using protocol-permitted vancomycin or linezolid in 18 and 7 patients (3.5% and 1.3%), respectively, in the ceftazidime-avibactam plus metronidazole group and 18 and 5 (3.4% and 1.0%), respectively, in the meropenem group. One patient in the meropenem group received daptomycin.
End Points
All criteria for the primary end point of noninferiority of ceftazidime-avibactam plus metronidazole compared with meropenem were met across all primary analysis populations (Table 3). Secondary end points at the end-of-treatment and late-follow-up visits were not formally assessed against a noninferiority margin; nonetheless, the lower limits of the 95% CI for the between-group difference in all analysis sets were numerically above −10%, consistent with the primary end point (Table 3).
Clinical Cure Rate at Test-of-Cure Visit (Primary End Point) and End-of-Treatment and Late-Follow-Up Visits (Secondary End Points) for Patients in the Microbiologically Modified Intention-to-Treat, Modified Intention-to-Treat, and Clinically Evaluable at Test-of-Cure Visit Analysis Populations
| End Points . | mMITTa . | MITTb . | CE at TOCb . | |||
|---|---|---|---|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 413) . | Meropenem (n = 410) . | Ceftazidime-Avibactam + Metronidazole (n = 520) . | Meropenem (n = 523) . | Ceftazidime-Avibactam + Metronidazole (n = 410) . | Meropenem (n = 416) . | |
| Primary | ||||||
| Cure at TOCc | 337 (81.6) | 349 (85.1) | 429 (82.5) | 444 (84.9) | 376 (91.7) | 385 (92.5) |
| Differenced (95% CI), % | −3.5 (−8.64 to 1.58) | −2.4 (−6.90 to 2.10) | −0.8 (−4.61 to 2.89) | |||
| Secondary | ||||||
| Cure at EOT, No. (%)e | 361 (87.4) | 379 (92.4) | 459 (88.3) | 482 (92.2) | 381 (92.9) | 396 (95.2) |
| Difference (95% CI), %d | −5.0 (−9.24 to −0.93) | −3.9 (−7.57 to −0.29) | Not reported | |||
| Cure at LFU visite | 340 (82.3) | 347 (84.6) | 429 (82.5) | 436 (83.4) | 369 (90.0) | 376 (90.4) |
| Difference (95% CI), %d | −2.3 (−7.41 to 2.79) | −0.9 (−5.45 to 3.72) | Not reported | |||
| End Points . | mMITTa . | MITTb . | CE at TOCb . | |||
|---|---|---|---|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 413) . | Meropenem (n = 410) . | Ceftazidime-Avibactam + Metronidazole (n = 520) . | Meropenem (n = 523) . | Ceftazidime-Avibactam + Metronidazole (n = 410) . | Meropenem (n = 416) . | |
| Primary | ||||||
| Cure at TOCc | 337 (81.6) | 349 (85.1) | 429 (82.5) | 444 (84.9) | 376 (91.7) | 385 (92.5) |
| Differenced (95% CI), % | −3.5 (−8.64 to 1.58) | −2.4 (−6.90 to 2.10) | −0.8 (−4.61 to 2.89) | |||
| Secondary | ||||||
| Cure at EOT, No. (%)e | 361 (87.4) | 379 (92.4) | 459 (88.3) | 482 (92.2) | 381 (92.9) | 396 (95.2) |
| Difference (95% CI), %d | −5.0 (−9.24 to −0.93) | −3.9 (−7.57 to −0.29) | Not reported | |||
| Cure at LFU visite | 340 (82.3) | 347 (84.6) | 429 (82.5) | 436 (83.4) | 369 (90.0) | 376 (90.4) |
| Difference (95% CI), %d | −2.3 (−7.41 to 2.79) | −0.9 (−5.45 to 3.72) | Not reported | |||
Abbreviations: CE, clinically evaluable; CI, confidence interval; EOT, end-of-treatment; LFU, late-follow-up; MITT, modified intention-to-treat; mMITT, microbiologically MITT; TOC, test-of-cure.
a Primary analysis population for the US Food and Drug Administration.
b Co–primary analysis populations for the European Medicines Agency.
c The primary end point was clinical cure at TOC. The sponsor concluded noninferiority if the lower limit of the 95% CI at TOC was above −12.5% for the MITT and CE. The sponsor accepts that the Food and Drug Administration will conclude noninferiority if the lower limit of the 95% CI is above −10% for all populations.
d Difference in clinical cure rates. CIs for group differences are calculated using the unstratified Miettinen and Nurminen method. For subjects reviewed by the surgical review panel, the clinical response was based on surgical review evaluation if it was different from the investigator's assessment.
e Secondary end points were not formally assessed against a noninferiority margin.
Clinical Cure Rate at Test-of-Cure Visit (Primary End Point) and End-of-Treatment and Late-Follow-Up Visits (Secondary End Points) for Patients in the Microbiologically Modified Intention-to-Treat, Modified Intention-to-Treat, and Clinically Evaluable at Test-of-Cure Visit Analysis Populations
| End Points . | mMITTa . | MITTb . | CE at TOCb . | |||
|---|---|---|---|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 413) . | Meropenem (n = 410) . | Ceftazidime-Avibactam + Metronidazole (n = 520) . | Meropenem (n = 523) . | Ceftazidime-Avibactam + Metronidazole (n = 410) . | Meropenem (n = 416) . | |
| Primary | ||||||
| Cure at TOCc | 337 (81.6) | 349 (85.1) | 429 (82.5) | 444 (84.9) | 376 (91.7) | 385 (92.5) |
| Differenced (95% CI), % | −3.5 (−8.64 to 1.58) | −2.4 (−6.90 to 2.10) | −0.8 (−4.61 to 2.89) | |||
| Secondary | ||||||
| Cure at EOT, No. (%)e | 361 (87.4) | 379 (92.4) | 459 (88.3) | 482 (92.2) | 381 (92.9) | 396 (95.2) |
| Difference (95% CI), %d | −5.0 (−9.24 to −0.93) | −3.9 (−7.57 to −0.29) | Not reported | |||
| Cure at LFU visite | 340 (82.3) | 347 (84.6) | 429 (82.5) | 436 (83.4) | 369 (90.0) | 376 (90.4) |
| Difference (95% CI), %d | −2.3 (−7.41 to 2.79) | −0.9 (−5.45 to 3.72) | Not reported | |||
| End Points . | mMITTa . | MITTb . | CE at TOCb . | |||
|---|---|---|---|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 413) . | Meropenem (n = 410) . | Ceftazidime-Avibactam + Metronidazole (n = 520) . | Meropenem (n = 523) . | Ceftazidime-Avibactam + Metronidazole (n = 410) . | Meropenem (n = 416) . | |
| Primary | ||||||
| Cure at TOCc | 337 (81.6) | 349 (85.1) | 429 (82.5) | 444 (84.9) | 376 (91.7) | 385 (92.5) |
| Differenced (95% CI), % | −3.5 (−8.64 to 1.58) | −2.4 (−6.90 to 2.10) | −0.8 (−4.61 to 2.89) | |||
| Secondary | ||||||
| Cure at EOT, No. (%)e | 361 (87.4) | 379 (92.4) | 459 (88.3) | 482 (92.2) | 381 (92.9) | 396 (95.2) |
| Difference (95% CI), %d | −5.0 (−9.24 to −0.93) | −3.9 (−7.57 to −0.29) | Not reported | |||
| Cure at LFU visite | 340 (82.3) | 347 (84.6) | 429 (82.5) | 436 (83.4) | 369 (90.0) | 376 (90.4) |
| Difference (95% CI), %d | −2.3 (−7.41 to 2.79) | −0.9 (−5.45 to 3.72) | Not reported | |||
Abbreviations: CE, clinically evaluable; CI, confidence interval; EOT, end-of-treatment; LFU, late-follow-up; MITT, modified intention-to-treat; mMITT, microbiologically MITT; TOC, test-of-cure.
a Primary analysis population for the US Food and Drug Administration.
b Co–primary analysis populations for the European Medicines Agency.
c The primary end point was clinical cure at TOC. The sponsor concluded noninferiority if the lower limit of the 95% CI at TOC was above −12.5% for the MITT and CE. The sponsor accepts that the Food and Drug Administration will conclude noninferiority if the lower limit of the 95% CI is above −10% for all populations.
d Difference in clinical cure rates. CIs for group differences are calculated using the unstratified Miettinen and Nurminen method. For subjects reviewed by the surgical review panel, the clinical response was based on surgical review evaluation if it was different from the investigator's assessment.
e Secondary end points were not formally assessed against a noninferiority margin.
No clinically meaningful trends in outcomes were observed between patient or disease baseline characteristic subgroups, except in patients with moderate renal impairment at baseline. Within this population, there was a response trend in favor of meropenem in the mMITT (between-group difference, −29.1%; 95% CI, −50.05 to −5.36) and MITT (−25.6%;−44.53 to −4.78) populations (Figure 2). Numerically, this trend was also seen in the clinically evaluable population (between-group difference −16.0; 95% CI, −38.23 to 6.87). Within the first 48–72 hours of dosing, 67.9% of patients with an estimated CrCl <50 mL/min at baseline across both study drug groups showed rapid improvement to an estimated CrCl >50 mL/min (ceftazidime-avibactam plus metronidazole, 67.5%; meropenem, 69.8%).
![Difference in clinical cure rates at test-of-cure (TOC) visit by renal function. Dark gray line represents −12.5% noninferiority margin required by the European Medicines Agency for the overall primary populations. Subgroups were not required to meet this noninferiority margin. Confidence intervals (CIs) were calculated using Miettinen and Nurminen method without adjustments. Renal function was based on CrCl reported by the site using the Cockcroft-Gault method [16] and based on local laboratory data. Patients with a baseline CrCl <31 mL/min were recorded as having a missing renal status and were not included in the renal status subgroup analyses shown. Clinical cure rates in patients with missing renal status receiving CAZ-AVI plus MTZ or MER, respectively, were 2 of 3 patients (66.6%) and 2 of 2 (100%) in the MITT population; 2 of 2 (100%) and 1 of 1 (100%) in the CE population; and 1 of 3 (33.3%) and 2 of 2 (100%) in the mMITT population. Abbreviations: CAZ-AVI, ceftazidime-avibactam; CE, clinically evaluable; CrCl, creatinine clearance; MER, meropenem; MITT, modified intention-to-treat; mMITT, microbiologically MITT; MTZ, metronidazole.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/62/11/10.1093_cid_ciw133/1/m_ciw13302.jpeg?Expires=1749850933&Signature=NlNyu~fTOUwQoP8c3L21-isiHLlac1r7ct5-8Pb69zGbhPa4zLEWQSHeSpRg849oht~0Tc0eILESh-TQfJBJC4ZrdCRCNvETQW-aDAUN8WyAvzPI1Ywx70-bpZbwlavhA0FBlroO1xYEGKEZ1GQrLFpzYtK-7zY3xKQuvVZ-VUkrm8I4SfJoXYpw5ikXvdD3B3dA5okvz8x4wRXYzNQ-sbItZH0Nniio-wJmjXG6uLFNDPVCsZYw~FixOR02ng-BIiUhRbCLwrrE0~d8mmSQsbmvp0YSeqnggkJ~fsT2CEixTJEtg3exq2yzABJKxnFWB-mkjTvq7Xk8~XJJlioeGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Difference in clinical cure rates at test-of-cure (TOC) visit by renal function. Dark gray line represents −12.5% noninferiority margin required by the European Medicines Agency for the overall primary populations. Subgroups were not required to meet this noninferiority margin. Confidence intervals (CIs) were calculated using Miettinen and Nurminen method without adjustments. Renal function was based on CrCl reported by the site using the Cockcroft-Gault method [16] and based on local laboratory data. Patients with a baseline CrCl <31 mL/min were recorded as having a missing renal status and were not included in the renal status subgroup analyses shown. Clinical cure rates in patients with missing renal status receiving CAZ-AVI plus MTZ or MER, respectively, were 2 of 3 patients (66.6%) and 2 of 2 (100%) in the MITT population; 2 of 2 (100%) and 1 of 1 (100%) in the CE population; and 1 of 3 (33.3%) and 2 of 2 (100%) in the mMITT population. Abbreviations: CAZ-AVI, ceftazidime-avibactam; CE, clinically evaluable; CrCl, creatinine clearance; MER, meropenem; MITT, modified intention-to-treat; mMITT, microbiologically MITT; MTZ, metronidazole.
Ceftazidime-avibactam plus metronidazole was effective in patients with ceftazidime-resistant Gram-negative pathogens, with clinical cure rates similar to meropenem (83.0% vs 85.9%, respectively) and to those seen in patients with ceftazidime-susceptible Gram-negative pathogens (82.0%) (Table 4). Meropenem was effective in 87.7% of patients with ceftazidime-susceptible Gram-negative isolates (Table 4).
Clinical Response at the Test-of-Cure Visit for Patients with Ceftazidime-Resistant and Ceftazidime-Susceptible Gram-Negative Pathogens (Microbiologically Modified Intention-to-Treat Analysis Population)
| Pathogen . | Ceftazidime Avibactam + Metronidazole (n = 413) . | Meropenem (n = 410) . | Between-Group Difference in Clinical Cure Rates, (95% CI), %a . | ||
|---|---|---|---|---|---|
| Patients, No. . | Clinical Cure, No. (%) . | Patients, No. . | Clinical Cure, No. (%) . | ||
| All | |||||
| Ceftazidime resistant | 47 | 39 (83.0) | 64 | 55 (85.9) | −3.0 (−17.89 to 10.60) |
| Ceftazidime susceptible | 289 | 237 (82.0) | 292 | 256 (87.7) | −5.7 (−11.57 to 0.17) |
| Enterobacteriaceae | |||||
| Ceftazidime resistant | 44 | 36 (81.8) | 62 | 53 (85.5) | −3.7 (−19.31 to 10.44) |
| Ceftazidime susceptible | 279 | 229 (82.1) | 280 | 245 (87.5) | −5.4 (−11.45 to 0.54) |
| Escherichia coli | |||||
| Ceftazidime resistant | 24 | 19 (79.2) | 37 | 31 (83.8) | −4·6 (−26.77 to 14.86) |
| Ceftazidime susceptible | 236 | 192 (81.4) | 239 | 210 (87.9) | −6.5 (−13.09 to −0.02) |
| Klebsiella pneumoniae | |||||
| Ceftazidime resistant | 13 | 10 (76.9) | 13 | 9 (69.2) | 7.7 (−27.10 to 40.96) |
| Ceftazidime susceptible | 34 | 28 (82.4) | 35 | 27 (77.1) | 5.2 (−14.43 to 24.56) |
| Non-Enterobacteriaceae | |||||
| Ceftazidime resistant | 4 | 4 (100.0) | 4 | 4 (100.0) | 0.0 (−52.33 to 52.33) |
| Ceftazidime susceptible | 35 | 31 (88.6) | 43 | 41 (95.3) | −6.8 (−22.10 to 5.99) |
| Pseudomonas aeruginosa | |||||
| Ceftazidime resistant | 2 | 2 (100.0) | 4 | 4 (100.0) | 0.0 (−69.74 to 53.54) |
| Ceftazidime susceptible | 30 | 27 (90.0) | 32 | 30 (93.8) | −3.8 (−20.55 to 11.90) |
| Pathogen . | Ceftazidime Avibactam + Metronidazole (n = 413) . | Meropenem (n = 410) . | Between-Group Difference in Clinical Cure Rates, (95% CI), %a . | ||
|---|---|---|---|---|---|
| Patients, No. . | Clinical Cure, No. (%) . | Patients, No. . | Clinical Cure, No. (%) . | ||
| All | |||||
| Ceftazidime resistant | 47 | 39 (83.0) | 64 | 55 (85.9) | −3.0 (−17.89 to 10.60) |
| Ceftazidime susceptible | 289 | 237 (82.0) | 292 | 256 (87.7) | −5.7 (−11.57 to 0.17) |
| Enterobacteriaceae | |||||
| Ceftazidime resistant | 44 | 36 (81.8) | 62 | 53 (85.5) | −3.7 (−19.31 to 10.44) |
| Ceftazidime susceptible | 279 | 229 (82.1) | 280 | 245 (87.5) | −5.4 (−11.45 to 0.54) |
| Escherichia coli | |||||
| Ceftazidime resistant | 24 | 19 (79.2) | 37 | 31 (83.8) | −4·6 (−26.77 to 14.86) |
| Ceftazidime susceptible | 236 | 192 (81.4) | 239 | 210 (87.9) | −6.5 (−13.09 to −0.02) |
| Klebsiella pneumoniae | |||||
| Ceftazidime resistant | 13 | 10 (76.9) | 13 | 9 (69.2) | 7.7 (−27.10 to 40.96) |
| Ceftazidime susceptible | 34 | 28 (82.4) | 35 | 27 (77.1) | 5.2 (−14.43 to 24.56) |
| Non-Enterobacteriaceae | |||||
| Ceftazidime resistant | 4 | 4 (100.0) | 4 | 4 (100.0) | 0.0 (−52.33 to 52.33) |
| Ceftazidime susceptible | 35 | 31 (88.6) | 43 | 41 (95.3) | −6.8 (−22.10 to 5.99) |
| Pseudomonas aeruginosa | |||||
| Ceftazidime resistant | 2 | 2 (100.0) | 4 | 4 (100.0) | 0.0 (−69.74 to 53.54) |
| Ceftazidime susceptible | 30 | 27 (90.0) | 32 | 30 (93.8) | −3.8 (−20.55 to 11.90) |
Abbreviations: CI, confidence interval; mMITT, microbiologically modified intention-to-treat.
a CIs for group differences were calculated using the unstratified Miettinen and Nurminen method. The analysis includes patients infected by ≥1 ceftazidime-resistant Gram-negative pathogen. Clinical cure rate for the mMITT analysis set was defined as the number of patients with a clinical response at the test-of-cure visit divided by the combined number with clinical cure, clinical failure, and indeterminate outcome. Clinical response was based on surgical review evaluation if it differed from the investigator's assessment. Ceftazidime resistance includes both the Clinical Laboratory Standards Institute (M100-S22) break point–defined resistant and intermediate categories. Percentages are based on the total number of patients in the subgroup.
Clinical Response at the Test-of-Cure Visit for Patients with Ceftazidime-Resistant and Ceftazidime-Susceptible Gram-Negative Pathogens (Microbiologically Modified Intention-to-Treat Analysis Population)
| Pathogen . | Ceftazidime Avibactam + Metronidazole (n = 413) . | Meropenem (n = 410) . | Between-Group Difference in Clinical Cure Rates, (95% CI), %a . | ||
|---|---|---|---|---|---|
| Patients, No. . | Clinical Cure, No. (%) . | Patients, No. . | Clinical Cure, No. (%) . | ||
| All | |||||
| Ceftazidime resistant | 47 | 39 (83.0) | 64 | 55 (85.9) | −3.0 (−17.89 to 10.60) |
| Ceftazidime susceptible | 289 | 237 (82.0) | 292 | 256 (87.7) | −5.7 (−11.57 to 0.17) |
| Enterobacteriaceae | |||||
| Ceftazidime resistant | 44 | 36 (81.8) | 62 | 53 (85.5) | −3.7 (−19.31 to 10.44) |
| Ceftazidime susceptible | 279 | 229 (82.1) | 280 | 245 (87.5) | −5.4 (−11.45 to 0.54) |
| Escherichia coli | |||||
| Ceftazidime resistant | 24 | 19 (79.2) | 37 | 31 (83.8) | −4·6 (−26.77 to 14.86) |
| Ceftazidime susceptible | 236 | 192 (81.4) | 239 | 210 (87.9) | −6.5 (−13.09 to −0.02) |
| Klebsiella pneumoniae | |||||
| Ceftazidime resistant | 13 | 10 (76.9) | 13 | 9 (69.2) | 7.7 (−27.10 to 40.96) |
| Ceftazidime susceptible | 34 | 28 (82.4) | 35 | 27 (77.1) | 5.2 (−14.43 to 24.56) |
| Non-Enterobacteriaceae | |||||
| Ceftazidime resistant | 4 | 4 (100.0) | 4 | 4 (100.0) | 0.0 (−52.33 to 52.33) |
| Ceftazidime susceptible | 35 | 31 (88.6) | 43 | 41 (95.3) | −6.8 (−22.10 to 5.99) |
| Pseudomonas aeruginosa | |||||
| Ceftazidime resistant | 2 | 2 (100.0) | 4 | 4 (100.0) | 0.0 (−69.74 to 53.54) |
| Ceftazidime susceptible | 30 | 27 (90.0) | 32 | 30 (93.8) | −3.8 (−20.55 to 11.90) |
| Pathogen . | Ceftazidime Avibactam + Metronidazole (n = 413) . | Meropenem (n = 410) . | Between-Group Difference in Clinical Cure Rates, (95% CI), %a . | ||
|---|---|---|---|---|---|
| Patients, No. . | Clinical Cure, No. (%) . | Patients, No. . | Clinical Cure, No. (%) . | ||
| All | |||||
| Ceftazidime resistant | 47 | 39 (83.0) | 64 | 55 (85.9) | −3.0 (−17.89 to 10.60) |
| Ceftazidime susceptible | 289 | 237 (82.0) | 292 | 256 (87.7) | −5.7 (−11.57 to 0.17) |
| Enterobacteriaceae | |||||
| Ceftazidime resistant | 44 | 36 (81.8) | 62 | 53 (85.5) | −3.7 (−19.31 to 10.44) |
| Ceftazidime susceptible | 279 | 229 (82.1) | 280 | 245 (87.5) | −5.4 (−11.45 to 0.54) |
| Escherichia coli | |||||
| Ceftazidime resistant | 24 | 19 (79.2) | 37 | 31 (83.8) | −4·6 (−26.77 to 14.86) |
| Ceftazidime susceptible | 236 | 192 (81.4) | 239 | 210 (87.9) | −6.5 (−13.09 to −0.02) |
| Klebsiella pneumoniae | |||||
| Ceftazidime resistant | 13 | 10 (76.9) | 13 | 9 (69.2) | 7.7 (−27.10 to 40.96) |
| Ceftazidime susceptible | 34 | 28 (82.4) | 35 | 27 (77.1) | 5.2 (−14.43 to 24.56) |
| Non-Enterobacteriaceae | |||||
| Ceftazidime resistant | 4 | 4 (100.0) | 4 | 4 (100.0) | 0.0 (−52.33 to 52.33) |
| Ceftazidime susceptible | 35 | 31 (88.6) | 43 | 41 (95.3) | −6.8 (−22.10 to 5.99) |
| Pseudomonas aeruginosa | |||||
| Ceftazidime resistant | 2 | 2 (100.0) | 4 | 4 (100.0) | 0.0 (−69.74 to 53.54) |
| Ceftazidime susceptible | 30 | 27 (90.0) | 32 | 30 (93.8) | −3.8 (−20.55 to 11.90) |
Abbreviations: CI, confidence interval; mMITT, microbiologically modified intention-to-treat.
a CIs for group differences were calculated using the unstratified Miettinen and Nurminen method. The analysis includes patients infected by ≥1 ceftazidime-resistant Gram-negative pathogen. Clinical cure rate for the mMITT analysis set was defined as the number of patients with a clinical response at the test-of-cure visit divided by the combined number with clinical cure, clinical failure, and indeterminate outcome. Clinical response was based on surgical review evaluation if it differed from the investigator's assessment. Ceftazidime resistance includes both the Clinical Laboratory Standards Institute (M100-S22) break point–defined resistant and intermediate categories. Percentages are based on the total number of patients in the subgroup.
In most patients, microbiological outcomes were presumed based on clinical outcome, because intra-abdominal cultures require an invasive procedure and were therefore only obtained if clinically indicated. Thus, microbiological outcomes in the mMITT population were similar to clinical responses (Supplementary Appendix).
AEs occurred at a similar frequency in the 2 treatment groups (Table 5), with the most frequent being in the system organ class gastrointestinal disorders. There were no clinically meaningful trends in laboratory values, and there was only Clostridium difficile enterocolitis event in each treatment group. Deaths occurred in 13 (2.5%) and 8 (1.5%) of the ceftazidime-avibactam plus metronidazole and meropenem groups, respectively. For these deaths, 4 (0.77%) and 1 (0.19%) of the patients receiving ceftazidime-avibactam plus metronidazole and meropenem, respectively, had moderate renal impairment at baseline and 6 (1.1%) and 3 (0.6%) of the deaths, respectively, were attributed to progression of cIAI. None were considered related to the study drug and there were no trends in cause of death.
Safety Evaluation up to Late Follow-Up Visit, 6–7 Weeks from Randomization (Safety Population)
| . | Patients, No. (%)a . | |
|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 529) . | Meropenem (n = 529) . | |
| Summary | ||
| Any AEb | 243 (45.9) | 227 (42.9) |
| Any AE of severe intensity | 30 (5.7) | 36 (6.8) |
| Any serious AE | 42 (7.9) | 40 (7.6) |
| Any AE leading to discontinuation | 14 (2.6) | 7 (1.3) |
| Any AE leading to death | 13 (2.5) | 8 (1.5) |
| AEs in ≥2% of patients in either treatment group by system organ class/preferred termc | ||
| Infections and infestations | ||
| Wound infection | 13 (2.5) | 11 (2.1) |
| Blood and lymphatic system disorders | ||
| Anemia | 11 (2.1) | 9 (1.7) |
| Nervous system disorders | ||
| Headache | 15 (2.8) | 9 (1.7) |
| Vascular disorders | ||
| Hypertension | 15 (2.8) | 24 (4.5) |
| Hypotension | 12 (2.3) | 12 (2.3) |
| Phlebitis | 10 (1.9) | 11 (2.1) |
| Respiratory disorders | ||
| Cough | 11 (2.1) | 13 (2.5) |
| Gastrointestinal disorders | ||
| Diarrhead | 40 (7.6) | 17 (3.2) |
| Nausea | 36 (6.8) | 24 (4.5) |
| Vomiting | 24 (4.5) | 10 (1.9) |
| Abdominal distension | 10 (1.9) | 11 (2.1) |
| Constipation | 8 (1.5) | 20 (3.8) |
| General disorders | ||
| Pyrexia | 24 (4.5) | 24 (4.5) |
| Asthenia | 10 (1·9) | 12 (2·3) |
| Safety topicse | ||
| Liver disorder | 11 (2.1) | 8 (1.5) |
| Diarrhea | 40 (7.6) | 18 (3.4) |
| Hypersensitivity/anaphylaxis disorder | 23 (4.3) | 16 (3.0) |
| Hematological disorder | 16 (3.0) | 15 (2.8) |
| Renal disorder | 12 (2.3) | 3 (0.6) |
| . | Patients, No. (%)a . | |
|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 529) . | Meropenem (n = 529) . | |
| Summary | ||
| Any AEb | 243 (45.9) | 227 (42.9) |
| Any AE of severe intensity | 30 (5.7) | 36 (6.8) |
| Any serious AE | 42 (7.9) | 40 (7.6) |
| Any AE leading to discontinuation | 14 (2.6) | 7 (1.3) |
| Any AE leading to death | 13 (2.5) | 8 (1.5) |
| AEs in ≥2% of patients in either treatment group by system organ class/preferred termc | ||
| Infections and infestations | ||
| Wound infection | 13 (2.5) | 11 (2.1) |
| Blood and lymphatic system disorders | ||
| Anemia | 11 (2.1) | 9 (1.7) |
| Nervous system disorders | ||
| Headache | 15 (2.8) | 9 (1.7) |
| Vascular disorders | ||
| Hypertension | 15 (2.8) | 24 (4.5) |
| Hypotension | 12 (2.3) | 12 (2.3) |
| Phlebitis | 10 (1.9) | 11 (2.1) |
| Respiratory disorders | ||
| Cough | 11 (2.1) | 13 (2.5) |
| Gastrointestinal disorders | ||
| Diarrhead | 40 (7.6) | 17 (3.2) |
| Nausea | 36 (6.8) | 24 (4.5) |
| Vomiting | 24 (4.5) | 10 (1.9) |
| Abdominal distension | 10 (1.9) | 11 (2.1) |
| Constipation | 8 (1.5) | 20 (3.8) |
| General disorders | ||
| Pyrexia | 24 (4.5) | 24 (4.5) |
| Asthenia | 10 (1·9) | 12 (2·3) |
| Safety topicse | ||
| Liver disorder | 11 (2.1) | 8 (1.5) |
| Diarrhea | 40 (7.6) | 18 (3.4) |
| Hypersensitivity/anaphylaxis disorder | 23 (4.3) | 16 (3.0) |
| Hematological disorder | 16 (3.0) | 15 (2.8) |
| Renal disorder | 12 (2.3) | 3 (0.6) |
Abbreviation: AE, adverse event.
a Patients with multiple AEs are counted once for each system organ class and/or preferred term.
b Each patient is counted only once within a treatment group in this overall summary.
c AEs are sorted by system organ class in international order and by preferred term in decreasing order of frequency in patients treated with ceftazidime-avibactam plus metronidazole.
d There was 1 toxin-positive case of Clostridium difficile enterocolitis in each treatment group, confirmed by a local laboratory. Both cases were considered nonserious and moderate in intensity.
e Each safety topic represents the aggregate of a group of preidentified relevant AE preferred terms based on those from previous a phase 2 study of ceftazidime-avibactam in complicated intra-abdominal infection.
Safety Evaluation up to Late Follow-Up Visit, 6–7 Weeks from Randomization (Safety Population)
| . | Patients, No. (%)a . | |
|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 529) . | Meropenem (n = 529) . | |
| Summary | ||
| Any AEb | 243 (45.9) | 227 (42.9) |
| Any AE of severe intensity | 30 (5.7) | 36 (6.8) |
| Any serious AE | 42 (7.9) | 40 (7.6) |
| Any AE leading to discontinuation | 14 (2.6) | 7 (1.3) |
| Any AE leading to death | 13 (2.5) | 8 (1.5) |
| AEs in ≥2% of patients in either treatment group by system organ class/preferred termc | ||
| Infections and infestations | ||
| Wound infection | 13 (2.5) | 11 (2.1) |
| Blood and lymphatic system disorders | ||
| Anemia | 11 (2.1) | 9 (1.7) |
| Nervous system disorders | ||
| Headache | 15 (2.8) | 9 (1.7) |
| Vascular disorders | ||
| Hypertension | 15 (2.8) | 24 (4.5) |
| Hypotension | 12 (2.3) | 12 (2.3) |
| Phlebitis | 10 (1.9) | 11 (2.1) |
| Respiratory disorders | ||
| Cough | 11 (2.1) | 13 (2.5) |
| Gastrointestinal disorders | ||
| Diarrhead | 40 (7.6) | 17 (3.2) |
| Nausea | 36 (6.8) | 24 (4.5) |
| Vomiting | 24 (4.5) | 10 (1.9) |
| Abdominal distension | 10 (1.9) | 11 (2.1) |
| Constipation | 8 (1.5) | 20 (3.8) |
| General disorders | ||
| Pyrexia | 24 (4.5) | 24 (4.5) |
| Asthenia | 10 (1·9) | 12 (2·3) |
| Safety topicse | ||
| Liver disorder | 11 (2.1) | 8 (1.5) |
| Diarrhea | 40 (7.6) | 18 (3.4) |
| Hypersensitivity/anaphylaxis disorder | 23 (4.3) | 16 (3.0) |
| Hematological disorder | 16 (3.0) | 15 (2.8) |
| Renal disorder | 12 (2.3) | 3 (0.6) |
| . | Patients, No. (%)a . | |
|---|---|---|
| Ceftazidime-Avibactam + Metronidazole (n = 529) . | Meropenem (n = 529) . | |
| Summary | ||
| Any AEb | 243 (45.9) | 227 (42.9) |
| Any AE of severe intensity | 30 (5.7) | 36 (6.8) |
| Any serious AE | 42 (7.9) | 40 (7.6) |
| Any AE leading to discontinuation | 14 (2.6) | 7 (1.3) |
| Any AE leading to death | 13 (2.5) | 8 (1.5) |
| AEs in ≥2% of patients in either treatment group by system organ class/preferred termc | ||
| Infections and infestations | ||
| Wound infection | 13 (2.5) | 11 (2.1) |
| Blood and lymphatic system disorders | ||
| Anemia | 11 (2.1) | 9 (1.7) |
| Nervous system disorders | ||
| Headache | 15 (2.8) | 9 (1.7) |
| Vascular disorders | ||
| Hypertension | 15 (2.8) | 24 (4.5) |
| Hypotension | 12 (2.3) | 12 (2.3) |
| Phlebitis | 10 (1.9) | 11 (2.1) |
| Respiratory disorders | ||
| Cough | 11 (2.1) | 13 (2.5) |
| Gastrointestinal disorders | ||
| Diarrhead | 40 (7.6) | 17 (3.2) |
| Nausea | 36 (6.8) | 24 (4.5) |
| Vomiting | 24 (4.5) | 10 (1.9) |
| Abdominal distension | 10 (1.9) | 11 (2.1) |
| Constipation | 8 (1.5) | 20 (3.8) |
| General disorders | ||
| Pyrexia | 24 (4.5) | 24 (4.5) |
| Asthenia | 10 (1·9) | 12 (2·3) |
| Safety topicse | ||
| Liver disorder | 11 (2.1) | 8 (1.5) |
| Diarrhea | 40 (7.6) | 18 (3.4) |
| Hypersensitivity/anaphylaxis disorder | 23 (4.3) | 16 (3.0) |
| Hematological disorder | 16 (3.0) | 15 (2.8) |
| Renal disorder | 12 (2.3) | 3 (0.6) |
Abbreviation: AE, adverse event.
a Patients with multiple AEs are counted once for each system organ class and/or preferred term.
b Each patient is counted only once within a treatment group in this overall summary.
c AEs are sorted by system organ class in international order and by preferred term in decreasing order of frequency in patients treated with ceftazidime-avibactam plus metronidazole.
d There was 1 toxin-positive case of Clostridium difficile enterocolitis in each treatment group, confirmed by a local laboratory. Both cases were considered nonserious and moderate in intensity.
e Each safety topic represents the aggregate of a group of preidentified relevant AE preferred terms based on those from previous a phase 2 study of ceftazidime-avibactam in complicated intra-abdominal infection.
DISCUSSION
The increasing incidence of multidrug resistance in ESBL-producing pathogens casts some doubt as to the long-term efficacy of existing agents in treating cIAI [17]. The current phase 3 investigations demonstrated ceftazidime-avibactam in combination with metronidazole is effective in the treatment of cIAI. Noninferiority compared with meropenem for the primary end point of clinical cure at the test-of-cure visit was consistently achieved across study populations, with the lower limit of the 95% CI for the between-group treatment difference in the mMITT, MITT, and clinically evaluable populations being above −10%, satisfying both the sponsor-defined margin (above −12.5%) and the FDA requirements (above −10%). Furthermore, the lower limit of the 95% CIs in the secondary end point analyses of clinical cure at the end-of-treatment and late-follow-up visits were numerically above −10%. Meropenem is a reference standard for treatment of serious ESBL-producing infections and demonstrated efficacy consistent with its known profile [10, 18].
In the ceftazidime-avibactam plus metronidazole group, 83.0% of patients with ceftazidime-resistant infections were classified as clinical cures. This was consistent with results seen in patients with ceftazidime-susceptible infections (82.0%) and those with ceftazidime-resistant infections in the meropenem group (85.9%). The ceftazidime-avibactam MIC was left-shifted compared with ceftazidime alone in ceftazidime-resistant isolates in the mMITT population at baseline, and almost all had a ceftazidime-avibactam MIC provisionally within the susceptible range (MIC, ≤8 mg/L). These data suggest that avibactam restores the efficacy of ceftazidime against ceftazidime-resistant pathogens.
The proportion of patients with clinical cure across patient and disease baseline subgroups was generally consistent between the 2 treatment arms, except in patients with moderate renal impairment at baseline, in whom there was a response trend in favor of meropenem. As with other drugs eliminated largely by renal excretion, renal function affects the pharmacokinetics of ceftazidime, avibactam, and meropenem [19–21]. To account for this, dose adjustments for these drugs were made for all patients with moderate renal impairment at baseline, with the aim of achieving drug plasma levels associated with target attainment while minimizing the risk of overexposure due to impaired renal clearance. Post hoc review of serum creatinine levels indicated rapid improvement of renal function in some patients after enrollment. Dose adjustments were made; however, some patients were potentially underdosed early in the study. Because the protocol-specified reduction in total daily dose in renal impairment for ceftazidime-avibactam (−66%) was greater than for meropenem (−33%), this increased the risk of underdosing in patients receiving ceftazidime-avibactam. These results should be interpreted with caution because patients with renal impairment at baseline represented only a small subgroup (8% of the MITT population); however, similar results have also been noted with ceftolozane-tazobactam, another β-lactamase/β-lactam combination [22].
Subsequent pharmacokinetic/pharmacodynamic modeling has been completed to calculate the probability of target attainment, taking into account improving renal function during the critical phase of treatment [23, 24]. Using these models, revised dose adjustments for moderate (and severe) renal impairment at baseline have been established, ensuring that optimal ceftazidime-avibactam plasma levels for target attainment are achieved in the future [12].
Considering the known safety profile for metronidazole, ceftazidime-avibactam had a safety and tolerability profile broadly similar to ceftazidime alone and meropenem. Most AEs were mild or moderate in both groups, with low incidences of discontinuations or deaths due to AEs and few serious AEs.
A potential limitation of the study is the enrollment of a high proportion of patients (>80%) with an APACHE II score ≤10 and a relatively large proportion of patients (approximately 40%) with cIAI related to the appendix. Nevertheless, these proportions were both somewhat lower in the present study than in a recent study evaluating the cephalosporin-β-lactamase inhibitor combination, ceftolozane-tazobactam, in patients with cIAI [25]. The source of infection and pathogens implicated are also similar to those observed in other cIAI clinical studies in recent years [26, 27]. There was a small imbalance between groups in the proportion of patients enrolled with bacteremia (4.2% in the ceftazidime-avibactam plus metronidazole group and 2.7% in the meropenem group). However, other markers of more critically ill patients, such as the APACHE II score, tended to be evenly distributed between groups.
In conclusion, ceftazidime-avibactam plus metronidazole was effective in treating hospitalized adults with cIAI, as demonstrated by noninferiority to meropenem. The clinical cure rate with ceftazidime-avibactam plus metronidazole was comparable to that with meropenem in patients with Gram-negative pathogens resistant to ceftazidime and similar to its own efficacy against ceftazidime-susceptible infections. There were no new safety signals, and the safety profile of ceftazidime-avibactam plus metronidazole was comparable to those of ceftazidime, metronidazole, and meropenem alone. These results support the use of ceftazidime-avibactam plus metronidazole as a potential alternative to carbapenems in the treatment of cIAI.
Notes
Acknowledgments. We thank all investigators and patients involved in these 2 studies. Medical writing support was provided by Catherine Savage of Prime Medica Ltd, funded by AstraZeneca.
Disclaimer. The design and conduct of the study, analysis of the study data, and opinions, conclusions, and interpretation of the data are the responsibility of the authors. The funder of the study was responsible for study design and data collection. All authors had full access to the data in the study.
Financial support. This work was supported by AstraZeneca. Ceftazidime-avibactam is being developed by AstraZeneca and Allergan (formerly Actavis).
Potential conflicts of interest. J. E. M. has received research support from AstraZeneca, Bayer, and Merck; consultant payments from Astra-Zeneca, Bayer, Cubist, and Pfizer; and a speaker's honorarium from Merck; and has served as a consultant on an advisory board sponsored by Allergan (formerly Actavis). L. B. G. is a former employee of AstraZeneca and was a shareholder of AstraZeneca at the time of the present studies. J. A., H. B., G. G. S., and P. N. are employees and shareholders of AstraZeneca. D. R. is a former employee of Actavis and current consultant to Allergan, and L. L. is a former employee of Actavis. J. P.'s institution has received funding from AstraZeneca for the current studies. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




