-
PDF
- Split View
-
Views
-
Cite
Cite
Angela Colbers, Brookie Best, Stein Schalkwijk, Jiajia Wang, Alice Stek, Carmen Hidalgo Tenorio, David Hawkins, Graham Taylor, Regis Kreitchmann, Sandra Burchett, Annette Haberl, Kabamba Kabeya, Marjo van Kasteren, Elizabeth Smith, Edmund Capparelli, David Burger, Mark Mirochnick, for the PANNA Network and the IMPAACT 1026 Study Team, M.E. van der Ende, MC Erasmus, A.J.A.M. van der Ven, J. Nellen, J. Moltó, E. Nicastri, C. Giaquinto, A. Gingelmaier, F. Lyons, J. Lambert, C. Wyen, G. Faetkenheuer, J.K. Rockstroh, C. Schwarze-Zander, S Tariq Sadiq, Y. Gilleece, C. Wood, Shelley Buschur, Chivon Jackson, Mary Paul, Claudia Florez, Patricia Bryan, Monica Stone, Mindy Katz, Raphaelle Auguste, Andrew Wiznia, Karen L. Bruder, Gail Lewis, Denise Casey, Marcelo H. Losso, Silvina A. Ivalo, Alejandro Hakim, Audra Deveikis, Jagmohan Batra, Janielle Jackson Alvarez, Katherine M. Knapp, Nina Sublette, Thomas Wride, Irma L. Febo, Ruth Santos, Vivian Tamayo, for the PANNA Network and the IMPAACT 1026 Study Team, Maraviroc Pharmacokinetics in HIV-1–Infected Pregnant Women, Clinical Infectious Diseases, Volume 61, Issue 10, 15 November 2015, Pages 1582–1589, https://doi.org/10.1093/cid/civ587
Close - Share Icon Share
Abstract
Objective. To describe the pharmacokinetics of maraviroc in human immunodeficiency virus (HIV)–infected women during pregnancy and post partum.
Methods. HIV-infected pregnant women receiving maraviroc as part of clinical care had intensive steady-state 12-hour pharmacokinetic profiles performed during the third trimester and ≥2 weeks after delivery. Cord blood samples and matching maternal blood samples were taken at delivery. The data were collected in 2 studies: P1026 (United States) and PANNA (Europe). Pharmacokinetic parameters were calculated.
Results. Eighteen women were included in the analysis. Most women (12; 67%) received 150 mg of maraviroc twice daily with a protease inhibitor, 2 (11%) received 300 mg twice daily without a protease inhibitor, and 4 (22%) had an alternative regimen. The geometric mean ratios for third-trimester versus postpartum maraviroc were 0.72 (90% confidence interval, .60–.88) for the area under the curve over a dosing interval (AUCtau) and 0.70 (0.58–0.85) for the maximum maraviroc concentration. Only 1 patient showed a trough concentration (Ctrough) below the suggested target of 50 ng/mL, both during pregnancy and post partum. The median ratio of maraviroc cord blood to maternal blood was 0.33 (range, 0.03–0.56). The viral load close to delivery was <50 copies/mL in 13 women (76%). All children were HIV negative at testing.
Conclusions. Overall maraviroc exposure during pregnancy was decreased, with a reduction in AUCtau and maximum concentration of about 30%. Ctrough was reduced by 15% but exceeded the minimum Ctrough target concentration. Therefore, the standard adult dose seems sufficient in pregnancy.
Clinical Trials Registration. NCT00825929 and NCT000422890.
It is estimated that worldwide 16 million women were living with human immunodeficiency virus (HIV) in 2013, with about 1.3 million of them women giving birth [1, 2]. HIV-infected pregnant women may receive antiretrovirals both to protect their own health and to reduce the risk of mother-to-child transmission of HIV [3]. Combination antiretroviral therapy (cART) has been shown to be a highly effective strategy for prevention of mother-to-child transmission of HIV, reducing the risk from 15%–40% to <2% [3].
Maraviroc is an antagonist to C-C chemokine receptor type 5 (CCR5), which plays an important role in blocking HIV-1 entry into susceptible cells [4]. It is effective for treatment of CCR5-tropic HIV-1 as part of cART therapy. The standard recommended dose for maraviroc therapy in adults or adolescents is 300 mg twice daily, unless coadministered with a boosted protease inhibitor, in which case the dose is reduced to 150 mg twice daily [5]. There are no data available describing maraviroc pharmacokinetics and safety for use during pregnancy, and the US Department of Health and Human Services perinatal guidelines include no recommendations regarding maraviroc therapy or dosing regimens during pregnancy [6].
Pregnancy is associated with a myriad of physical changes that affect the pharmacokinetics of drugs [7, 8], mostly resulting in a reduction in drug exposure during pregnancy [9]. Decreased antiretroviral concentrations may lead to inadequate viral suppression and/or development of antiretroviral resistance and may increase the risk of mother-to-child transmission in HIV-infected pregnant women [3, 10]. Data describing maraviroc pharmacokinetics in pregnancy have not been published to our knowledge.
Specific safety issues for maraviroc during pregnancy include its influence on pregnancy duration and fetal development. Maraviroc is assigned to the Food and Drug Administration pregnancy category B, because animal reproduction studies failed to demonstrate a risk to the fetus but no adequate and well-controlled studies have been performed in pregnant women. Reports on the safety of maraviroc during human pregnancy are limited. The most recent Antiretroviral Pregnancy Registry interim report (through 31 January 2015) [11] includes only a few patients who received maraviroc, who did not exhibit any birth defects with exposure to maraviroc in the first trimester (n = 18) or second and third trimesters (n = 5). No data are available concerning the influence of maraviroc on pregnancy duration to our knowledge.
Current available data on transfer of maraviroc across the human placenta are limited to a single case report and data from an ex vivo placenta perfusion study both indicating minor placental transfer [12, 13]. Fetal antiretroviral exposure via placental transfer may provide preexposure prophylaxis against HIV infection for the fetus and newborn, possibly helping to prevent perinatal transmission of HIV but also possibly resulting in drug-related fetal teratogenicity and/or toxicity [6].
Overall, available data are too limited to make grounded recommendations regarding maraviroc use and dosing regimens during pregnancy, highlighting the exclusion of pregnant women from clinical trials during the development of new drugs, mainly owing to concerns about potential risks to the fetus [14]. As a result, HIV-infected pregnant women are currently receiving maraviroc as part of clinical care in the absence of pregnancy specific safety and pharmacokinetic data. Two protocols—the IMPAACT (International Maternal Pediatric Adolescent AIDS Clinical Trials) Network P1026 protocol and the PANNA (Study on Pharmacokinetics of Newly Developed Antiretroviral Agents in HIV-Infected Pregnant Women) Network—have been developed to study the safety and pharmacokinetics during pregnancy of antiretroviral drugs used for clinical care that lack safety and pharmacokinetic data obtained during pregnancy. For the current report, these networks have collaborated to describe the pharmacokinetics, transplacental transfer, and safety of maraviroc in pregnant HIV-infected women.
METHODS
The data presented in this study were collected in 2 studies. Both were nonrandomized, open-label, parallel-group, multicenter phase-IV studies in HIV-infected pregnant women. PANNA recruited patients from HIV treatment centers in Europe. IMPAACT recruited patients from sites in the Americas. Here, we report data from pregnant HIV-infected patients treated with maraviroc as part of their cART.
The studies were conducted in compliance with the principles of the Declaration of Helsinki. Informed consent was obtained from each participant before study entrance. The studies were approved by the medical ethical committee from each individual center involved and by the national authorities if applicable. The studies were registered at ClinicalTrials.gov (NCT00825929 and NCT000422890).
Patient Eligibility
Patient eligibility included being HIV-infected, pregnant, ≥18 years of age at screening and treated with a cART regimen containing maraviroc as prescribed for clinical care for ≥2 weeks before the day of first pharmacokinetic evaluation. Subjects continued to take their prescribed medications throughout pregnancy. Women continued on study until the completion of postpartum pharmacokinetic sampling. Patients were excluded if they had a past medical history or current condition that might interfere with drug absorption, distribution, metabolism or excretion (such as renal failure or hepatic failure) or presented with grade III/IV anemia (ie, hemoglobin <4.6 mmol/L or <7.4 g/dL) at screening (PANNA specific) or multiple pregnancy (IMPAACT specific).
Safety Assessments and Viral Load
Inclusion screening consisted of: medical history, physical examination, serum biochemistry and hematology, HIV-1 RNA load determination and CD4+ T-cell count. Analyses for safety assessments were performed by local laboratories. Blood samples for safety assessments were further taken at the visits for pharmacokinetic blood sampling and at delivery. Patients were asked for adverse events at each visit and serum biochemistry, hematology, HIV-1 RNA load and CD4+ T-cell count. The HIV-status of the infants was assessed.
Pharmacokinetic Blood Sampling
The 12- or 24-hour intensive pharmacokinetic profiles were performed in the third trimester between 30 and 36 weeks of gestation. Pharmacokinetic sampling was repeated ≥2 weeks (preferably 4–6 weeks) post partum. At delivery (if possible) a cord blood and a maternal blood sample were taken. Evaluation at visits included sampling before and at 1, 2, 4, 6, 8 and 12 hours after medication intake on all pharmacokinetic study days. Subjects in the PANNA study also had samples collected 0.5 and 3 hours after dosing, and subjects receiving once-daily doses had samples collected 24 hours after dosing. Plasma was separated and stored at ≤−18°C until shipment on dry ice to the central laboratory for analysis. Information collected included the time of the 2 prior doses of maraviroc and maternal weight.
Analytical Methods
Concentrations of maraviroc in plasma were analyzed by 2 centers.
The PANNA samples were analyzed at the Ottawa Hospital Research Institute, Ontario, Canada, and the IMPAACT samples at the Pediatric Clinical Pharmacology Laboratory, University of California, San Diego. Both laboratories used a validated liquid chromatography mass spectrometry/mass spectrometry method. The lower limits of quantification were 0.005 mg/L (PANNA) and 0.0039 mg/L (IMPAACT). The linear calibration ranges in plasma were 0.005–1.0 mg/L (PANNA) and 0.0039–2.0 mg/L (IMPAACT). Both pharmacology laboratories participate in the AIDS Clinical Trial Group pharmacology quality control (precision testing) program in the United States, which performs standardized interlaboratory testing twice a year [15].
Pharmacokinetic Assessments
Pharmacokinetic parameters were determined using a noncompartmental model in WinNonlin software (version 6.3; Pharsight). For each individual curve the following were determined: the area under the curve of a dosing period (AUCtau), obtained using the trapezoidal rule; the trough concentration (Ctrough), defined as the sample taken at 12 or 24 hours; the average plasma concentration (Cavg); the maximum concentration (Cmax); the elimination half-life; the time of Cmax; apparent clearance (dose/AUCtau); and the apparent volume of distribution (apparent clearance/elimination rate constant).
Statistical Analysis and Data Handling
Patients with evaluable pharmacokinetic data during pregnancy were included in demographic and safety analyses and descriptive statistics of the pharmacokinetic parameters. Pharmacokinetic parameters are reported as geometric means with 95% intervals for the 150-mg twice-daily maraviroc dose with a protease inhibitor. The pharmacokinetic parameters for the other dosing regimens were reported for each individual, because the data were too limited to be described statistically. Geometric mean ratios with 90% confidence intervals for individual third-trimester and postpartum pharmacokinetic parameters were determined for all parameters by combining data from all dosing regimens, provided that the same maraviroc dose was used during and after pregnancy. A paired t test on the natural log–transformed pharmacokinetic parameters was performed to compare third-trimester and postpartum data. The Mann–Whitney U test was used to compare AUCtau ratios between studies (PANNA vs IMPAACT), testing for between-study differences. Cord blood–maternal blood concentration ratios were determined and recorded.
RESULTS
Eighteen HIV-infected pregnant women (IMPAACT 11; PANNA 7) completed third-trimester sampling between 31 and 38 weeks of gestation, and 14 completed sampling between 4 and 15 weeks post partum. Four subjects withdrew from the study and did not undergo postpartum sampling.
The characteristics of the women and their pregnancy outcomes are shown in Table 1. For the maraviroc regimens, 12 women (67%) received 150 mg twice daily with a protease inhibitor; 2 (11%) received 300 mg twice daily without a protease inhibitor, 2 (11%) received 300 mg once daily with a protease inhibitor, and 2 (11%) received 300 mg twice daily with a protease inhibitor. The protease inhibitors used were darunavir-ritonavir (14 women), lopinavir-ritonavir (1 woman), and atazanavir-ritonavir (1 woman); 10 patients also received raltegravir. Eleven women started maraviroc treatment before pregnancy, 6 started during pregnancy (2 in the first and 4 in the second trimester), and the start date was not known for 1 patient. None of the women used other medication possibly interacting with maraviroc.
| Characteristic . | Finding . |
|---|---|
| Patients included, No. | 18 (11 IMPAACT; 7 PANNA) |
| Age at delivery, median (range), y | 25 (20–41) |
| Race/ethnicity, No. (%) | |
| Black, non-Hispanic | 9 (50) |
| Hispanic | 6 (33) |
| White | 2 (11) |
| Other or >1 race | 1 (6) |
| Smoking, No. (%) | 7 (21) |
| Alcohol use, No (%) | 4 (12) |
| Maraviroc regimen, No. (%) | |
| 150 mg twice daily with PI | 12 (67) |
| 300 mg twice daily without PI | 2 (11) |
| 300 mg once daily with PI | 2 (11) |
| 300 mg twice daily with PI | 2 (11) |
| NRTIs used (No. [%]) | Combivir (4 [22]), Truvada (3 [17]), tenofovir DF alone (2 [11]), lamivudine alone (1 [6]), abacavir alone (1 [6]), zidovudine alone (1 [6]), and NRTI-free regimen (6 [33]) |
| Other ARVs used (No. [%]) | Raltegravir (10 [56]), etravirine (1 [6]) plus PI, and enfuvirtide (1 [6]) |
| Maraviroc started during pregnancy, No. (%) | 6/17 (35) (unknown in 1) |
| 1st trimester | 2 (12) |
| 2nd trimester | 4 (24) |
| 3rd trimester (n = 18) | |
| Gestational age, median (range), wk | 34 (31–38) |
| Weight, median (range), kg | 75 (48–128) |
| HIV RNA undetectable <50 copies/mL, No. (%) | 13 (72) |
| HIV RNA <400 copies/mL, No. (%) | 15 (83) |
| CD4+ count, median (range), cells/mm3 | 481 (66–1030) |
| Post partum (n = 14) | |
| Time after delivery, median (range), wk | 7 (4–15) |
| Weight, median (range), kg | 73 (56–121) |
| HIV RNA undetectable <50, No. (%) | 11 (73) (missing in 3) |
| HIV RNA <400, No. (%) | 14 (93) (missing in 3) |
| CD4+ count, median (range), cells/ mm3 | 521 (66–1465) |
| Pregnancy outcomes (n = 18) | |
| Gestational age, median (range), wk | 39 (37–41) |
| Cesarean delivery | 12 (67) (unknown in 1) |
| Maternal HIV RNA undetectable <50, No. (%) | 13 (76) (missing in 1) |
| Maternal HIV RNA undetectable <400, No. (%) | 14 (82) (missing in 1) |
| Birth weight, median (range), g | 3215 (2350–3750) |
| Infant uninfected, No. (%) | 18 (100) |
| Characteristic . | Finding . |
|---|---|
| Patients included, No. | 18 (11 IMPAACT; 7 PANNA) |
| Age at delivery, median (range), y | 25 (20–41) |
| Race/ethnicity, No. (%) | |
| Black, non-Hispanic | 9 (50) |
| Hispanic | 6 (33) |
| White | 2 (11) |
| Other or >1 race | 1 (6) |
| Smoking, No. (%) | 7 (21) |
| Alcohol use, No (%) | 4 (12) |
| Maraviroc regimen, No. (%) | |
| 150 mg twice daily with PI | 12 (67) |
| 300 mg twice daily without PI | 2 (11) |
| 300 mg once daily with PI | 2 (11) |
| 300 mg twice daily with PI | 2 (11) |
| NRTIs used (No. [%]) | Combivir (4 [22]), Truvada (3 [17]), tenofovir DF alone (2 [11]), lamivudine alone (1 [6]), abacavir alone (1 [6]), zidovudine alone (1 [6]), and NRTI-free regimen (6 [33]) |
| Other ARVs used (No. [%]) | Raltegravir (10 [56]), etravirine (1 [6]) plus PI, and enfuvirtide (1 [6]) |
| Maraviroc started during pregnancy, No. (%) | 6/17 (35) (unknown in 1) |
| 1st trimester | 2 (12) |
| 2nd trimester | 4 (24) |
| 3rd trimester (n = 18) | |
| Gestational age, median (range), wk | 34 (31–38) |
| Weight, median (range), kg | 75 (48–128) |
| HIV RNA undetectable <50 copies/mL, No. (%) | 13 (72) |
| HIV RNA <400 copies/mL, No. (%) | 15 (83) |
| CD4+ count, median (range), cells/mm3 | 481 (66–1030) |
| Post partum (n = 14) | |
| Time after delivery, median (range), wk | 7 (4–15) |
| Weight, median (range), kg | 73 (56–121) |
| HIV RNA undetectable <50, No. (%) | 11 (73) (missing in 3) |
| HIV RNA <400, No. (%) | 14 (93) (missing in 3) |
| CD4+ count, median (range), cells/ mm3 | 521 (66–1465) |
| Pregnancy outcomes (n = 18) | |
| Gestational age, median (range), wk | 39 (37–41) |
| Cesarean delivery | 12 (67) (unknown in 1) |
| Maternal HIV RNA undetectable <50, No. (%) | 13 (76) (missing in 1) |
| Maternal HIV RNA undetectable <400, No. (%) | 14 (82) (missing in 1) |
| Birth weight, median (range), g | 3215 (2350–3750) |
| Infant uninfected, No. (%) | 18 (100) |
Abbreviations: ARVs, antiretrovirals; DF, disoproxil fumarate; HIV, human immunodeficiency virus; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
| Characteristic . | Finding . |
|---|---|
| Patients included, No. | 18 (11 IMPAACT; 7 PANNA) |
| Age at delivery, median (range), y | 25 (20–41) |
| Race/ethnicity, No. (%) | |
| Black, non-Hispanic | 9 (50) |
| Hispanic | 6 (33) |
| White | 2 (11) |
| Other or >1 race | 1 (6) |
| Smoking, No. (%) | 7 (21) |
| Alcohol use, No (%) | 4 (12) |
| Maraviroc regimen, No. (%) | |
| 150 mg twice daily with PI | 12 (67) |
| 300 mg twice daily without PI | 2 (11) |
| 300 mg once daily with PI | 2 (11) |
| 300 mg twice daily with PI | 2 (11) |
| NRTIs used (No. [%]) | Combivir (4 [22]), Truvada (3 [17]), tenofovir DF alone (2 [11]), lamivudine alone (1 [6]), abacavir alone (1 [6]), zidovudine alone (1 [6]), and NRTI-free regimen (6 [33]) |
| Other ARVs used (No. [%]) | Raltegravir (10 [56]), etravirine (1 [6]) plus PI, and enfuvirtide (1 [6]) |
| Maraviroc started during pregnancy, No. (%) | 6/17 (35) (unknown in 1) |
| 1st trimester | 2 (12) |
| 2nd trimester | 4 (24) |
| 3rd trimester (n = 18) | |
| Gestational age, median (range), wk | 34 (31–38) |
| Weight, median (range), kg | 75 (48–128) |
| HIV RNA undetectable <50 copies/mL, No. (%) | 13 (72) |
| HIV RNA <400 copies/mL, No. (%) | 15 (83) |
| CD4+ count, median (range), cells/mm3 | 481 (66–1030) |
| Post partum (n = 14) | |
| Time after delivery, median (range), wk | 7 (4–15) |
| Weight, median (range), kg | 73 (56–121) |
| HIV RNA undetectable <50, No. (%) | 11 (73) (missing in 3) |
| HIV RNA <400, No. (%) | 14 (93) (missing in 3) |
| CD4+ count, median (range), cells/ mm3 | 521 (66–1465) |
| Pregnancy outcomes (n = 18) | |
| Gestational age, median (range), wk | 39 (37–41) |
| Cesarean delivery | 12 (67) (unknown in 1) |
| Maternal HIV RNA undetectable <50, No. (%) | 13 (76) (missing in 1) |
| Maternal HIV RNA undetectable <400, No. (%) | 14 (82) (missing in 1) |
| Birth weight, median (range), g | 3215 (2350–3750) |
| Infant uninfected, No. (%) | 18 (100) |
| Characteristic . | Finding . |
|---|---|
| Patients included, No. | 18 (11 IMPAACT; 7 PANNA) |
| Age at delivery, median (range), y | 25 (20–41) |
| Race/ethnicity, No. (%) | |
| Black, non-Hispanic | 9 (50) |
| Hispanic | 6 (33) |
| White | 2 (11) |
| Other or >1 race | 1 (6) |
| Smoking, No. (%) | 7 (21) |
| Alcohol use, No (%) | 4 (12) |
| Maraviroc regimen, No. (%) | |
| 150 mg twice daily with PI | 12 (67) |
| 300 mg twice daily without PI | 2 (11) |
| 300 mg once daily with PI | 2 (11) |
| 300 mg twice daily with PI | 2 (11) |
| NRTIs used (No. [%]) | Combivir (4 [22]), Truvada (3 [17]), tenofovir DF alone (2 [11]), lamivudine alone (1 [6]), abacavir alone (1 [6]), zidovudine alone (1 [6]), and NRTI-free regimen (6 [33]) |
| Other ARVs used (No. [%]) | Raltegravir (10 [56]), etravirine (1 [6]) plus PI, and enfuvirtide (1 [6]) |
| Maraviroc started during pregnancy, No. (%) | 6/17 (35) (unknown in 1) |
| 1st trimester | 2 (12) |
| 2nd trimester | 4 (24) |
| 3rd trimester (n = 18) | |
| Gestational age, median (range), wk | 34 (31–38) |
| Weight, median (range), kg | 75 (48–128) |
| HIV RNA undetectable <50 copies/mL, No. (%) | 13 (72) |
| HIV RNA <400 copies/mL, No. (%) | 15 (83) |
| CD4+ count, median (range), cells/mm3 | 481 (66–1030) |
| Post partum (n = 14) | |
| Time after delivery, median (range), wk | 7 (4–15) |
| Weight, median (range), kg | 73 (56–121) |
| HIV RNA undetectable <50, No. (%) | 11 (73) (missing in 3) |
| HIV RNA <400, No. (%) | 14 (93) (missing in 3) |
| CD4+ count, median (range), cells/ mm3 | 521 (66–1465) |
| Pregnancy outcomes (n = 18) | |
| Gestational age, median (range), wk | 39 (37–41) |
| Cesarean delivery | 12 (67) (unknown in 1) |
| Maternal HIV RNA undetectable <50, No. (%) | 13 (76) (missing in 1) |
| Maternal HIV RNA undetectable <400, No. (%) | 14 (82) (missing in 1) |
| Birth weight, median (range), g | 3215 (2350–3750) |
| Infant uninfected, No. (%) | 18 (100) |
Abbreviations: ARVs, antiretrovirals; DF, disoproxil fumarate; HIV, human immunodeficiency virus; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Pharmacokinetics
The mean plasma concentration-time profiles of maraviroc for the regimen of 150 mg twice daily with a protease inhibitor in the third trimester and post partum are presented in Figure 1 [16]; summary statistics for the pharmacokinetic parameters for that regimen are listed in Table 2. Individual pharmacokinetic parameters for the other maraviroc treatment regimens can be found in Table 3. Figure 2 presents the individual AUCtau ratios (third trimester/post partum) for the different maraviroc treatment regimens. Individual ratios did not indicate obvious differences between these regimens. When the ratio between pregnancy and postpartum pharmacokinetic parameters for all subjects were compared (with separate regimens pooled together), significant reductions were seen in AUCtau (28%; P = .008) and Cmax (30%; P = .007) (Table 4). The concentration at the last time point (Clast) was reduced by 15% (P = .10), and the elimination half-life was marginally increased (4%, P = .41). The AUCtau ratios did not differ significantly between women in PANNA and the IMPAACT studies (P = .76), indicating no between-study differences, although statistical power to exclude a difference between the cohorts was limited. In all women except 1, maraviroc concentrations both during pregnancy and post partum were above the suggested minimum target Ctrough values of 50 ng/mL for antiretroviral therapy–experienced patients with resistant HIV-1 strains [5]. Furthermore, all patients had a Cavg above the suggested threshold of 75 ng/mL [17]. The patient with the subtherapeutic Ctrough showed Cavg values just above the threshold (76 and 83 ng/mL). The woman whose Ctrough did not exceed the target Ctrough received maraviroc 300 mg twice daily without a protease inhibitor. Her third-trimester and postpartum 12-hour concentrations were 29.7 and 33.4 ng/mL, respectively. The predose concentrations were 24.6 ng/mL (third trimester) and 24.2 ng/mL (post partum,) respectively.
Pharmacokinetic Parameters During Pregnancy and Post Partum for Standard Maraviroc Regimena
| Parameter . | Geometric Mean (95% CI) . | |
|---|---|---|
| 3rd Trimester (n = 12) . | Post Partum (n = 10) . | |
| AUCtau, ng ⋅ mL/h | 2717 (2038–3622) | 3645 (2429–5469) |
| Cmax, ng/mL | 448 (318–632) | 647 (408–1025) |
| tmax, hb | 3.09 (1–6) | 2.03 (0.98–4) |
| t½ , h | 5.7 (4.2–7.7) | 5.5 (4.1–7.5) |
| Clast, ng/mL | 108 (81–145) | 128 (89–184) |
| CLss/F, L/h | 55 (41–74) | 44 (28–69) |
| Vdss/F, L | 456 (293–711) | 352 (181–683) |
| Cavg, ng/mL | 226 (170–302) | 304 (202–456) |
| Parameter . | Geometric Mean (95% CI) . | |
|---|---|---|
| 3rd Trimester (n = 12) . | Post Partum (n = 10) . | |
| AUCtau, ng ⋅ mL/h | 2717 (2038–3622) | 3645 (2429–5469) |
| Cmax, ng/mL | 448 (318–632) | 647 (408–1025) |
| tmax, hb | 3.09 (1–6) | 2.03 (0.98–4) |
| t½ , h | 5.7 (4.2–7.7) | 5.5 (4.1–7.5) |
| Clast, ng/mL | 108 (81–145) | 128 (89–184) |
| CLss/F, L/h | 55 (41–74) | 44 (28–69) |
| Vdss/F, L | 456 (293–711) | 352 (181–683) |
| Cavg, ng/mL | 226 (170–302) | 304 (202–456) |
Abbreviations: AUCtau, area under the curve of a dosing period; Cavg, average concentration; CI, confidence interval; CL, clearance; Clast, concentration at last time point (12 or 24 h after dosing); Cmax, maximum concentration; F, oral bioavailability; t½, half-life; tmax, time of maximum concentration; Vd, apparent volume of distribution.
a Most patients (n = 12) received 150 mg of maraviroc twice daily, with a protease inhibitor.
btmax was reported as median (range).
Pharmacokinetic Parameters During Pregnancy and Post Partum for Standard Maraviroc Regimena
| Parameter . | Geometric Mean (95% CI) . | |
|---|---|---|
| 3rd Trimester (n = 12) . | Post Partum (n = 10) . | |
| AUCtau, ng ⋅ mL/h | 2717 (2038–3622) | 3645 (2429–5469) |
| Cmax, ng/mL | 448 (318–632) | 647 (408–1025) |
| tmax, hb | 3.09 (1–6) | 2.03 (0.98–4) |
| t½ , h | 5.7 (4.2–7.7) | 5.5 (4.1–7.5) |
| Clast, ng/mL | 108 (81–145) | 128 (89–184) |
| CLss/F, L/h | 55 (41–74) | 44 (28–69) |
| Vdss/F, L | 456 (293–711) | 352 (181–683) |
| Cavg, ng/mL | 226 (170–302) | 304 (202–456) |
| Parameter . | Geometric Mean (95% CI) . | |
|---|---|---|
| 3rd Trimester (n = 12) . | Post Partum (n = 10) . | |
| AUCtau, ng ⋅ mL/h | 2717 (2038–3622) | 3645 (2429–5469) |
| Cmax, ng/mL | 448 (318–632) | 647 (408–1025) |
| tmax, hb | 3.09 (1–6) | 2.03 (0.98–4) |
| t½ , h | 5.7 (4.2–7.7) | 5.5 (4.1–7.5) |
| Clast, ng/mL | 108 (81–145) | 128 (89–184) |
| CLss/F, L/h | 55 (41–74) | 44 (28–69) |
| Vdss/F, L | 456 (293–711) | 352 (181–683) |
| Cavg, ng/mL | 226 (170–302) | 304 (202–456) |
Abbreviations: AUCtau, area under the curve of a dosing period; Cavg, average concentration; CI, confidence interval; CL, clearance; Clast, concentration at last time point (12 or 24 h after dosing); Cmax, maximum concentration; F, oral bioavailability; t½, half-life; tmax, time of maximum concentration; Vd, apparent volume of distribution.
a Most patients (n = 12) received 150 mg of maraviroc twice daily, with a protease inhibitor.
btmax was reported as median (range).
Pharmacokinetic Parameters During Pregnancy and Post Partum for Alternative Maraviroc Regimens
| Parameters by Maraviroc Regimen . | 3rd Trimester . | Post Partum . | ||
|---|---|---|---|---|
| Individual1 . | Individual2 . | Individual1 . | Individual2 . | |
| Regimen: 300 mg twice daily (no PI) | ||||
| AUCtau, ng ⋅ mL/h | 911 | 1601 | 995 | NA |
| Cmax, ng/mL | 339 | 349 | 368 | NA |
| tmax, h | 1.92 | 6.03 | 0.83 | NA |
| t½ , h | 8.3 | 3.3 | 12.0 | NA |
| Clast, ng/mL | 30 | 91 | 33 | NA |
| CLss/F, L/h | 329 | 187 | 301 | NA |
| Vdss/F, L | 3934 | 890 | 5227 | NA |
| Cavg, ng/mL | 76 | 133 | 83 | NA |
| 3rd Trimester | Post Partum | |||
| Individual3 | Individual4 | Individual3 | Individual4 | |
| Regimen: 300 mg once daily plus PI | ||||
| AUCtau, ng ⋅ mL/h | 5548 | 10 289 | 7368 | 12 990 |
| Cmax, ng/mL | 906 | 1173 | 835 | 1796 |
| tmax, h | 3.00 | 2.05 | 2.08 | 3.00 |
| t½ , h | 7.9 | 7.2 | 7.9 | 5.1 |
| Clast, ng/mL | 56 | 115 | 74 | 90 |
| CLss/F, L/h | 54 | 29 | 41 | 23 |
| Vdss/F, L | 620 | 303 | 466 | 170 |
| Cavg, ng/mL | 231 | 429 | 307 | 541 |
| 3rd Trimester | Post Partum | |||
| Individual5 | Individual6 | Individual5 | Individual6 | |
| Regimen: 300 mg twice daily plus PI | ||||
| AUCtau, ng ⋅ mL/h | 8400 | 5784 | 15 447 | NA |
| Cmax, ng/mL | 1143 | 1246 | 2161 | NA |
| tmax, h | 4.00 | 2.08 | 4.00 | NA |
| t½ , h | 5.1 | 4.0 | 3.7 | NA |
| Clast, ng/mL | 314 | 136 | 482 | NA |
| CLss/F, L/h | 36 | 26 | 19 | NA |
| Vdss/F, L | 264 | 148 | 104 | NA |
| Cavg, ng/mL | 700 | 482 | 1287 | NA |
| Parameters by Maraviroc Regimen . | 3rd Trimester . | Post Partum . | ||
|---|---|---|---|---|
| Individual1 . | Individual2 . | Individual1 . | Individual2 . | |
| Regimen: 300 mg twice daily (no PI) | ||||
| AUCtau, ng ⋅ mL/h | 911 | 1601 | 995 | NA |
| Cmax, ng/mL | 339 | 349 | 368 | NA |
| tmax, h | 1.92 | 6.03 | 0.83 | NA |
| t½ , h | 8.3 | 3.3 | 12.0 | NA |
| Clast, ng/mL | 30 | 91 | 33 | NA |
| CLss/F, L/h | 329 | 187 | 301 | NA |
| Vdss/F, L | 3934 | 890 | 5227 | NA |
| Cavg, ng/mL | 76 | 133 | 83 | NA |
| 3rd Trimester | Post Partum | |||
| Individual3 | Individual4 | Individual3 | Individual4 | |
| Regimen: 300 mg once daily plus PI | ||||
| AUCtau, ng ⋅ mL/h | 5548 | 10 289 | 7368 | 12 990 |
| Cmax, ng/mL | 906 | 1173 | 835 | 1796 |
| tmax, h | 3.00 | 2.05 | 2.08 | 3.00 |
| t½ , h | 7.9 | 7.2 | 7.9 | 5.1 |
| Clast, ng/mL | 56 | 115 | 74 | 90 |
| CLss/F, L/h | 54 | 29 | 41 | 23 |
| Vdss/F, L | 620 | 303 | 466 | 170 |
| Cavg, ng/mL | 231 | 429 | 307 | 541 |
| 3rd Trimester | Post Partum | |||
| Individual5 | Individual6 | Individual5 | Individual6 | |
| Regimen: 300 mg twice daily plus PI | ||||
| AUCtau, ng ⋅ mL/h | 8400 | 5784 | 15 447 | NA |
| Cmax, ng/mL | 1143 | 1246 | 2161 | NA |
| tmax, h | 4.00 | 2.08 | 4.00 | NA |
| t½ , h | 5.1 | 4.0 | 3.7 | NA |
| Clast, ng/mL | 314 | 136 | 482 | NA |
| CLss/F, L/h | 36 | 26 | 19 | NA |
| Vdss/F, L | 264 | 148 | 104 | NA |
| Cavg, ng/mL | 700 | 482 | 1287 | NA |
Abbreviations: AUCtau, area under the curve over a dosing period; Cavg, average concentration; CI, confidence interval; CL, clearance; Clast, concentration at last time point (12 or 24 hours after dosing); Cmax, maximum concentration; F, oral bioavailability; NA, not available; PI, protease inhibitor; t½, half-life; tmax, time of maximum concentration; Vd, apparent volume of distribution.
Pharmacokinetic Parameters During Pregnancy and Post Partum for Alternative Maraviroc Regimens
| Parameters by Maraviroc Regimen . | 3rd Trimester . | Post Partum . | ||
|---|---|---|---|---|
| Individual1 . | Individual2 . | Individual1 . | Individual2 . | |
| Regimen: 300 mg twice daily (no PI) | ||||
| AUCtau, ng ⋅ mL/h | 911 | 1601 | 995 | NA |
| Cmax, ng/mL | 339 | 349 | 368 | NA |
| tmax, h | 1.92 | 6.03 | 0.83 | NA |
| t½ , h | 8.3 | 3.3 | 12.0 | NA |
| Clast, ng/mL | 30 | 91 | 33 | NA |
| CLss/F, L/h | 329 | 187 | 301 | NA |
| Vdss/F, L | 3934 | 890 | 5227 | NA |
| Cavg, ng/mL | 76 | 133 | 83 | NA |
| 3rd Trimester | Post Partum | |||
| Individual3 | Individual4 | Individual3 | Individual4 | |
| Regimen: 300 mg once daily plus PI | ||||
| AUCtau, ng ⋅ mL/h | 5548 | 10 289 | 7368 | 12 990 |
| Cmax, ng/mL | 906 | 1173 | 835 | 1796 |
| tmax, h | 3.00 | 2.05 | 2.08 | 3.00 |
| t½ , h | 7.9 | 7.2 | 7.9 | 5.1 |
| Clast, ng/mL | 56 | 115 | 74 | 90 |
| CLss/F, L/h | 54 | 29 | 41 | 23 |
| Vdss/F, L | 620 | 303 | 466 | 170 |
| Cavg, ng/mL | 231 | 429 | 307 | 541 |
| 3rd Trimester | Post Partum | |||
| Individual5 | Individual6 | Individual5 | Individual6 | |
| Regimen: 300 mg twice daily plus PI | ||||
| AUCtau, ng ⋅ mL/h | 8400 | 5784 | 15 447 | NA |
| Cmax, ng/mL | 1143 | 1246 | 2161 | NA |
| tmax, h | 4.00 | 2.08 | 4.00 | NA |
| t½ , h | 5.1 | 4.0 | 3.7 | NA |
| Clast, ng/mL | 314 | 136 | 482 | NA |
| CLss/F, L/h | 36 | 26 | 19 | NA |
| Vdss/F, L | 264 | 148 | 104 | NA |
| Cavg, ng/mL | 700 | 482 | 1287 | NA |
| Parameters by Maraviroc Regimen . | 3rd Trimester . | Post Partum . | ||
|---|---|---|---|---|
| Individual1 . | Individual2 . | Individual1 . | Individual2 . | |
| Regimen: 300 mg twice daily (no PI) | ||||
| AUCtau, ng ⋅ mL/h | 911 | 1601 | 995 | NA |
| Cmax, ng/mL | 339 | 349 | 368 | NA |
| tmax, h | 1.92 | 6.03 | 0.83 | NA |
| t½ , h | 8.3 | 3.3 | 12.0 | NA |
| Clast, ng/mL | 30 | 91 | 33 | NA |
| CLss/F, L/h | 329 | 187 | 301 | NA |
| Vdss/F, L | 3934 | 890 | 5227 | NA |
| Cavg, ng/mL | 76 | 133 | 83 | NA |
| 3rd Trimester | Post Partum | |||
| Individual3 | Individual4 | Individual3 | Individual4 | |
| Regimen: 300 mg once daily plus PI | ||||
| AUCtau, ng ⋅ mL/h | 5548 | 10 289 | 7368 | 12 990 |
| Cmax, ng/mL | 906 | 1173 | 835 | 1796 |
| tmax, h | 3.00 | 2.05 | 2.08 | 3.00 |
| t½ , h | 7.9 | 7.2 | 7.9 | 5.1 |
| Clast, ng/mL | 56 | 115 | 74 | 90 |
| CLss/F, L/h | 54 | 29 | 41 | 23 |
| Vdss/F, L | 620 | 303 | 466 | 170 |
| Cavg, ng/mL | 231 | 429 | 307 | 541 |
| 3rd Trimester | Post Partum | |||
| Individual5 | Individual6 | Individual5 | Individual6 | |
| Regimen: 300 mg twice daily plus PI | ||||
| AUCtau, ng ⋅ mL/h | 8400 | 5784 | 15 447 | NA |
| Cmax, ng/mL | 1143 | 1246 | 2161 | NA |
| tmax, h | 4.00 | 2.08 | 4.00 | NA |
| t½ , h | 5.1 | 4.0 | 3.7 | NA |
| Clast, ng/mL | 314 | 136 | 482 | NA |
| CLss/F, L/h | 36 | 26 | 19 | NA |
| Vdss/F, L | 264 | 148 | 104 | NA |
| Cavg, ng/mL | 700 | 482 | 1287 | NA |
Abbreviations: AUCtau, area under the curve over a dosing period; Cavg, average concentration; CI, confidence interval; CL, clearance; Clast, concentration at last time point (12 or 24 hours after dosing); Cmax, maximum concentration; F, oral bioavailability; NA, not available; PI, protease inhibitor; t½, half-life; tmax, time of maximum concentration; Vd, apparent volume of distribution.
Geometric Mean Ratios for Maraviroc Pharmacokinetics: Third Trimester Versus Post Partum (n = 14)
| Parameter . | Ratio (90% CI), % . | P Valuea . |
|---|---|---|
| AUCtau | 72 (60–88) | .008 |
| Cmax | 70 (58–85) | .007 |
| t½ | 104 (86–127) | .41 |
| Clast | 85 (72–101) | .10 |
| CLss/F | 131 (105–164) | .04 |
| Vd/F | 139 (95–204) | .12 |
| Cavg | 72 (60–88) | .008 |
| Parameter . | Ratio (90% CI), % . | P Valuea . |
|---|---|---|
| AUCtau | 72 (60–88) | .008 |
| Cmax | 70 (58–85) | .007 |
| t½ | 104 (86–127) | .41 |
| Clast | 85 (72–101) | .10 |
| CLss/F | 131 (105–164) | .04 |
| Vd/F | 139 (95–204) | .12 |
| Cavg | 72 (60–88) | .008 |
Abbreviations: AUCtau, area under the curve of a dosing period; Cavg, average concentration; CI, confidence interval; CL, clearance; Clast, concentration at last time point (12 or 24 hours after dosing); Cmax, maximum concentration; F, oral bioavailability; t½ , half-life; Vd, apparent volume of distribution.
aP values from paired-sample t tests on natural log–transformed parameters.
Geometric Mean Ratios for Maraviroc Pharmacokinetics: Third Trimester Versus Post Partum (n = 14)
| Parameter . | Ratio (90% CI), % . | P Valuea . |
|---|---|---|
| AUCtau | 72 (60–88) | .008 |
| Cmax | 70 (58–85) | .007 |
| t½ | 104 (86–127) | .41 |
| Clast | 85 (72–101) | .10 |
| CLss/F | 131 (105–164) | .04 |
| Vd/F | 139 (95–204) | .12 |
| Cavg | 72 (60–88) | .008 |
| Parameter . | Ratio (90% CI), % . | P Valuea . |
|---|---|---|
| AUCtau | 72 (60–88) | .008 |
| Cmax | 70 (58–85) | .007 |
| t½ | 104 (86–127) | .41 |
| Clast | 85 (72–101) | .10 |
| CLss/F | 131 (105–164) | .04 |
| Vd/F | 139 (95–204) | .12 |
| Cavg | 72 (60–88) | .008 |
Abbreviations: AUCtau, area under the curve of a dosing period; Cavg, average concentration; CI, confidence interval; CL, clearance; Clast, concentration at last time point (12 or 24 hours after dosing); Cmax, maximum concentration; F, oral bioavailability; t½ , half-life; Vd, apparent volume of distribution.
aP values from paired-sample t tests on natural log–transformed parameters.
Ten umbilical cord blood samples were collected with matching maternal blood samples. The median time between the reported last dose and delivery was 10 hours (range, 2–16 hours); the median time between cord blood sample and maternal sample was 2.5 minutes (0–79 minutes). The median concentrations in the maternal and cord blood samples were 222 (range, 26–597) and 52 (4–209) ng/mL, respectively, and the median ratio of cord blood to maternal blood was 0.33 (range, 0.03–0.56).
![Mean concentration-time profiles for maraviroc (150 mg twice daily with protease inhibitor) during pregnancy (third trimester) and post partum. Values represent mean maraviroc concentrations at steady state (with standard deviations); reference concentrations (dashed line) are from Kakuda et al [16].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/61/10/10.1093/cid/civ587/2/m_civ58701.jpeg?Expires=1750313736&Signature=M1NBbHuWezuoza-T2zp4xIvwtg02yH5fsZErUXiG7iy1fqNMlzSsijIsbIHWim8ShlqURvxs2PXE3UBE4DbP-Gp7laHpkOsJf9rI6eILr7Z1oNvS0pu3M8sA-krnmmJvD3ikDlS37PWWz4RDhpzTET1W1sOWTPlVZrINw14cXXE28qaGpnYXqLn8uF68Spp2QoPRv2JRuuENqLVXafgql391aqX0Nqq1m4zMSEsBkZEtD6tg7Ct60TH~AdukDQeWGUOdqSE~pX56XfP6Bmc2CWDOL3~IlF5e~1H-i-QNu2rQVt70lylbAGpjwDdJl8xjJHfmwDstDS4U9AEtc4oLcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Mean concentration-time profiles for maraviroc (150 mg twice daily with protease inhibitor) during pregnancy (third trimester) and post partum. Values represent mean maraviroc concentrations at steady state (with standard deviations); reference concentrations (dashed line) are from Kakuda et al [16].
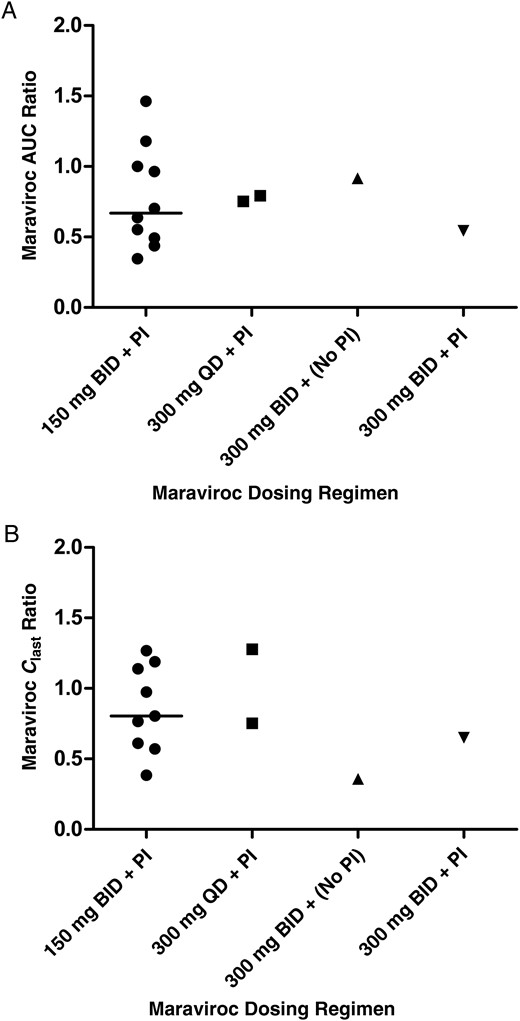
Individual ratios of third-trimester to postpartum values for maraviroc area under the curve (AUC) (A) and concentration at the last time point (Clast) (B), by dosing regimen. For the 150-mg regimen, the medians are shown (line). Abbreviations: BID, twice daily; PI, protease inhibitor; QD, once daily.
Pregnancy Outcome and Safety
The median gestational age at delivery was 39 (range, 37–41), weeks and the median birth weight was 3215 (2350–3750) g. One baby was low birth weight, weighing 2350 g after delivery at 37.9 weeks gestational age. All children tested negative for HIV. Two congenital abnormalities were reported: congenital pulmonary airway malformation (or cystic adenomatoid malformation), for which the relationship to maternal antiretroviral use could not be judged, and sacral dimple, assessed as not related to maternal antiretroviral use.
A serious adverse event occurred in 2 mothers. One had a 3-day hospital admission due to hemoptysis at 38 weeks gestation and was diagnosed with a respiratory tract infection. Another mother was admitted to a psychiatric hospital. These serious adverse event were assessed as not related to maraviroc administration. Two grade 3 or 4 laboratory events were reported: abnormal glucose and potassium levels. HIV viral loads were detectable (>50 copies/mL) for 4 patients around delivery: 55, 2106, 3547, and 5110 copies/mL. These patients received maraviroc at 150 mg twice daily with a protease inhibitor (darunavir/ritonavir) and etravirine (n = 2) and/or raltegravir (n = 3), enfuvirtide (n = 1), and a nucleoside reverse-transcriptase inhibitor backbone (n = 3). Three of these 4 patients had relatively low maraviroc exposure (AUCtau) and Clast in the third trimester. However, 3 patients with an even lower AUCtau and Clast did not show a detectable viral load.
DISCUSSION
We describe the pharmacokinetics of maraviroc in 18 pregnant HIV-infected patients during the third trimester of pregnancy and after delivery. The data were collected by 2 networks: PANNA (Europe) and IMPAACT (United States and Argentina). Overall, maraviroc concentrations were reduced in the third trimester, with reductions in AUCtau (28%), Cmax (30%), and Clast (15%). Transplacental passage of maraviroc was low, with a median ratio of cord blood to maternal blood of 0.33.
In our population of mainly cART-experienced women, 76% had undetectable HIV RNA levels at delivery, and none of the children tested HIV positive. Maraviroc was well tolerated during pregnancy, and infant outcomes were good. The median gestational age at delivery was 39 weeks, with no preterm births.
The maraviroc postpartum pharmacokinetic profiles observed in this study for a maraviroc regimen of 150 mg twice daily with a protease inhibitor were similar to those reported in the literature for nonpregnant adults (mixed sex) [16]. The postpartum curves for the other dosing regimens (300 mg twice daily without a protease inhibitor or 300 mg once daily with a protease inhibitor) were also consistent with the nonpregnant reference pharmacokinetic profiles [18]. Most patients received 150 mg of maraviroc twice daily with a protease inhibitor, and the individual ratios of the patients receiving other regimens fell within the range of ratios reported for this regimen. Consistent with previous assessments of antiretroviral pharmacokinetics in pregnancy, there was substantial interpatient variability. Because the number of patients using alternative regimens were low, we could not directly compare the effect of pregnancy on the different regimens.
Only 1 subject showed maraviroc Ctrough values below the suggested minimum target concentration for antiretroviral therapy–experienced patients with resistant HIV-1 strains of 50 ng/mL, both during pregnancy and post partum [5]. She was receiving the 300-mg twice-daily regimen without a protease inhibitor but with tenofovir and raltegravir. Plasma concentrations of tenofovir were also very low on both occasions, whereas raltegravir exposure was not abnormal (data not shown). This patient reported being adherent for at least the week before pharmacokinetic assessment, and she did not use other medication concomitantly. Her below-target Ctrough values for maraviroc in both during pregnancy and post partum suggests that the low troughs were patient specific and not caused by pregnancy. Despite these low levels, she had an undetectable viral load in the third trimester and post partum, in line with a recent study in which no significant relationship between maraviroc exposure and antiviral response was found [19].
Four subjects had a detectable viral load around delivery. In 3 of them, the maraviroc exposure was relatively low in the third trimester, but 3 other patients showed even lower exposure. Therefore, a relationship between exposure and a detectable viral load could not be demonstrated. The 4 patients with detectable viral loads had complicated treatment regimens (including ≥3 classes of antiretrovirals) and long treatment histories, indicating that they were difficult to treat.
Maraviroc is a CYP450 3A4 substrate, and exposure is increased when it is taken together with the strong CYP3A4 inhibitor ritonavir, leading to the recommendation that maraviroc doses should be decreased when administered along with a boosted protease inhibitor regimen. A limitation of our study is the heterogeneous population and the different doses and antiretroviral regimens used, a consequence of the opportunistic design of both studies. The populations studied by PANNA and IMPAACT mainly received the recommended adult maraviroc dose of 150 mg with a protease inhibitor (67%) or 300 mg without a protease inhibitor (11%) However, our subjects received other maraviroc regimens as well, including 300 mg once or twice daily (both 11%) with a protease inhibitor.
We observed a decrease of about 30% in Cavg and peak concentrations in the third trimester of pregnancy. According to our knowledge, no other data on the influence of human pregnancy on maraviroc pharmacokinetics have been published or presented. A study in rhesus macaques found unchanged maraviroc pharmacokinetics after a single intrapartum dose, compared with results in nonpregnant animals [20]. However, this study is of limited value because it is difficult to compare data from nonhuman primates with human data and to extrapolate exposure with a single dose to long term exposure.
The decrease in exposure to maraviroc observed in our subjects during pregnancy is similar in magnitude to that seen with the protease inhibitors atazanavir [21], darunavir [22], and lopinavir [23]. Several metabolism-related mechanisms could explain the lower maraviroc exposure in pregnancy: increased CYP450 3A4 activity in the gut and liver leading to decreased gastrointestinal absorption due to increased gut metabolism and increased hepatic clearance and/or less boosting by ritonavir associated with lower ritonavir exposure in pregnancy. Unfortunately, paired third-trimester and postpartum data are available for only a single patient not using a protease inhibitor. For this patient, the third-trimester/postpartum ratio of the maraviroc AUCtau was 0.92, compared with 0.35–1.46 for patients receiving a protease inhibitor. Therefore, we cannot conclude that less boosting in pregnancy is the major cause of the lower maraviroc exposure. Other mechanisms may also explain the lower exposure, including reduced intestinal motility, larger plasma volume, and increased hepatic blood flow.
Placenta passage of maraviroc is limited, with a median cord blood–maternal blood ratio of 0.33. This is consistent with the ratio of 0.37 in a single case report [12] and higher than that reported for an ex vivo placenta perfusion model [13]. ABC efflux transporters may play a role, resulting in limiting maraviroc transfer across the placenta to the fetus [13].
In conclusion, although overall maraviroc exposure is 28%–30% lower during pregnancy, Ctrough was reduced to a lesser extent. All except 1 of the subjects met the target Ctrough during pregnancy for antiretroviral resistant HIV-1, suggesting that the standard adult dose seems to be sufficient during pregnancy.
Notes
Acknowledgments. We thank the patients for participating in the studies and the laboratory personnel at the Laboratory of the Ottawa Hospital Research Institute, Ontario, Canada for analyzing the pharmacokinetic samples. We thank the staff from the centers participating in the PANNA network: M. E. van der Ende, MD, PhD, Erasmus MC Rotterdam, the Netherlands; A. J. A. M. van der Ven, MD, PhD, Radboud university medical center, Nijmegen, the Netherlands; J. Nellen, MD, PhD, Academisch Medisch Centrum, Amsterdam, the Netherlands; J. Moltó, MD, PhD, Hospital Universitari Germans Trias I Pujol, Badalona, Spain; A. Antinori, MD, IRCSS, Rome, Italy; E. Nicastri, MD, National Institute for Infectious Diseases “L. Spallanzani,” Rome; C. Giaquinto, MD, PhD, University of Padua, Italy; K. Weizsäcker, MD, PhD, Klinik für Geburtsmedizin, Charité Universitätsmedizin, Berlin, Germany; A. Gingelmaier, MD, PhD, Klinikum der Universität München, Frauenklinik Innenstadt, Munich, Germany; F. Lyons, MD, St James's Hospital Dublin, Ireland; J. Lambert, MD, PhD, Mater Misericordiae University Hospital Dublin; C. Wyen MD, PhD, and G. Faetkenheuer, MD, PhD, University of Cologne, Germany; J. K. Rockstroh MD, PhD, and C. Schwarze-Zander, MD, University of Bonn, Germany; S. Tariq Sadiq, MD, PhD, Institution for Infection and Immunity, St George's, University of London, London, United Kingdom; Y. Gilleece, MD, Brighton and Sussex University Hospitals NHS Trust, Brighton, United Kingdom; and C. Wood, MD, North Middlesex Hospital, London.
The IMPAACT investigators included the following: 3801 Texas Children's Hospital (Clinical Research Center [CRS]) (Shelley Buschur, RN, CNM; Chivon Jackson, RN, BSN, ADN; Mary Paul, MD); 4201 University of Miami Pediatric Perinatal HIV/AIDS (CRS) (Claudia Florez, MD; Patricia Bryan, BSN, MPH; Monica Stone, MD); 5013 Jacobi Medical Center, Bronx (National Institute of Child Health and Human Development [NICHD] CRS) (Mindy Katz, MD; Raphaelle Auguste, RN; Andrew Wiznia, MD); 5018 University of South Florida–Tampa (NICHD CRS) (Karen L. Bruder, MD; Gail Lewis, RN; Denise Casey, RN); 5082 Hospital General de Agudos, Buenos Aires, Argentina (NICHD CRS) (Marcelo H. Losso, MD; Silvina A. Ivalo, MD; Alejandro Hakim, MD); 5093 Miller Children's Hospital (NICHD CRS) (Audra Deveikis, MD; Jagmohan Batra, MD; Janielle Jackson Alvarez, RN); 6501 St Jude Memphis, Tennessee (CRS) (Katherine M. Knapp, MD; Nina Sublette, FNP, PhD; Thomas Wride, MS); 6601 University of Puerto Rico Pediatric HIV/AIDS Research Program (CRS) (Irma L. Febo MD; Ruth Santos, RN, MPH; Vivian Tamayo, MD).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. The PANNA network is supported by the European AIDS Treatment Network, the European Commission, DG Research, Sixth Framework Programme (contract LSHP-CT-2006-037570), Bristol-Myers Squibb, Merck Sharp & Dohme, and Janssen Research. Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases, NIH (grants UM1AI068632 [IMPAACT Leadership and Operations Center], UM1AI068616 [IMPAACT Statistical and Data Management Center], and UM1AI106716 [IMPAACT Laboratory Center]), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




