-
Views
-
Cite
Cite
Taishi Yoshida, Yoshiki Oda, Kaito Sasaki, Mafuyu Ichimura, Yosuke Okamura, Shinichi Koguchi, Yu Nagase, Masashi Higuchi, Naoki Shinyashiki, Tsukasa Ichikawa, Nobukatsu Nemoto, Takeru Ito, Organic–inorganic hybrid composites based on polysiloxane ionic-liquid and polyoxotungstates exhibiting improved thermal stability, Chemistry Letters, Volume 54, Issue 4, April 2025, upaf054, https://doi.org/10.1093/chemle/upaf054
Close - Share Icon Share
Abstract
Organic–inorganic hybrid composites were successfully prepared utilizing polysiloxane ionic-liquid and polyoxometalate inorganic clusters. Imidazolium-based polysiloxane ionic-liquid (denoted as [HPIm6]-Cl) having a cationic skeleton was employed for the anion-exchange reaction to introduce polyoxotungstate ([PW12O40]3− (PW12) or [SiW12O40]4− (SiW12)). The obtained [HPIm6]-PW12 was thermally stable over 450 °C, exhibiting drastically improved thermal stability as compared with the pristine [HPIm6]-Cl. The conductivity of [HPIm6]-PW12 was 2.5× 10−6 S cm−1 at 210 °C without humidification, which will be promising as a polymer electrolyte working over 100 °C under low humidified conditions.
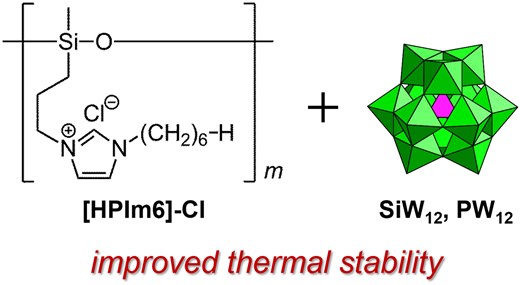
Organic-inorganic hybrid composites were obtained by using polysiloxane ionic-liquid and polyoxometalate. The hybridization drastically improved thermal stability of the composite, which is promising as a proton conductor working over 100 °C.




