-
PDF
- Split View
-
Views
-
Cite
Cite
Aimin Hu, Xiaoqiong Tong, Lijun Yang, Zijuan Shi, Qingwen Long, Maoqing Chen, Yujun Lee, Gender differences in the functional language networks at birth: a resting-state fNIRS study, Cerebral Cortex, Volume 34, Issue 5, May 2024, bhae196, https://doi.org/10.1093/cercor/bhae196
Close - Share Icon Share
Abstract
Numerous studies reported inconsistent results concerning gender influences on the functional organization of the brain for language in children and adults. However, data for the gender differences in the functional language networks at birth are sparse. Therefore, we investigated gender differences in resting-state functional connectivity in the language-related brain regions in newborns using functional near-infrared spectroscopy. The results revealed that female newborns demonstrated significantly stronger functional connectivities between the superior temporal gyri and middle temporal gyri, the superior temporal gyri and the Broca’s area in the right hemisphere, as well as between the right superior temporal gyri and left Broca’s area. Nevertheless, statistical analysis failed to reveal functional lateralization of the language-related brain areas in resting state in both groups. Together, these results suggest that the onset of language system might start earlier in females, because stronger functional connectivities in the right brain in female neonates were probably shaped by the processing of prosodic information, which mainly constitutes newborns’ first experiences of speech in the womb. More exposure to segmental information after birth may lead to strengthened functional connectivities in the language system in both groups, resulting in a stronger leftward lateralization in males and a more balanced or leftward dominance in females.
Introduction
Numerous studies have demonstrated that men and women differ in language performance and language development on average. Although inconsistent results have been obtained concerning gender differences on language tasks such as vocabulary and verbal analogies (Hyde and Linn 1988; Clarke et al. 1990), gender differences favoring women in the tests of speech production and verbal fluency have been clearly shown (Halpern 1992). Furthermore, girls have an earlier and faster development of verbal abilities—talking earlier (Murray et al. 1990), acquiring vocabulary at a faster speed (Roulstone et al. 2002), and so on. Apart from these gender discrepancies in language among general population, gender-related differences are also identified in developmental language-related disorders like dyslexia and stuttering, with lower prevalence for women than men (Wallentin 2009). Furthermore, after left cerebral stroke, females seem to have a better recovery from aphasia than males (Pedersen et al. 1995).
From the perspective of clinical linguistics, research of gender-related differences in language development is vital for diagnosing and treating speech and language disorders effectively (Beitchman et al. 1990; Molini et al. 2017; Lindsay and Kolne 2023). For example, Molini et al. (2017) investigated the risk factors for speech–language disorders in children up to 5 yr of age. They found that there was also a significant association between certain speech–language disorders and male gender, with more boys presenting these disorders than girls. Besides, understanding these gender-specific differences is essential for clinicians to customize intervention strategies that address the unique needs of each gender (Lindsay et al. 2019; Herrmann et al. 2023). For example, a review study by Lindsay et al. (2019) evaluated the efficacy of gender-sensitivity training programs for healthcare providers and addressed the necessity for such training in eradicating gender-based health inequalities. They found that an ongoing effort to integrate gender-sensitivity training within healthcare professional development is needed, though the evidence to firmly deduce its effectiveness remains lacking. They called for more robust, well-designed research to ascertain the long-term impact of such training on healthcare providers’ behaviors and practices, across a wider spectrum of healthcare specializations.
Besides, gender-related differences in language have intrigued many researchers to explore their neurophysiological basis. Nevertheless, the empirical evidence available fails to reach an agreement on the gender differences in brain activation during language tasks. For instance, concerning macroscopic language lateralization, certain task-elicited studies have indicated gender disparities in lateralization patterns (Shaywitz et al. 1995; Hill et al. 2006). Conversely, research employing sizable samples (Frost et al. 1999; Hirnstein et al. 2013) along with multiple meta-analyses (Sommer et al. 2004, 2008; Sato 2020) have revealed predominant gender similarities in the cerebral structuring for language. These inconsistent results may be attributed to some paradigmatic and methodological issues, hindering replication and generalization of prior studies. Among them, task heterogeneity and social and cultural divergence of a particular demographic might be 2 of the factors interfering the evaluation of gender-related disparities on cortical activity and brain functions.
Recently, the resting-state functional connectivity (RSFC) has emerged as 1 of the important approaches to the understanding of the intrinsic mechanism of human brain (Biswal et al. 1995; Honey et al. 2009; van den Heuvel et al. 2009). With RSFC, the intervening factor of task heterogeneity can be excluded. A large number of previous functional magnetic resonance imaging (fMRI) studies have already demonstrated that gender is a significant determinant in the resting functional connectivity, which might lead to behavioral and cognitive differences among individuals (Biswal et al. 2010; Zuo et al. 2010; Tian et al. 2011; Wang et al. 2012; Satterthwaite et al. 2015). Nevertheless, it remains largely unexplored whether gender differences in brain connectivity exist within language-related brain regions in resting state, especially among participants as young as newborns.
Functional near-infrared spectroscopy (fNIRS), another noninvasive brain imaging technique monitoring hymodynamic activity by measurement of changes in the concentration of cerebral hemoglobin, has been increasingly used in the assessment of RSFC (Lu et al. 2009, Zhang et al. 2010). With resting-state fNIRS, neuroscientists would be able to detect brain activity in infants or patients for whom going through long experiments or performing complicated cognitive tasks might be impossible. Moreover, it is also easier for researchers to replicate and compare results due to the ease of the procedure and its relative short duration. Notably, fNIRS is particularly applicable to newborns and very young infants due to its safe, noninvasive, portable, and silent characteristics as well as its relative resilience to motion artifacts. Consequently, it is widely used in the investigation of the origins and developmental patterns of neural mechanisms, especially those pertaining to language development (Gervain et al. 2011).
Gender differences in RSFC using fNIRS, nevertheless, have not been substantially examined. One study investigating gender differences in functional connectivity of the prefrontal cortex (PFC) in adults found that male group displayed stronger connectivity in the PFC areas. As to lateralization, there was leftward dominance in the male participants while bilateral dominance in the female participant (Chuang and Sun 2014). In another study on the functional activity of 4-month-old infants, frontoparietal, somatomotor, visual, and dorsal networks displayed stronger differences across gender (Wang et al. 2023). Despite these 2 studies which reported gender differences in RSFC involving language areas like Broca’s area and frontoparietal regions, effects of gender on functional connectivity of other language-related regions, such as the temporoal gyrus, have not been addressed. Furthermore, psychosocial variables might interfere the evaluation of gender-related disparities on cortical activity and brain functions in adults or 4-month-old infants.
This study, therefore, investigates the effects of gender on RSFC of language-related regions at birth using fNIRS. RSFC in the language system has already been identified in adults and infants in previous fNIRS studies (Zhang et al. 2010; Homae et al. 2011). Language networks in infancy, though still plastic, resemble the organization of adults. Newborns possess the white matter tracts, encompassing the arcuate fasciculus, which link brain areas of the mature language network (Sket et al. 2019). Compared to previous neuroimaging studies on adults, children, and infants, the test of newborns makes it possible to minimize the effect of the social–cultural factors on any gender differences of the human brain and approach the biological origin of any gender differences. In the meantime, compared to the task-elicited studies, examination of the RSFC of newborns’ brain can circumvent the aforementioned intervening factor brought by task heterogeneity. Furthermore, the user-friendly technique of fNIRS is especially suitable for newborns and its application in resting-state conditions further enhances the reproducibility and comparability of results. In this study, the gender effects on both the RSFC of the language-related areas encompassing superior temporal gyri (STG), middle temporal gyri (MTG) and Broca’s area in the bilateral brain and their functional lateralization pattern, if any, will be examined. We hope it can provide insight into the understanding of human brain at birth across gender.
Materials and methods
Participants
Fifty-two healthy, full-term newborns from the Division of Neonatology at the Affiliated Hospital of North Sichuan Medical College participated this study. Of these, data from 36 newborns (mean age: 3 d, range: 0 to 6 d; 18 females) were preserved for final analyses. Sixteen participants were excluded for lack of time duration of 6 min (n = 1) or poor data quality (n = 15). Informed consent was obtained from the newborns’ parents or legal guardians prior to participation. The study was approved by the ethics boards of the Affiliated Hospital of North Sichuan Medical College, with approval number 2023015.
Data acquisition
The fNIRS signals were collected by a multichannel fNIRS system (NirSmartII-3000A, Danyang Huichuang Medical Equipment Co., Ltd, China) with 2 wavelengths of 730 nm and 850 nm at a sampling rate of 11 Hz. Newborns were sleeping in a supine position in the crib in a dimly lit, quiet room at the Affiliated Hospital of North Sichuan Medical College throughout the test session, with 1 parent or grandparent, 1 nurse, and 1 or 2 experimenters present. The babies were placed with a stretchable head cap which covered the brain regions including the prefrontal and temporal lobes. The cap consists of 13 sources and 14 detectors (source–detector separation: 25 mm) forming 36 measurement channels. The spatial sites of sources, detectors, and anchor points (located at Nz, Cz, Al, Ar, lz, and the standard international 10–20 system of electrode placement) were measured on brain template using an electromagnetic 3D digitizer device (Patriot, Polhemus, United States of America). The obtained coordinates were subsequently transformed into Montreal Neurological Institute coordinates (MNI) and afterwards mapped to the MNI standard brain template by spatial registration method in NirSpace (Danyang Huichuang Medical Equipment Co., Ltd, China). Figure 1 demonstrates the mapping between the measurement regions of interest (ROIs) and brain areas. Resting-state data were gathered for more than 8 min for each group, but the first 2-min data was discarded for each participant and only 6 min of data was used for further analysis to maintain the balance between the duration required for the functional connectivity and the maximum number of participants included.
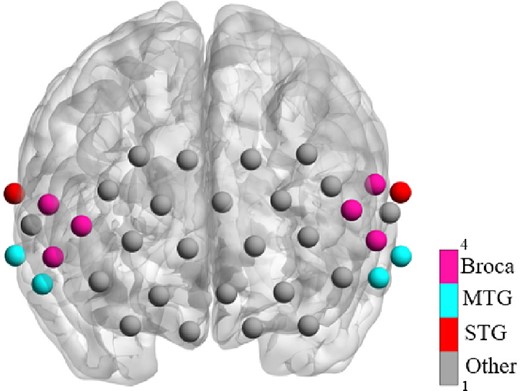
The measurement ROIs used in this study and the corresponding brain areas.
Data processing and statistical analysis
The NirSpark software (HuiChuang, China) package was applied to preprocess the acquired data. Motion artifacts were removed using spline interpolation method. Then, data were bandpass filtered from 0.01 to 0.1 Hz to remove the noise caused by physiological fluctuations such as respiration. Subsequently, using the modified Beer–Lambert law with a differential path length factor of 6 for each wavelength (Kocsis et al. 2006), the relative changes in the concentration of oxygenated hemoglobin, deoxygenated hemoglobin, and total hemoglobin (total-Hb) of each participant were calculated based on the filtered data from the 2 wavelengths (730 and 850 nm). Because a previous study found that total-Hb yielded more robust results to experimental noise (White et al. 2022), this study centered on the concentration changes of total-Hb. After the preprocessing of the data, group-level analysis was conducted to compare the resting functional connectivity and cortical lateralization between males and females.
Functional connectivity
The functional connectivity can be defined as the temporal correlation between brain regions embodied by the detection channels of fNIRS. In this study, 12 channels were divided into 3 ROIs based on their locations (see Fig. 1), encompassing the left superior temporal gyrus, the left middle temporal gyrus, the left Broca’s area (L-Broca), or BA 44 and BA 45 in the left inferior PFC, the right STG (R-STG), the right middle temporal gyrus (R-MTG), and the right Broca’s area (R-Broca).
The software NirSpark was used to extract the low-frequency change of total hemoglobin concentration at various time points, and the transformed total-Hb sequences were employed to establish and evaluate the correlation between 12 channels through the calculation of the Pearson’s correlation coefficient. Then, a 36 × 36 functional connectivity matrix could be obtained from each participant. Later, between-ROI connectivity was analyzed at the group level. To improve normality, Fisher’s r-to-z transformation was performed to change the correlation coefficients to z-scores. Correlation analysis was conducted for each channel in the selected regions to understand the gender-specific effects of RSFC.
Cortical lateralization
To access the degree of hemispheric dominance of fNIRS data, laterality index (LI) was used. LI means the contributions of oxygenation dynamic response made by the volumes of left and right hemispheres. The LI can be defined as LI = (Tl − Tr)/(Tl + Tr), where Tl and Tr represent the mean concentration of total-Hb within the ROI in the left and right hemisphere in resting state, respectively. The LI varies from −1 to 1, with a positive value of L1 signifying leftward dominance and vice versa. Bilateral dominance depending on statistical analysis is indicated by a near-zero value of L1. A 2-tailed t-test was conducted to decide whether L1 is a near-zero value or not. After that, the 1-tailed t-test was conducted to decide a leftward or rightward dominance. Then, 2-sample t-test was performed to determine whether there are significant gender differences in cortical lateralization.
Results
Functional connectivity
The RSFC strengths of the ROIs, which are reflected by total-Hb for the male and female groups, were shown in Fig. 2a and b, respectively. It can be seen from Fig. 2 that intrahemispheric and, especially, adjacent ROIs demonstrated stronger RSFC in both groups. For males, stronger correlations between STG and MTG, and between MTG and Broca’s area in the left hemisphere, and weaker correlations among ROIs in the right hemisphere and the bilateral regions can be observed in Fig. 2a. For females, while the strengths of RSFC in the left hemisphere were similar to those of males, stronger RSFCs among ROIs in the right hemisphere can be observed (Fig. 2b). Further, compared to male neonates, stronger connectivity strengths among bilateral ROIs in female neonates were also shown.
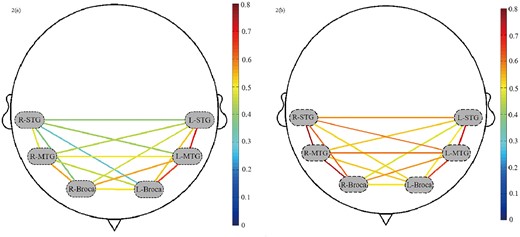
RSFC of ROIs: a) and b) represent the RSFC of ROIs of male and female group respectively. The dashed ovals signify 6 ROIs. The redder lines represent stronger RSFC between the ROIs, and the bluer ones represent the weaker RSFC between the ROIs.
To compare the significantly different ROIs of total-Hb between male and female groups, 2-sample t-tests with P value ≤ 0.05 and false discovery rate were employed. The comparisons of RSFC strengths among the ROIs between the males and females were shown in Fig. 3. As can be seen, the RSFC strengths between the R-STG and R-Broca (P ≤ 0.01), R-STG and R-MTG (P ≤ 0.01), and L-Broca and R-STG (P ≤ 0.05) were significantly different, with stronger connections in these regions in the female group.
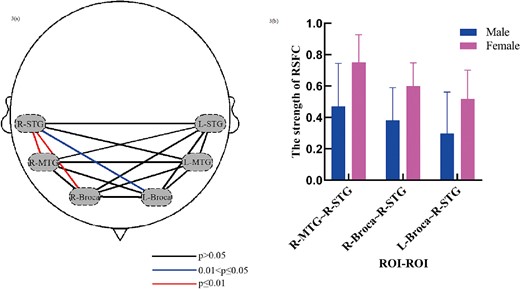
Significant gender differences in brain networks. a) The P values of the inter-group t-test of the RSFC strengths among the ROIs. The dashed ovals signify 6 ROIs. b) The distribution of the strengths of RSFC between the ROIs with significant differences.
Cortical lateralization
Most of the male neonates (n = 12) revealed left-lateralized activation pattern (LI > 0), while most of the female neonates (n = 12) revealed right-lateralized activation pattern (LI < 0; Fig. 4). However, statistics did not show significant hemispheric asymmetry in both groups (males: t = 0.504, P = 0.621; females: t = 1.685, P = 0.11). Two-sample t-tests also failed to show significant differences in the cortical lateralization of the language-related areas between groups (t = 1.284, P = 0.208).

Laterality index distribution of the male and female group. No significant hemipheric asymmetry in both groups was observed (males: P = 0.621; females: P = 0.11) and no significant difference between the 2 distributions was found (P = 0.208).
Discussion
In this study, we employed resting state fNIRS techniques to examine the gender influences on functional connectivity and hemispheric asymmetry of the language-related brain regions—STG, MTG, and Broca’s area. As to functional connectivity, contrary to an fMRI study that did not find any significant gender effect on the intrinsic connections of language-related brain regions in adults (Xu et al. 2020), the present study has provided evidence for gender influences on these language-related brain areas in newborns. Specifically, we observed significant differences in the connectivity between the right STG and MTG, right STG and Broca’s area, as well as between the left Broca’s area and right STG. These differences might partly explain the different language performance between men and women (Wirth et al. 2007; Meyerhoff 2014). At a hemispheric level, however, statistical analysis did not reveal significant hemispheric asymmetry within either group, nor were there significant differences in hemispheric asymmetry between the groups for the selected ROIs. We will discuss these findings in greater detail below.
Both groups showed stronger intrahemispheric connectivities compared with interhemispheric connection in resting state, with greater overall connectivities in females newborns. Our study contrasts with an fMRI study, which demonstrated strong interhemispheric functional connectivities, rather than intrahemispheric connectivities, within the language networks of newborns (Perani et al. 2011). Our result suggests that the adult pattern of predominant intrahemispheric connectivities start to emerge from birth, forming a strong biological basis for its full maturation with more language exposure and brain development.
The data of male group support a proposal by a diffusion tensor imaging (DTI) study that males are optimized for within-hemispheric connectivities (Ingalhalikar et al. 2014). The stronger intrahemispheric and interhemispheric connectivities in females, compared to males, align with another DTI study which showed overall cortical connectivity in adult females (Gong et al. 2009). However, previous DTI studies also proposed that female brains are optimized for interhemispheric connections (Ingalhalikar et al. 2014). Our study, which found stronger intrahemispheric functional connectivities rather than interhemispheric connection in female newborns, seemingly runs counter to this observation. Nevertheless, compared to males, stronger bilateral connectivity in females could be observed. In our study, the connectivity strength between the L-Broca and R-STG in the female group, though not as strong as that between some other regions, significantly outperformed the connectivity strength in the corresponding areas in male counterpart. Our study suggests that the resting-state cortical pattern with stronger interhemispheric connectivities in adult females (Ritchie et al. 2018) than in adult males emerges at birth.
Besides the significant connectivity strength between the L-Broca and R-STG, female newborns demonstrated significantly stronger functional connectivity between the right STG and MTG, and between the right STG and Broca’s area. This finding suggests a stronger neural basis for the processing of prosodic information in female newborns. In adults, fMRI studies have exposed that inferior frontal and temporal regions in the right hemisphere predominantly process prosodic information (Meyer et al. 2002; Gandour et al. 2004). Similarly, in newborns, the right primary and secondary auditory cortex is activated more strongly compared with the left auditory cortex, indicating that newborns rely more on prosodic than segmental information (Perani et al. 2011). Moreover, research has shown that newborns predominantly activate their right primary and secondary auditory cortex in response to music (Perani et al. 2010). Our result extends the previous studies by demonstrating the existence of gender differences in newborns in the right inferior frontal and temporal regions in resting state. Since the first auditory experiences of speech start in utero (Gervain 2015, 2018), this finding suggests that, compared with their males counterparts, female newborns might be more prone to the influences of their first experiences with speech, which mainly involve prosodic information during the gestational period. Further exposure to more language information, such as segmental information primarily processed in the left hemisphere, might encourage stronger functional connectivities in the left brain later in their life compared to those in the first days of life. This finding also suggests that the interplay of genetic origins and environmental influences in the womb or several days after birth is likely to shape the significant gender differences in the functional connectivity between the R-STG and R-MTG, and between the R-STG and R-Broca in newborns.
As to hemispheric lateralization of the language-related regions, statistics suggest rather bilateral dominance of the selected ROIs in both groups, failing to provide strong evidence for cortical lateralization. This study is in alignment with several meta-analyses by Sommer et al. (Sommer et al. 2004, 2008) and some other functional imaging studies which did not observe any gender differences in language lateralization in adults (Springer et al. 1999; Knecht et al. 2000). However, it runs counter to several behavioral studies (Frost et al. 1999), neuroanatomic studies (Witelson and Kigar 1992; Kulynych et al. 1994), positron emission tomography (PET) studies (Wood et al. 1991; Jaeger et al. 1998), and fMRI studies (Shaywitz et al. 1995; Pugh et al. 1996), which demonstrated more bilateral representation of language in adult females than in males. Hirnstein et al. (2013) argued that studies with relatively small sample sizes could have a higher chance of reaching significant results; nevertheless, mere coincidence cannot serve as solid proof for the significant gender differences obtained by several studies with different neuroimaging tools, despite their small sample sizes. Moreover, we need to notice that, though near-zero value of LI was reported in our study, positive LI value indicating left-lateralized activation pattern was shown in most male neonates and negative LI indicating right-lateralized activation pattern was demonstrated in most female counterparts. Whether these lateralized patterns will be strengthened with more language experiences and brain development is yet to know. Therefore, taken together, more studies are required to address potential gender influences on hemispheric lateralization of the language system.
Conclusion
This study reveals gender differences in the RSFC of the language-related regions in the newborns’ brain using the fNIRS technique, providing valuable insights into the neural mechanisms of the language system at birth and offering important clinical implications for language disorders. However, this study failed to observe hemispheric lateralization in both groups, and no significant gender differences in hemispheric lateralization were found. Future studies are suggested to employ larger sample sizes to explore gender differences in functional connectivities and hemispheric laterality of language-related areas and beyond, both in resting state and task-elicited paradigms in newborns, providing further insight into the neural mechanisms of human brain.
Acknowledgments
The authors thank all participants and their guardians for participating in this study. We also thank the reviewers for their constructive comments and suggestions. It is important to note that the authors take full responsibility for any errors that may arise.
Author contributions
Aimin Hu (Conceptualization, Data collection, Methodology, Formal analysis, Writing—original draft, Writing—review & editing), Xiaoqiong Tong (Funding acquisition, Investigation, Methodology, Project administration, Writing—review & editing), Lijun Yang (Investigation, Data collection, resources, Writing—review & editing), Zijuan Shi (Data collection, Software, Methodology, Writing—original draft, Writing—review & editing), Qingwen Long (Data collection, Visualization, Writing—review & editing), Maoqing Chen (Methodology, Validation, Writing—review & editing), and Yujun Lee (Conceptualization, Funding acquisition, resources, Project administration, Supervision, Writing—review & editing).
Funding
This work was supported by the National Social Science Foundation (grant number 18BYY091), the North Sichuan Medical University Affiliated Hospital (grant number 2021JC012), and the 14th Five-Year Plan for Social Science Research in Nanchong City (NC23C141).
Conflict of interest statement: None declared.
Data availability
Generated statement: the original contributions presented in the study are included in the article and further inquiries can be directed to the corresponding author.
References
Author notes
Aimin Hu and Xiaoqiong Tong contributed equally to this study.

