-
PDF
- Split View
-
Views
-
Cite
Cite
Shari S Bassuk, JoAnn E Manson, for the VITAL Research Group, Marine omega-3 fatty acid supplementation and prevention of cardiovascular disease: update on the randomized trial evidence, Cardiovascular Research, Volume 119, Issue 6, May 2023, Pages 1297–1309, https://doi.org/10.1093/cvr/cvac172
Close - Share Icon Share
Abstract
To date, the VITamin D and OmegA-3 TriaL (VITAL) is the only large-scale randomized trial of marine omega-3 fatty acid (n−3 FA) supplementation for cardiovascular disease (CVD) prevention in a general population unselected for elevated cardiovascular risk. We review the findings of VITAL, as well as results from recent secondary prevention trials and updated meta-analyses of n−3 FA trials in the primary and secondary prevention of CVD. In VITAL, a nationwide sample of 25 871 US adults aged 50 and older, including 5106 African Americans, were randomized in a 2 × 2 factorial design to n−3 FAs (1 g/day; 1.2:1 ratio of eicosapentaenoic to docosahexaenoic acid) and vitamin D3 (2000 IU/day) for a median of 5.3 years. Compared with an olive oil placebo, the n−3 FA intervention did not significantly reduce the primary endpoint of major CVD events [composite of myocardial infarction (MI), stroke, and CVD mortality; hazard ratio (HR) = 0.92 (95% confidence interval 0.80–1.06)] but did significantly reduce total MI [HR = 0.72 (0.59–0.90)], percutaneous coronary intervention [HR = 0.78 (0.63–0.95)], fatal MI [HR = 0.50 (0.26–0.97)], and recurrent (but not first) hospitalization for heart failure [HR = 0.86 (0.74–0.998)]. The intervention neither decreased nor increased risk of atrial fibrillation. African Americans derived the greatest treatment benefit for MI and for recurrent hospitalization for heart failure (P interaction < 0.05 for both outcomes). Meta-analyses that include VITAL and high-risk or secondary prevention n−3 FA trials show coronary, but generally not stroke, risk reduction. More research is needed to determine which individuals may be most likely to derive net benefit. (VITAL clinicaltrials.gov identifier: NCT01169259).
1. Introduction
Clarifying the cardiovascular and other benefits and risks of supplemental marine omega-3 fatty acids (n−3 FAs) taken for primary prevention of cardiovascular disease (CVD) is important. Although n−3 FA supplementation has long been recommended for heart health in patients with coronary heart disease (CHD) not meeting target intakes for fatty fish rich in n−3 FAs,1,2 many individuals also take such supplements in the hopes of preventing a first cardiovascular event. In the USA, n−3 FAs have ranked among the most widely used supplements for nearly two decades.3–5 Globally, sales of n−3 FA supplements reached $4.1 billion in 2019 and are projected to double by 2025.6
The VITamin D and OmegA-3 TriaL (VITAL) is a completed investigator-initiated study that tested the effectiveness of supplemental n−3 FAs and, independently, vitamin D for prevention of CVD, cancer, and other outcomes in a general population unselected for elevated cardiovascular risk. At the time of the trial’s initiation, ecologic, laboratory, and observational study data supporting the use of n−3 FAs for primary prevention of CVD were promising but inconclusive and insufficient to establish causality.7 Some8–10 although not all11–13 trials in secondary prevention or high-risk settings had found coronary and CVD risk reductions, but large trials in a general population selected only on age (and not on high risk for CVD) were lacking. VITAL was designed to fill this knowledge gap and remains the only primary prevention trial of its kind to date. In this article, we summarize the trial’s design and results for the n−3 FA intervention. We focus on the trial’s primary and ancillary cardiovascular findings and discuss these data in relation to relevant recent research. We also present the results for an array of non-cardiovascular endpoints as these influence the overall balance of benefits and risks of n−3 FA supplementation in a usual-risk population. Findings from the trial have been published in numerous journals over the past several years, but no prior publication has summarized the results for all available outcomes in one article.
2. Overview of VITAL
VITAL was a nationwide, randomized, double-blind, placebo-controlled trial of the benefits and risks of supplemental marine n−3 FAs [1 g/day Omacor® fish oil capsule with 840 mg of n−3 FAs as ethyl esters, including eicosapentaenoic acid (EPA, 460 mg) and docosahexaenoic acid (DHA, 380 mg)] and vitamin D3 (2000 IU/day) for the primary prevention of CVD and cancer among 25 871 US men aged ≥50 years and women aged ≥55 years.7,14–17 A similar number of men (n = 12 786) and women (n = 13 085) were recruited, and African Americans were oversampled (n = 5106), to allow examination of treatment effects according to sex and race.18 Eligibility criteria were as follows: (i) no prior myocardial infarction (MI), stroke, transient ischaemic attack, coronary revascularization, or cancer (non-melanoma skin cancer was allowed); (ii) no hypercalcaemia or parathyroid disorders, renal failure or dialysis, severe liver disease, anticoagulant use, or other conditions that could present safety issues; (iii) willingness to forego the use of non-study fish-oil supplements and to restrict vitamin D and calcium intakes from multivitamins and other supplements to no more than the recommended dietary allowances for older adults19 of 800 IU/day and 1200 mg/day, respectively; and (iv) successful completion of a placebo run-in lasting 3 months. Participants were randomized to vitamin D, n−3 FAs, both active agents, or both placebos (the placebo for the n−3 FA trial was olive oil) in a 2 × 2 factorial design (Figure 1). The first participant was randomized in November 2011 and the last in March 2014. Randomized treatment ended on schedule on 31 December 2017, yielding a median intervention period of 5.3 years (range, 3.8–6.1 years). Participants are now being followed observationally.
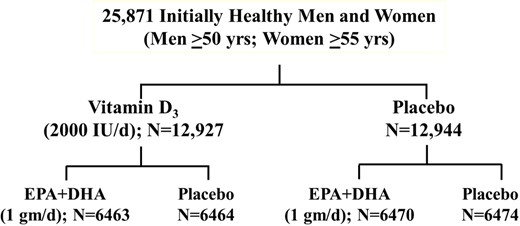
VITAL factorial design. Adapted from Bassuk et al.14 Copyright © 2016, Elsevier. Used with permission.
At baseline, participants answered questionnaires on clinical and lifestyle risk factors for CVD, cancer, and other endpoints of interest. During follow-up, they answered yearly questionnaires to update their risk factor status and provide information on their compliance with treatment and occurrence of endpoints. Participants who reported an endpoint of interest were asked to provide consent for medical record review. VITAL physicians blinded to treatment group reviewed records using established criteria to confirm or disconfirm reported endpoints.20–22 The National Death Index-Plus and other sources were used to ascertain deaths. Pre-randomization blood samples were obtained from willing participants [16 956 of 25 871 randomized participants (66%)], and 1- to 5-year follow-up samples were obtained from ∼6000 participants in ancillary studies. Some participants (n = 1054) who lived within driving distance to a Clinical and Translational Science Center clinic in Boston, Massachusetts, USA, completed in-clinic assessments at baseline and 1, 2, or 4 years later. Ancillary studies examined the effects of the study agents on an array of cardiovascular-related and other outcomes, with the aim of assessing the overall balance of benefits and risks of supplementation. Other than the blood collections, clinic assessments, and selected ancillary studies, VITAL has been conducted pragmatically via postal and electronic communications.
Table 1 shows the baseline characteristics of the study population. During the intervention period, the average response rate to yearly questionnaires was 93%; mortality follow-up exceeded 98%; and adherence to randomized treatment was high.15,16 Among the ∼15 500 participants who had analyzable baseline blood samples, the mean plasma n−3 index, defined as EPA + DHA as a percent of total fatty acids,23 was 2.6% [standard deviation (SD), 0.9%]. A large post-randomization difference in the n−3 index between the active n−3 FA and placebo groups (55% increase) was seen throughout the treatment period. In participants with follow-up measurements, the mean achieved plasma n−3 index with active n−3 FA was ∼4.1%, with negligible change over time in the placebo group. Of note, African American participants had a higher baseline plasma n−3 index than did non-Hispanic white participants [2.83% (SD: 0.93) vs. 2.58% (SD: 0.90), P interaction < 0.001]24 and also had higher baseline intakes of dark-meat fish [1.4 (SD: 3.1) vs. 0.9 (SD: 1.1) servings/week, P interaction < 0.001] and white-meat fish [1.6 (SD: 4.4) vs. 1.0 (SD: 1.2) servings/week, P interaction < 0.001].24
Baseline characteristics of the 25 871 VITAL participants, according to randomized treatment assignment
| Baseline characteristic . | All participants . | n−3 fatty acids . | |
|---|---|---|---|
| Active . | Placebo . | ||
| Total number | 25 871 | 12 933 | 12 938 |
| Female sex—number (%) | 13 085/25 871 (50.6) | 6547 (50.6) | 6538 (50.5) |
| Age, years, mean ± SD | 67.1 ± 7.1 | 67.2 ± 7.1 | 67.1 ± 7.1 |
| Race/ethnicity—number (%)a | |||
| Non-Hispanic White | 18 046/25 304 (71.3) | 9044 (71.5) | 9002 (71.2) |
| African American | 5106/25 304 (20.2) | 2549 (20.1) | 2557 (20.2) |
| Hispanic (not African American) | 1013/25 304 (4.0) | 491 (3.9) | 522 (4.1) |
| Asian/Pacific Islander | 388/25 304 (1.5) | 200 (1.6) | 188 (1.5) |
| Native American | 228/25 304 (0.9) | 120 (0.9) | 108 (0.9) |
| Other or unknown | 523/25 304 (2.1) | 249 (2.0) | 274 (2.2) |
| Body mass index, kg/m2, mean ± SDb | 28.1 (5.7) | 28.1 ± 5.7 | 28.1 ± 5.8 |
| Current smoking—number (%) | 1836/25 485 (7.2) | 920 (7.2) | 916 (7.2) |
| Hypertension treated with medication—number (%) | 12 791/25 698 (49.8) | 6338 (49.3) | 6453 (50.2) |
| Cholesterol-lowering medication, current use—number (%) | 9524/25 428 (37.5) | 4788 (37.7) | 4736 (37.2) |
| Diabetes—number (%) | 3549/25 828 (13.7) | 1799 (13.9) | 1750 (13.5) |
| Current regular aspirin use—number (%)c | 11 570/25 497 (45.4) | 5771 (45.3) | 5799 (45.5) |
| Baseline characteristic . | All participants . | n−3 fatty acids . | |
|---|---|---|---|
| Active . | Placebo . | ||
| Total number | 25 871 | 12 933 | 12 938 |
| Female sex—number (%) | 13 085/25 871 (50.6) | 6547 (50.6) | 6538 (50.5) |
| Age, years, mean ± SD | 67.1 ± 7.1 | 67.2 ± 7.1 | 67.1 ± 7.1 |
| Race/ethnicity—number (%)a | |||
| Non-Hispanic White | 18 046/25 304 (71.3) | 9044 (71.5) | 9002 (71.2) |
| African American | 5106/25 304 (20.2) | 2549 (20.1) | 2557 (20.2) |
| Hispanic (not African American) | 1013/25 304 (4.0) | 491 (3.9) | 522 (4.1) |
| Asian/Pacific Islander | 388/25 304 (1.5) | 200 (1.6) | 188 (1.5) |
| Native American | 228/25 304 (0.9) | 120 (0.9) | 108 (0.9) |
| Other or unknown | 523/25 304 (2.1) | 249 (2.0) | 274 (2.2) |
| Body mass index, kg/m2, mean ± SDb | 28.1 (5.7) | 28.1 ± 5.7 | 28.1 ± 5.8 |
| Current smoking—number (%) | 1836/25 485 (7.2) | 920 (7.2) | 916 (7.2) |
| Hypertension treated with medication—number (%) | 12 791/25 698 (49.8) | 6338 (49.3) | 6453 (50.2) |
| Cholesterol-lowering medication, current use—number (%) | 9524/25 428 (37.5) | 4788 (37.7) | 4736 (37.2) |
| Diabetes—number (%) | 3549/25 828 (13.7) | 1799 (13.9) | 1750 (13.5) |
| Current regular aspirin use—number (%)c | 11 570/25 497 (45.4) | 5771 (45.3) | 5799 (45.5) |
There were no significant differences in the baseline characteristics between the groups.
SD, standard deviation.
Race and ethnic group were reported by participants.
For body mass index, data were missing for 2.4% of participants.
At least monthly.
Adapted from Manson et al.16
Baseline characteristics of the 25 871 VITAL participants, according to randomized treatment assignment
| Baseline characteristic . | All participants . | n−3 fatty acids . | |
|---|---|---|---|
| Active . | Placebo . | ||
| Total number | 25 871 | 12 933 | 12 938 |
| Female sex—number (%) | 13 085/25 871 (50.6) | 6547 (50.6) | 6538 (50.5) |
| Age, years, mean ± SD | 67.1 ± 7.1 | 67.2 ± 7.1 | 67.1 ± 7.1 |
| Race/ethnicity—number (%)a | |||
| Non-Hispanic White | 18 046/25 304 (71.3) | 9044 (71.5) | 9002 (71.2) |
| African American | 5106/25 304 (20.2) | 2549 (20.1) | 2557 (20.2) |
| Hispanic (not African American) | 1013/25 304 (4.0) | 491 (3.9) | 522 (4.1) |
| Asian/Pacific Islander | 388/25 304 (1.5) | 200 (1.6) | 188 (1.5) |
| Native American | 228/25 304 (0.9) | 120 (0.9) | 108 (0.9) |
| Other or unknown | 523/25 304 (2.1) | 249 (2.0) | 274 (2.2) |
| Body mass index, kg/m2, mean ± SDb | 28.1 (5.7) | 28.1 ± 5.7 | 28.1 ± 5.8 |
| Current smoking—number (%) | 1836/25 485 (7.2) | 920 (7.2) | 916 (7.2) |
| Hypertension treated with medication—number (%) | 12 791/25 698 (49.8) | 6338 (49.3) | 6453 (50.2) |
| Cholesterol-lowering medication, current use—number (%) | 9524/25 428 (37.5) | 4788 (37.7) | 4736 (37.2) |
| Diabetes—number (%) | 3549/25 828 (13.7) | 1799 (13.9) | 1750 (13.5) |
| Current regular aspirin use—number (%)c | 11 570/25 497 (45.4) | 5771 (45.3) | 5799 (45.5) |
| Baseline characteristic . | All participants . | n−3 fatty acids . | |
|---|---|---|---|
| Active . | Placebo . | ||
| Total number | 25 871 | 12 933 | 12 938 |
| Female sex—number (%) | 13 085/25 871 (50.6) | 6547 (50.6) | 6538 (50.5) |
| Age, years, mean ± SD | 67.1 ± 7.1 | 67.2 ± 7.1 | 67.1 ± 7.1 |
| Race/ethnicity—number (%)a | |||
| Non-Hispanic White | 18 046/25 304 (71.3) | 9044 (71.5) | 9002 (71.2) |
| African American | 5106/25 304 (20.2) | 2549 (20.1) | 2557 (20.2) |
| Hispanic (not African American) | 1013/25 304 (4.0) | 491 (3.9) | 522 (4.1) |
| Asian/Pacific Islander | 388/25 304 (1.5) | 200 (1.6) | 188 (1.5) |
| Native American | 228/25 304 (0.9) | 120 (0.9) | 108 (0.9) |
| Other or unknown | 523/25 304 (2.1) | 249 (2.0) | 274 (2.2) |
| Body mass index, kg/m2, mean ± SDb | 28.1 (5.7) | 28.1 ± 5.7 | 28.1 ± 5.8 |
| Current smoking—number (%) | 1836/25 485 (7.2) | 920 (7.2) | 916 (7.2) |
| Hypertension treated with medication—number (%) | 12 791/25 698 (49.8) | 6338 (49.3) | 6453 (50.2) |
| Cholesterol-lowering medication, current use—number (%) | 9524/25 428 (37.5) | 4788 (37.7) | 4736 (37.2) |
| Diabetes—number (%) | 3549/25 828 (13.7) | 1799 (13.9) | 1750 (13.5) |
| Current regular aspirin use—number (%)c | 11 570/25 497 (45.4) | 5771 (45.3) | 5799 (45.5) |
There were no significant differences in the baseline characteristics between the groups.
SD, standard deviation.
Race and ethnic group were reported by participants.
For body mass index, data were missing for 2.4% of participants.
At least monthly.
Adapted from Manson et al.16
3. Cardiovascular results of VITAL
3.1 Main effects
Results for CVD endpoints are shown in Table 2. In the total VITAL study population, supplemental n−3 FAs, compared with an olive-oil placebo, did not significantly reduce the primary endpoint of major CVD events [a composite of MI, stroke, and CVD mortality; hazard ratio (HR) = 0.92 [95% confidence interval (CI): 0.80–1.06)] but did reduce total MI, a prespecified secondary endpoint, by a significant 28% [HR = 0.72 (0.59–0.90)]. This benefit emerged after year 1 and persisted throughout the intervention phase (Figure 2). In addition, significant reductions in risk of the exploratory endpoints of percutaneous coronary intervention [PCI; HR = 0.78 (0.63–0.95)], fatal MI [HR = 0.50 (0.26–0.97)], and total CHD [HR = 0.83 (0.71–0.97)] were seen. On the other hand, the n−3 FA intervention was not associated with significantly reduced risks of coronary artery bypass grafting, stroke, CVD mortality, or an expanded CVD endpoint consisting of major CVD events plus coronary revascularization.
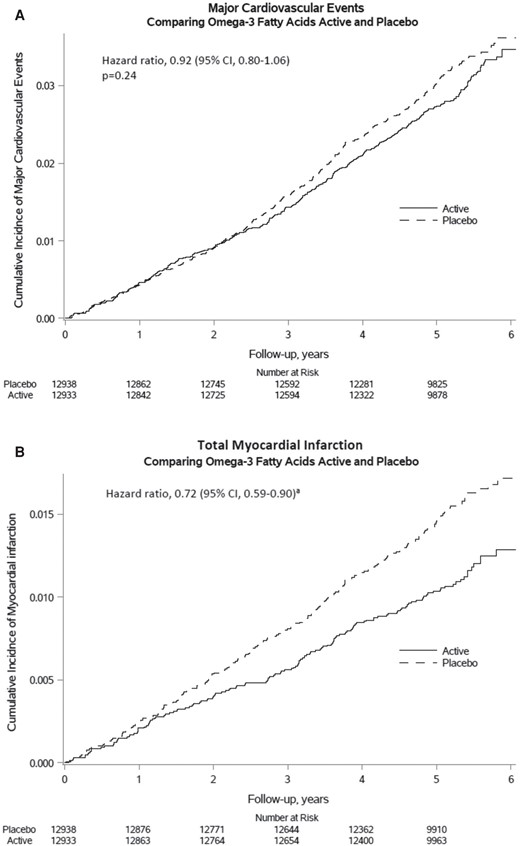
Cumulative incidence rates of major cardiovascular events and total myocardial infarction by year of follow-up, in the n−3 fatty acid group and the placebo group. Adapted from Manson et al.16 Copyright © 2019, Massachusetts Medical Society. Used with permission.
Hazard ratios (HR) and 95% confidence intervals (CI) of cardiovascular outcomes by randomized assignment to n−3 fatty acids (n−3 FAs)a
| Endpoint . | n−3 FAs (N = 12 933) . | Placebo (N = 12 938) . | HR . | 95% CI . |
|---|---|---|---|---|
| No. of participants w/event . | ||||
| Cardiovascular disease (CVD), primary and secondary outcomes | ||||
| Major CVD eventb,c | 386 | 419 | 0.92 | 0.80–1.06 |
| Expanded CVD eventd | 527 | 567 | 0.93 | 0.82–1.04 |
| Total myocardial infarction (MI) | 145 | 200 | 0.72 | 0.59–0.90 |
| Total stroke | 148 | 142 | 1.04 | 0.83–1.31 |
| Cardiovascular mortality | 142 | 148 | 0.96 | 0.76–1.21 |
| Other vascular outcomese | ||||
| Percutaneous coronary intervention (PCI) | 162 | 208 | 0.78 | 0.63–0.95 |
| Coronary artery bypass graft (CABG) | 85 | 86 | 0.99 | 0.73–1.33 |
| Fatal MI | 13 | 26 | 0.50 | 0.26–0.97 |
| Coronary heart disease (CHD) mortality | 37 | 49 | 0.76 | 0.49–1.16 |
| Total CHDf | 308 | 370 | 0.83 | 0.71–0.97 |
| Ischaemic stroke | 111 | 116 | 0.96 | 0.74–1.24 |
| Hemorrhagic stroke | 25 | 19 | 1.32 | 0.72–2.39 |
| Fatal stroke | 22 | 20 | 1.10 | 0.60–2.01 |
| Ancillary outcomes | ||||
| First hospitalization for heart failure | 244 | 255 | 0.96 | 0.80–1.14 |
| Recurrent hospitalization for heart failure | 326 | 379 | 0.86 | 0.74–0.998 |
| Atrial fibrillation | 469 | 431 | 1.09 | 0.96–1.24 |
| Excluding the first two years of follow-up: | ||||
| Major CVD event | 269 | 301 | 0.89 | 0.76–1.05 |
| Total MI | 94 | 131 | 0.72 | 0.55–0.93 |
| Total stroke | 103 | 112 | 0.92 | 0.70–1.20 |
| Endpoint . | n−3 FAs (N = 12 933) . | Placebo (N = 12 938) . | HR . | 95% CI . |
|---|---|---|---|---|
| No. of participants w/event . | ||||
| Cardiovascular disease (CVD), primary and secondary outcomes | ||||
| Major CVD eventb,c | 386 | 419 | 0.92 | 0.80–1.06 |
| Expanded CVD eventd | 527 | 567 | 0.93 | 0.82–1.04 |
| Total myocardial infarction (MI) | 145 | 200 | 0.72 | 0.59–0.90 |
| Total stroke | 148 | 142 | 1.04 | 0.83–1.31 |
| Cardiovascular mortality | 142 | 148 | 0.96 | 0.76–1.21 |
| Other vascular outcomese | ||||
| Percutaneous coronary intervention (PCI) | 162 | 208 | 0.78 | 0.63–0.95 |
| Coronary artery bypass graft (CABG) | 85 | 86 | 0.99 | 0.73–1.33 |
| Fatal MI | 13 | 26 | 0.50 | 0.26–0.97 |
| Coronary heart disease (CHD) mortality | 37 | 49 | 0.76 | 0.49–1.16 |
| Total CHDf | 308 | 370 | 0.83 | 0.71–0.97 |
| Ischaemic stroke | 111 | 116 | 0.96 | 0.74–1.24 |
| Hemorrhagic stroke | 25 | 19 | 1.32 | 0.72–2.39 |
| Fatal stroke | 22 | 20 | 1.10 | 0.60–2.01 |
| Ancillary outcomes | ||||
| First hospitalization for heart failure | 244 | 255 | 0.96 | 0.80–1.14 |
| Recurrent hospitalization for heart failure | 326 | 379 | 0.86 | 0.74–0.998 |
| Atrial fibrillation | 469 | 431 | 1.09 | 0.96–1.24 |
| Excluding the first two years of follow-up: | ||||
| Major CVD event | 269 | 301 | 0.89 | 0.76–1.05 |
| Total MI | 94 | 131 | 0.72 | 0.55–0.93 |
| Total stroke | 103 | 112 | 0.92 | 0.70–1.20 |
Analyses were from Cox regression models that were controlled for age, sex, and randomization group in the vitamin D portion of the trial. Analyses were not adjusted for multiple comparisons.
Primary outcomes.
A composite of MI, stroke, and cardiovascular mortality.
A composite of MI, stroke, cardiovascular mortality, and coronary revascularization (CABG, PCI).
Not prespecified as primary or secondary outcomes.
A composite of MI, coronary revascularization (CABG, PCI), and CHD death.
Adapted from Manson et al.16
Hazard ratios (HR) and 95% confidence intervals (CI) of cardiovascular outcomes by randomized assignment to n−3 fatty acids (n−3 FAs)a
| Endpoint . | n−3 FAs (N = 12 933) . | Placebo (N = 12 938) . | HR . | 95% CI . |
|---|---|---|---|---|
| No. of participants w/event . | ||||
| Cardiovascular disease (CVD), primary and secondary outcomes | ||||
| Major CVD eventb,c | 386 | 419 | 0.92 | 0.80–1.06 |
| Expanded CVD eventd | 527 | 567 | 0.93 | 0.82–1.04 |
| Total myocardial infarction (MI) | 145 | 200 | 0.72 | 0.59–0.90 |
| Total stroke | 148 | 142 | 1.04 | 0.83–1.31 |
| Cardiovascular mortality | 142 | 148 | 0.96 | 0.76–1.21 |
| Other vascular outcomese | ||||
| Percutaneous coronary intervention (PCI) | 162 | 208 | 0.78 | 0.63–0.95 |
| Coronary artery bypass graft (CABG) | 85 | 86 | 0.99 | 0.73–1.33 |
| Fatal MI | 13 | 26 | 0.50 | 0.26–0.97 |
| Coronary heart disease (CHD) mortality | 37 | 49 | 0.76 | 0.49–1.16 |
| Total CHDf | 308 | 370 | 0.83 | 0.71–0.97 |
| Ischaemic stroke | 111 | 116 | 0.96 | 0.74–1.24 |
| Hemorrhagic stroke | 25 | 19 | 1.32 | 0.72–2.39 |
| Fatal stroke | 22 | 20 | 1.10 | 0.60–2.01 |
| Ancillary outcomes | ||||
| First hospitalization for heart failure | 244 | 255 | 0.96 | 0.80–1.14 |
| Recurrent hospitalization for heart failure | 326 | 379 | 0.86 | 0.74–0.998 |
| Atrial fibrillation | 469 | 431 | 1.09 | 0.96–1.24 |
| Excluding the first two years of follow-up: | ||||
| Major CVD event | 269 | 301 | 0.89 | 0.76–1.05 |
| Total MI | 94 | 131 | 0.72 | 0.55–0.93 |
| Total stroke | 103 | 112 | 0.92 | 0.70–1.20 |
| Endpoint . | n−3 FAs (N = 12 933) . | Placebo (N = 12 938) . | HR . | 95% CI . |
|---|---|---|---|---|
| No. of participants w/event . | ||||
| Cardiovascular disease (CVD), primary and secondary outcomes | ||||
| Major CVD eventb,c | 386 | 419 | 0.92 | 0.80–1.06 |
| Expanded CVD eventd | 527 | 567 | 0.93 | 0.82–1.04 |
| Total myocardial infarction (MI) | 145 | 200 | 0.72 | 0.59–0.90 |
| Total stroke | 148 | 142 | 1.04 | 0.83–1.31 |
| Cardiovascular mortality | 142 | 148 | 0.96 | 0.76–1.21 |
| Other vascular outcomese | ||||
| Percutaneous coronary intervention (PCI) | 162 | 208 | 0.78 | 0.63–0.95 |
| Coronary artery bypass graft (CABG) | 85 | 86 | 0.99 | 0.73–1.33 |
| Fatal MI | 13 | 26 | 0.50 | 0.26–0.97 |
| Coronary heart disease (CHD) mortality | 37 | 49 | 0.76 | 0.49–1.16 |
| Total CHDf | 308 | 370 | 0.83 | 0.71–0.97 |
| Ischaemic stroke | 111 | 116 | 0.96 | 0.74–1.24 |
| Hemorrhagic stroke | 25 | 19 | 1.32 | 0.72–2.39 |
| Fatal stroke | 22 | 20 | 1.10 | 0.60–2.01 |
| Ancillary outcomes | ||||
| First hospitalization for heart failure | 244 | 255 | 0.96 | 0.80–1.14 |
| Recurrent hospitalization for heart failure | 326 | 379 | 0.86 | 0.74–0.998 |
| Atrial fibrillation | 469 | 431 | 1.09 | 0.96–1.24 |
| Excluding the first two years of follow-up: | ||||
| Major CVD event | 269 | 301 | 0.89 | 0.76–1.05 |
| Total MI | 94 | 131 | 0.72 | 0.55–0.93 |
| Total stroke | 103 | 112 | 0.92 | 0.70–1.20 |
Analyses were from Cox regression models that were controlled for age, sex, and randomization group in the vitamin D portion of the trial. Analyses were not adjusted for multiple comparisons.
Primary outcomes.
A composite of MI, stroke, and cardiovascular mortality.
A composite of MI, stroke, cardiovascular mortality, and coronary revascularization (CABG, PCI).
Not prespecified as primary or secondary outcomes.
A composite of MI, coronary revascularization (CABG, PCI), and CHD death.
Adapted from Manson et al.16
In ancillary analyses, supplemental n−3 FAs were predictive of a significant reduction in risk of recurrent hospitalization for heart failure [HR = 0.86 (0.74–0.998); P = 0.048] but not risk of first hospitalization for this condition [HR = 0.96 (0.80–1.14); P = 0.61].25 Supplemental n−3 FAs neither decreased nor increased the risk of atrial fibrillation [HR = 1.09 (0.96–1.24); P = 0.19].26 Among 197 participants who suffered a non-fatal stroke during the trial and who completed questionnaires assessing stroke outcomes a median of 1.4 years after diagnosis, those randomized to n−3 FAs had a non-significantly lower risk of functional limitations [as assessed by a physical performance scale adapted from Nagi:27 odds ratio (OR) = 0.55 (0.28–1.09)] and physical disability [Rosow-Breslau Functional Health scale:28 OR = 0.56 (0.31–1.02); modified Katz Activities of Daily Living scale:29 OR = 0.32 (0.50–1.67)] than did those randomized to placebo.30
Biomarker analyses were performed to elucidate potential mechanisms for protective cardiovascular effects of n−3 FAs. Among 1561 participants with 1-year follow-up blood samples, supplemental n−3 FAs had no significant effect on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, tumour necrosis factor receptor 2).31 In addition, n−3 FA supplementation led to a small but significant reduction in triglycerides but was not associated with change in high-density lipoprotein or low-density lipoprotein cholesterol (Olga Demler, unpublished data). In a substudy of 200 of these participants, n−3 FA supplementation led to changes in the bioactive lipidome (a panel of n−3 and n−6 FAs, oxylipins, and related bioactive lipids), which, in turn, had varied associations with changes in the aforementioned downstream lipid and inflammatory biomarkers.32 Further study is needed to clarify the potential importance of the bioactive lipidome for cardioprotection.
3.2 Subgroup effects
Baseline characteristics examined as potential modifiers of n−3 FA treatment effects in VITAL included sex; race/ethnicity; age; traditional cardiovascular risk factors (smoking, diabetes, hypertension, high cholesterol, and parental history of early MI); statin use; aspirin use; dietary fish intake; plasma n−3 index; and randomization to active vitamin D. Of these characteristics, only dietary fish intake significantly modified the effect of supplemental n−3 FAs on both major CVD events and, as shown in Figure 3, total MI (P interaction < 0.05 for each endpoint). Supplemental n−3 FAs were associated with a 19% reduction in major CVD events [HR = 0.81 (0.67–0.98)] and a 40% reduction in MI [HR = 0.60 (0.45–0.81)] in individuals whose fish intake was less than the cohort median of 1½ servings/week but not in those with higher fish intake [major CVD events: HR = 1.08 (0.88–1.32); MI: HR = 0.94 (0.67–1.31)].16 Consistent with this result, treatment-associated reductions in high-sensitivity C-reactive protein were greater in those with low fish intake than in those with high fish intake (P interaction = 0.06).31 Other characteristics did not significantly affect the association between supplemental n−3 FAs and risk of major CVD events. However, there was a suggestive signal for protection in African Americans [HR = 0.74 (0.53–1.03)] that was absent in members of other racial/ethnic groups [non-Hispanic whites: HR = 1.00 (0.85–1.18); others: HR = 0.94 (0.55–1.59); P interaction = 0.26].16 For MI, the presence of traditional cardiovascular risk factors and race/ethnicity were also significant modifiers of the treatment effect. Individuals with a greater number of traditional cardiovascular risk factors were more likely to derive an MI benefit from n−3 FA supplementation than those with fewer risk factors; the treatment-associated HR for those with two or more risk factors was 0.57 (0.41–0.81) but HRs were neutral for those with fewer risk factors (P interaction = 0.047).16 In analyses that considered each risk factor separately, there were greater reductions in MI with supplemental n−3 FAs in participants with hypertension [HR = 0.58 (0.44–0.78)] than in those without hypertension [HR = 1.01 (0.72–1.40); P interaction = 0.014] and in participants with diabetes [HR = 0.40 (0.22–0.74)] than in those without diabetes [HR = 0.80 (0.63–1.00); P interaction = 0.041]. With respect to race/ethnicity, African Americans had a significant 77% reduction in MI with n−3 FA supplementation [HR = 0.23 (0.11–0.47)], whereas other racial/ethnic groups had smaller reductions [non-Hispanic whites: HR = 0.93 (0.73–1.18); others: HR = 0.54 (0.23–1.26); P interaction = 0.001]. African Americans had an MI benefit regardless of fish intake, but non-Hispanic whites benefitted only when their fish intake was low.16 Moreover, the treatment benefit for MI in African Americans persisted after adjustment for presence of cardiovascular risk factors [HR = 0.19 (0.07–0.50)]. In addition to the MI benefit, African Americans also experienced significant treatment-associated reductions in coronary revascularization [HR = 0.51 (0.28–0.92)] and total CHD [HR = 0.61 (0.43–0.88)] but non-Hispanic whites did not [HR = 1.00 (0.82–1.21), P interaction = 0.001, and HR = 1.00 (0.85–1.17), P interaction = 0.004, respectively].

Hazard ratios (HR) and 95% confidence intervals (CI) of total myocardial infarction according to subgroups, comparing n−3 fatty acid and placebo groups. (From Cox regression models controlling for age, sex, and vitamin D randomization group). HR, hazard ratio; CI, confidence interval; MI, myocardial infarction. *Premature MI in a parent (before age 60 in father and before 65 in mother). From: Manson et al.16 Copyright © 2019, Massachusetts Medical Society. Used with permission.
For the ancillary endpoint of heart failure, the presence/absence of type 2 diabetes at baseline modified the effect of n−3 FA supplementation on incidence of first heart failure hospitalization, with HRs of 0.69 (0.50–0.95) and 1.09 (0.88–1.34) in those with and without diabetes, respectively (P interaction = 0.019).33 Diabetes status also modified the treatment effect on incidence of recurrent heart failure hospitalization, with HRs of 0.53 (0.41–0.69) and 1.07 (0.89–1.28) in those with and without diabetes, respectively (P interaction < 0.0001). Race did not modify the effect of supplemental n−3 FAs on incidence of first heart failure hospitalization [HRs of 0.87 (0.60–1.25) and 0.95 (0.77–1.18) in African Americans and non-Hispanic whites, respectively], but it did do so for incidence of recurrent heart failure hospitalization [HRs of 0.65 (0.49–0.88) and 0.90 (0.75–1.08) in African Americans and non-Hispanic whites, respectively; P interaction = 0.0497].33 These findings are consistent with the greater treatment-associated reductions in MI among those with diabetes than in those without diabetes, and in African Americans than in white participants, in VITAL.
4. Discussion of VITAL’s cardiovascular results in context of other research
VITAL’s finding that supplemental n−3 FAs confer significant coronary benefits aligns with results from laboratory and animal studies, and human trials of cardiovascular biomarker endpoints, which suggest that n−3 FAs not only reduce triglycerides but also inflammation, thrombosis, heart rate, blood pressure, and progression of atherosclerotic plaque; and promote nitric-oxide-induced endothelial relaxation and membrane stabilization (Figure 4).7,34–36 Experimental studies point to relevant molecular and gene-regulatory effects.34,36 However, a full understanding of the biologic effects of n−3 FAs, including differences between EPA and DHA, remains elusive. Dose–response curves for some effects plateau at n−3 FA doses of 1 g/day or less,37 but dose–response gradients for major CVD events and/or MI have been found in meta-analyses of available n−3 FA trials38–40 (see below). In observational studies, fish intake,41 dietary or supplemental EPA + DHA intakes,42,43 and n−3 FA biomarkers42,44 show inversely associations with coronary outcomes.
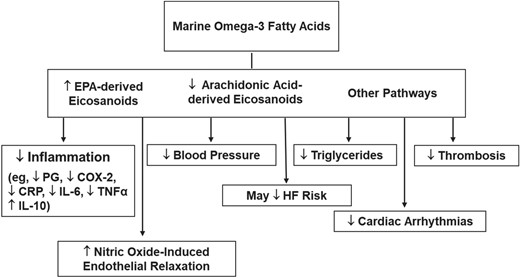
Mechanisms by which marine omega-3 fatty acids may lower cardiovascular disease risk. COX-2, cyclooxygenase-2; CRP, C-reactive protein; EPA, eicosapentaenoic acid; HF, heart failure; IL-6, interleukin-6; IL-10, interleukin-10; PG, prostaglandin; TNFα, tumour necrosis factor-α. Adapted from Manson et al.7 Copyright © 2012, Elsevier. Used with permission.
4.1 Trials of n−3 FAs in high-risk or secondary prevention populations
The treatment benefit on MI and PCI in VITAL—the only large trial of n−3 FAs for primary prevention of CVD in a usual-risk population—is consistent with the collective findings of prior n−3 FA trials testing doses of at least 1 g/day in patients with or at high risk for CVD,45,46 which indicate at least modest effects on coronary endpoints.
4.1.1 ‘First-wave’ trials
Results of the ‘first wave’ of large (n ≥ 5000 participants) n−3 FA trials were published between 1999 and 2013 and are summarized here in chronologic order.
4.1.1.1 GISSI-Prevenzione and JELIS
Supplemental n−3 FAs offered significant coronary benefit in two early open-label trials among individuals with heart disease or at high risk for it. In the GISSI-Prevenzione trial8 (published 1999), conducted among 11 324 patients with recent MI in Italy, supplemental n−3 FAs (850–882 mg EPA and DHA as ethyl esters in the ratio of EPA/DHA 1:2) lowered the risk for a subsequent coronary event (CHD death and non-fatal MI) by 13% (1–24%), cardiac death by 22% (8–35%), and coronary death by 20% (4–33%) over 3.5 years.8 In the Japan EPA Lipid Intervention Study9 (JELIS; published 2007), conducted among 18 645 participants [all were on lipid-lowering (statin) therapy and 20% had a history of CHD at baseline], EPA supplementation (1.8 g/day) reduced the risk for major coronary events (sudden cardiac death, fatal or non-fatal MI, unstable angina pectoris, and coronary revascularization) by 19% (5–31%; P = 0.01) over a mean follow-up of 4.6 years. There were reductions in all components of the primary endpoint except for sudden cardiac death, including a 25% reduction in non-fatal MI (P = 0.086), a 24% reduction in unstable angina (P = 0.014), a 21% reduction in fatal MI (P = 0.56), and a 14% reduction in coronary revascularization (P = 0.135). In the 14 981 participants without a history of CHD at baseline, EPA supplementation was associated with an 18% reduction in major coronary events (P = 0.13). The control groups in these studies, however, were not given placebos, so the findings cannot be considered conclusive.
4.1.1.2 GISSI-HF
The placebo-controlled GISSI-HF trial10 (published 2008), which enrolled 6975 patients with heart failure (>90% with reduced ejection fraction) in Italy, found that n−3 FA supplementation (850–882 mg EPA and DHA as ethyl esters in the average ratio of 1:1.2), added to standard therapy, favourably affected clinical outcomes compared with a placebo oil of unspecified type, reducing cardiovascular-related hospitalizations and CVD mortality by a significant 7% (1–13%) and 10% (1–19%), respectively, and MI by a suggestive 18% (−6% to +37%; P = 0.12), over a median follow-up of 3.9 years.10
4.1.1.3 ORIGIN and Risk and Prevention Study
On the other hand, neither the Outcome Reduction with an Initial Glargine Intervention trial47 (ORIGIN; published 2012), a 40-country 2 × 2 factorial trial that tested 6.2 years of n−3 FAs (1 g/day) and, independently, insulin glargine, against an olive-oil placebo among 12 356 patients with dysglycaemia and at high risk of CVD, nor the Risk and Prevention Study48 (published 2013), which tested 5 years of n−3 FAs (1 g/day) against an olive-oil placebo, among 12 513 patients in Italy with multiple cardiovascular risk factors or atherosclerotic vascular disease but not MI, found protective effects for n−3 FAs on future CVD events,47,48 total MI,47 and/or fatal or non-fatal MI.48
4.1.1.4 Smaller trials
Smaller first-wave trials (n = 2500 to <5000 participants),11–13,49 some of which tested lower n−3 FA doses11,12 or utilized short treatment periods,13 also failed to find cardiovascular or coronary benefits.
4.1.1.5 Meta-analyses of first-wave trials
A number of meta-analyses of first-wave trials were published between 2014 and 2018;42,43,45,46,50 although findings were not entirely consistent, several found modest benefit for n−3 FAs on coronary outcomes. For example, in a 2018 meta-analysis by the Omega-3 Treatment Trialists’ Collaboration of the aforementioned five large trials plus five smaller ones (the 10 trials together had 12 001 incident major vascular events among 77 917 participants), supplemental n−3 FAs (EPA dose range, 226–1800 mg/day; all but JELIS9 tested an EPA–DHA combination) for a mean of 4.4 years (range, 1.0–6.2 years) did not reduce incident major vascular events, major CHD events, stroke, or revascularization,45 although subdividing major CHD events into non-fatal MI and CHD death revealed a borderline-significant 7% reduction in the latter outcome [relative risk (RR) = 0.93 [(99% CI: 0.83–1.03); P = 0.053].51
4.1.2 ‘Second-wave’ trials
Results from subsequent large trials in high-risk populations have been mixed. The findings from these ‘second-wave’ trials were published between 2018 and 2021 and raise new questions about the importance of n−3 FA formulation vs. dose.
4.1.2.1 ASCEND
A Study of Cardiovascular Events in Diabetes (ASCEND), which tested the same EPA–DHA dose and formulation as in VITAL against an olive-oil placebo for a mean of 7.4 years in 15 480 UK adults with diabetes (94% with type 2 diabetes), reported non-significant results for the primary endpoint of serious CVD events [HR = 0.97 (0.87–1.08)] but a significant reduction in vascular death [HR = 0.81 (0.67–0.99)].52 With regard to coronary outcomes, treatment-associated HRs for coronary death, non-fatal MI, and the composite of these two endpoints were 0.79 (0.61–1.02), 0.93 (0.76–1.14), and 0.89 (0.75–1.04), respectively.
4.1.2.2 REDUCE-IT
The Reduction of Cardiovascular Events with Icosapent Ethyl—Intervention Trial (REDUCE-IT), an 11-country study conducted in Europe, North America, Asia, Africa, Australia, and New Zealand (study population was 90% white), tested a median of 4.9 years of high-dose highly purified and stable EPA [icosapent ethyl (4 g/day)] vs. a mineral-oil placebo in 8179 statin users with elevated triglycerides and at high CVD risk (70% with prior CVD, and the rest with diabetes plus other cardiovascular risk factors) and found significant and large reductions in major CVD events [MI, stroke, and CVD death: HR = 0.74 (0.65–0.83)], including MI [HR = 0.69 (0.58–0.81)], stroke [HR = 0.72 (0.55–0.93)], and CVD death [HR = 0.80 (0.66–0.98)].53 The intervention also significantly reduced PCI [HR = 0.68 (0.59–0.79)] and coronary artery bypass grafting [HR = 0.61 (0.45–0.81)].54 For the trial’s primary composite end point of cardiovascular death, non-fatal MI, non-fatal stroke, coronary revascularization, or unstable angina, the treatment-associated HR was 0.75 (0.68–0.83), with a slightly but non-significantly stronger benefit in the secondary prevention cohort [n = 5785 participants; HR = 0.73 (0.65–0.81)] than in the high-risk primary prevention cohort [n = 2394 participants; HR = 0.88 (0.70–1.10); P interaction = 0.14].
4.1.2.3 STRENGTH
The Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH), conducted in North America, Europe, South America, Asia, Australia, New Zealand, and South Africa (study population was 82% white, 10% Asian, 3% Black, and 5% other), tested a high-dose intervention [n−3 carboxylic acids (4 g/day), EPA–DHA ratio of 2.75:1] vs. a corn-oil placebo for a median of 3.5 years in 13 078 statin users with high triglycerides, low high-density lipoprotein cholesterol, and either established atherosclerotic disease or elevated CVD risk.55 The trial was stopped early because of a lack of CVD benefit and an increased risk of atrial fibrillation (see below).55 However, despite null results for most CVD endpoints, there was a suggestive 9% reduction in coronary events in the overall study population [HR = 0.91 (0.81–1.02); P = 0.09] and a significant 15% reduction in coronary events in participants who entered the trial with established CVD [HR = 0.85 (0.75–0.97); P = 0.02].
4.1.2.4 OMEMI
Although not a large study, another recent trial of higher-dose n−3 FAs is worthy of mention. The 2-year Omega-3 Fatty acids in Elderly with Myocardial Infarction trial (OMEMI) in Norway, which tested 1.8 g/day of n−3 FAs (930 mg EPA + 660 mg DHA) vs. a corn-oil placebo in 1027 participants aged 70–82 years with recent MI, had (perhaps not surprisingly, as the trial was underpowered) null results for the primary composite endpoint of non-fatal MI, unscheduled revascularization, stroke, all-cause death, and heart failure hospitalization [HR = 1.08 (0.82–1.41)].56
4.2 Largely concordant findings for stroke in trials of n−3 FAs
Of note, neither VITAL nor the majority of the first- or second-wave secondary prevention trials found a benefit for stroke (the strong reduction in REDUCE-IT is one exception), indicating that future investigations should be designed and powered to analyze potential effects of n−3 FAs on coronary outcomes as distinct endpoints. Interestingly, observational studies also show more consistent effects of n−3 FAs on coronary outcomes than on stroke. As noted earlier, high dietary intakes and high blood levels of n−3 FAs both are associated with reduced coronary risk in observational studies. In contrast, high fish intakes57,58 are more consistently inversely associated with ischaemic stroke risk than are high levels of n−3 FA biomarkers.58–61
4.3 Potential explanations for divergent coronary findings in trials of n−3 FAs
Potential reasons16 for greater coronary benefit in VITAL than in most of the trials of patients with or at high risk for CVD include greater attenuation of incremental n−3 FA benefit by cardiovascular medications such as statins in secondary prevention populations, where medication use is more prevalent; more advanced atherosclerotic disease in high-risk individuals, necessitating more powerful interventions than n−3 FAs or higher n−3 FA doses to avert clinical events; differences in dietary fish intake (few trials have studied this variable); and differences in the racial and ethnic composition of study populations (few trials have included an appreciable number of Black participants, a group with the greatest benefits in VITAL). The discrepancy between the findings of REDUCE-IT and those of other secondary prevention trials, particularly STRENGTH, also highlight uncertainties regarding optimal dosage, EPA–DHA balance, and formulation or chemical structure of the n−3 FA supplement; the role of composition of the placebo; and/or other factors.
Recent meta-analyses38–40 have synthesized the data and attempted to pinpoint reasons for the discrepant findings. A 2021 meta-analysis by Bernasconi et al.39,40 that included all of the aforementioned first- and second-wave trials plus 33 other trials (the 42 trials together had a total of 149 359 participants) reported significant reductions for CHD events [RR = 0.91 (0.85–0.97)], total MI [RR = 0.87 (0.80–0.96)], fatal MI [RR = 0.65 (0.46–0.91)], and CHD death [RR = 0.91 (0.85–0.98)] but not for CVD events [a composite outcome of MI, angina, stroke, heart failure, peripheral artery disease, sudden death, non-scheduled cardiovascular surgical interventions; RR = 0.96 (0.91–1.00)]. Each additional 1 g/day of n−3 FAs was associated with a 9.0% (3.8–13.9%) reduction in MI. (Another recent meta-analysis38 of many of these trials found a statistically significant linear dose–response relationship between n−3 FA dose and risk of major CVD events.) The statistical significance of the treatment effect on total MI and CHD events, though not on fatal MI and CHD death, persisted in ‘leave-one-trial-out’ sensitivity analyses. After adjusting for the effect of dose, a significant interaction by baseline cardiovascular risk emerged, such that, for equal doses, n−3 FA supplementation appeared to be more effective for prevention of total MI in lower risk populations (as seen in VITAL) but, paradoxically, also more effective for prevention of fatal MI in higher-risk populations. [As shown in Table S1 (Supplement B, online), stratified analyses of primary cardiovascular end point data from trials in secondary prevention or high-risk settings generally showed little effect modification by baseline CVD status or vascular risk scores.] In both dose-adjusted and dose-unadjusted analyses, the effects of n−3 FA supplementation appeared to be independent of year of study publication, a finding that does not support the hypothesis that the rising background use of cardiovascular medications in recent decades reduced the opportunity for n−3 FAs to provide incremental benefit. Although the Bernasconi et al. meta-analysis did not directly test for treatment interactions with statins or other cardiovascular medications, the earlier Omega-3 Treatment Trialists’ meta-analysis, ASCEND, and VITAL found no variation in results by statin use. Moreover, in STRENGTH, there was a significant protective effect of the n−3 FA intervention in participants with ezetimibe use but not in those without such use (P interaction = 0.008), and, in REDUCE-IT, there was a significant protective effect of the n−3 FA intervention in participants with high- or moderate-intensity statin use but not in those with low-intensity use (P interaction = 0.12). However, interpretation of the REDUCE-IT data is complicated by the trial’s use of a mineral-oil placebo. It has been argued that use of such a placebo may have interfered with the absorption of, and thus the salutary effects of, statins62 and artificially inflated the n−3 FA benefit by elevating event rates in the comparison group, although a U.S. Food and Drug Administration review panel did not find this explanation convincing.63 In dose-adjusted analyses presented by Bernasconi et al., treatment effects did not vary according to whether only EPA or a combination of EPA–DHA was tested. However, disentangling the effects of n−3 FA dose and composition is challenging because of their strong correlation in available trials (trials testing higher doses have tended to use EPA only, whereas trials testing lower doses have tended to use an EPA–DHA combination).
The above uncertainties are important to resolve. However, findings in secondary prevention settings may not generalize to primary prevention populations, so results of meta-analyses that combine VITAL with trials in higher-risk cohorts may not be helpful in developing or refining guidelines relating to n−3 FA supplementation in usual-risk populations.
4.3.1 Racial/ethnic considerations
Moreover, the combined meta-analyses obscure VITAL’s promising results in African Americans, which are important because of the potential for reducing health disparities. That African Americans, who had a higher baseline plasma n−3 index and fish intake than non-Hispanic whites, were more likely than the latter group to have a treatment benefit for coronary outcomes, including heart failure, was unanticipated and warrants additional study. With rare exceptions,9 trials of n−3 FAs for CVD prevention have enrolled largely white populations and thus have been unable to assess race-specific treatment effects.45 Intriguingly, although the number of non-white participants was modest (∼800) and the number of Black participants was not specified, REDUCE-IT also found a suggestively greater treatment-associated reduction in risk of major CVD events in non-whites [HR = 0.55 (0.38–0.82)] than in whites [HR = 0.76 (0.67–0.86); P interaction = 0.13].53 Moreover, a pooled analysis of 19 observational cohorts from 16 countries found racial variation in relationships between various marine- and plant-derived n−3 FA biomarkers and incident coronary disease, including a significantly stronger inverse association between α-linolenic acid and non-fatal MI in Blacks than in whites.44 Observational data also suggest that variation in genes involved in FA metabolism, including the FA desaturase genes FADS1 and FADS2, may interact with dietary FA intakes to affect risk of CHD and other endpoints,64–66 and that FADS variation in African-ancestry groups differs from such variation in European-ancestry groups.66–68 Clarifying the role of genetic factors may help explain the protective effect of n−3 FA supplementation on MI in African American participants. Also, although statistical adjustment for traditional cardiovascular risk factors did not eliminate the stronger treatment benefits in African Americans as compared with non-Hispanic whites, it is conceivable that racial variation in other characteristics (e.g. clinical, dietary and other lifestyle, socioenvironmental) may at least in part account for this result. For instance, n−3 FAs may counteract the harmful effects of air pollution,69 an environmental factor that affects African Americans70 disproportionately and increases cardiovascular risk.71
4.3.2 Dose considerations
The fact that coronary benefits of supplementation in VITAL were limited to participants with low baseline fish intake (in non-Hispanic white individuals and in the total cohort) suggests that further benefits in primary prevention populations may not accrue beyond a threshold dose. However, VITAL tested only one n−3 FA dose and thus could not determine whether treatment effects vary according to this parameter. Although the dose tested in VITAL is higher than that recommended by the American Heart Association for cardiovascular protection in healthy individuals (based on fish consumption of 1–2 servings per week) and is also the dose recommended by this agency for cardioprotection in patients with CHD,1,2 additional primary prevention trials of higher doses of n−3 FAs are warranted.
4.4 Trials of n−3 FAs and heart failure
The aforementioned meta-analyses did not consider the endpoints of heart failure and atrial fibrillation. With respect to heart failure, results of prospective observational studies of initially healthy populations generally show significant inverse associations between baseline fish or n−3 FA intakes or n−3 FA biomarker levels and incidence of this endpoint.72–74 VITAL is the first large trial of n−3 FAs assessing prevention of heart failure in a population unselected for elevated cardiovascular risk. The VITAL results showed a protective effect against recurrent heart failure hospitalization (in African Americans and in the total cohort) as well as a protective effect against both first and recurrent heart failure hospitalization in people with diabetes. Regarding potential mechanisms for protective effects specifically in individuals with diabetes, a small trial of n−3 FAs among patients with diabetic nephropathy found that the treatment reduced serum advanced glycation end products,75 which promote the onset and progression of heart failure, and improved insulin sensitivity.76 However, meta-analyses of other small, short-term n−3 FA trials in patients with diabetes77 or healthy individuals78 have shown neutral or inconsistent treatment effects on glucose or insulin-related biomarkers. Results of n−3 FA trials for heart failure protection in higher-risk populations have been mixed. Supplemental n−3 FAs reduced risk of heart failure hospitalization by a significant 33% (13–48%) in the Risk and Prevention Study48 and also, as described earlier, reduced the risk of adverse cardiovascular sequelae in individuals with heart failure in the GISSI-HF trial.10 Meta-analyses of small, short-term n−3 FA trials in patients with heart failure79,80 showed improvements in cardiac function, ventricular remodelling, inflammation, and/or fibrosis, as did a 6-month trial in 358 post-MI patients.81 In a mouse model of heart failure, EPA supplementation prevented contractile dysfunction and fibrosis.82,83 On the other hand, n−3 FAs did not lower risk of heart failure (endpoint was heart failure hospitalization unless noted) in ORIGIN47 [HR = 1.02 (0.88–1.19)], ASCEND52 [‘any heart failure’: HR = 0.89 (0.68–1.17)], REDUCE-IT53 [HR = 0.97 (0.77–1.22)],53 STRENGTH55 [‘hospitalization or urgent outpatient visit for heart failure’: HR = 1.12 (0.88–1.42)], or OMEMI56 [HR = 1.19 (0.62–2.26)]. A recent meta-analysis84 of 12 trials (many of the above trials, including VITAL, were included) found no treatment benefit for heart failure hospitalization.
4.5 Trials of n−3 FAs and atrial fibrillation
Regarding atrial fibrillation, n−3 FA supplementation was not significantly associated with this outcome in VITAL or in other trials testing a 1 g/day dose, although the effect estimates were consistently in the direction of harm [ASCEND:52,85 HR = 1.23 (0.98–1.54); Risk and Prevention Study:48 HR = 1.22 (0.93–1.61); GISSI-HF:10,86 HR = 1.10 (0.96–1.26)]. On the other hand, trials testing higher doses found significant [REDUCE-IT:53 5.3% vs. 3.9%, P = 0.003; STRENGTH:55 HR = 1.69 (1.29–2.21), P < 0.001] or near-significant [OMEMI:56 HR = 1.84 (0.98–3.45); P = 0.06] increases in risk of atrial fibrillation. A 2022 meta-analysis87 of these seven trials reported a pooled HR of 1.25 (1.07–1.46), with the risk elevation significantly greater in the trials testing doses exceeding 1 g/day [HR = 1.49 (1.04–2.15); P = 0.013] than in the trials testing doses of 1 g/day or less [HR = 1.12 (1.03–1.22); P = 0.024; P interaction < 0.001]. Observational studies of fish or n−3 FA intakes or n−3 FA biomarkers in relation to this endpoint in initially healthy populations have yielded largely neutral results.88–92 Trials of n−3 FAs have not shown benefit for the prevention of recurrent atrial fibrillation93–95 or postoperative atrial fibrillation in cardiac surgery patients.96,97 It is possible that treatment-associated risk elevations in atrial fibrillation, which is a risk factor for stroke, may offset treatment benefits on other pathways that would be expected to reduce stroke, leading to attenuated benefits on the latter outcome. The findings for atrial fibrillation highlight the need for improved understanding of the balance of dose-specific benefits and risks of supplemental n−3 FAs.
5. Non-cardiovascular results of VITAL
5.1 Cancer
Supplemental n−3 FAs were not associated with VITAL’s co-primary endpoint of incidence of total cancer [HR = 1.03 (0.93–1.13)] or with the secondary endpoints of incident breast [HR = 0.90 (0.70–1.16)], prostate [HR = 1.15 (0.94–1.39)], or colorectal [HR = 1.23 (0.83–1.83)] cancer; or cancer mortality [HR = 0.97 (0.79–1.20)] in the total study population. Baseline dietary fish intake tended to modify the intervention’s effect on cancer incidence (P interaction = 0.09), with a neutral effect in those with low fish intakes [HR = 0.96 (0.84–1.09)] but a trend toward harm in those with high fish intakes [HR = 1.13 (0.98–1.31)]. Sex was also an effect modifier, with treatment-associated HRs for incident cancer of 0.90 (0.78–1.05) and 1.13 (1.00–1.29) in men and women, respectively (P interaction = 0.024).
In an ancillary study of colorectal adenomas,24n−3 FA supplementation did not reduce the risk of either conventional adenomas or serrated polyps in the overall study sample. However, the intervention was associated with a lower risk of conventional adenomas among those with a low plasma n−3 index (below the cohort median of 2.5%) at baseline [OR = 0.76 (0.57–1.02); P interaction by n−3 index = 0.03] and among African Americans [OR = 0.59 (0.35–1.00); P interaction by race = 0.11]. This potential benefit requires confirmation in future studies.
The VITAL findings for cancer agree with those from trials of n−3 FAs for secondary prevention of CVD, which have reported neutral effects or slight (but non-significant) elevations in cancer incidence45,98 and neutral effects or borderline-significant reductions in cancer mortality.10,52,99 (REDUCE-IT and STRENGTH did not report cancer-related endpoints.)
5.2 All-cause mortality
Supplemental n−3 FAs were not associated with all-cause mortality [HR = 1.02 (0.90–1.15)] in the total study population. Baseline dietary fish intake significantly modified the intervention’s effect on all-cause mortality (P interaction = 0.02), with a suggestive protective effect in those with low fish intakes [HR = 0.87 (0.73–1.04)] but a trend toward adverse effects in those with high fish intakes [HR = 1.19 (0.99–1.44)].
The absence of a significant treatment effect in VITAL for all-cause mortality agrees with results of meta-analyses of earlier trials,45,46 ASCEND52 [HR = 0.95 (0.86–1.05)], REDUCE-IT53 [HR = 0.87 (0.74–1.02)], STRENGTH55 [HR = 1.13 (0.97–1.31)], and OMEMI56 [HR = 1.01 (0.60–1.71)]. However, longer follow-up may be needed to detect a benefit, should one exist.
5.3 Safety outcomes
With respect to side effects, the n−3 FA intervention was well tolerated in VITAL, with no treatment-associated increase in bleeding or gastrointestinal symptoms. Regarding bleeding, an excess risk was observed in trials that tested higher-dose EPA (JELIS and REDUCE-IT) but not in trials that tested higher-dose EPA–DHA (STRENGTH and OMEMI) or lower-dose EPA–DHA (VITAL, ORIGIN, Risk and Prevention Study, and ASCEND). (GISSI-Prevenzione and GISSI-HF did not report data on bleeding.) Whether higher-dose EPA formulations are more likely to increase bleeding risk than higher-dose EPA–DHA formulations requires further study.
5.4 Other endpoints
Results of VITAL ancillary studies of other outcomes, including autoimmune disorders,100 age-related macular degeneration (AMD),101 dry eye disease,102 chronic knee pain,103 migraine frequency and severity,104 cognition,105 depression,106 and kidney function in type 2 diabetes107 have been published (Table 3). Supplemental n−3 FAs did not significantly lower the incidence of confirmed autoimmune disease in the total study population [HR = 0.85 (0.67–1.08), P = 0.19], although the beneficial effect was significantly greater among those with a family history of autoimmune disease [HR = 0.66 (0.43–0.99)] than among those with no family history [HR = 1.14 (0.82–1.58); P interaction = 0.03].100 Moreover, in analyses of an expanded endpoint of confirmed plus probable autoimmune disease, n−3 FA supplementation was associated with a significant reduction in risk [HR = 0.82 (0.68–0.99); P = 0.04] in the overall study population, with a significant trend of increasing protection over time (P interaction = 0.04). Although supplemental n−3 FAs did not protect against the development of AMD [HR = 0.93 (0.73–1.17)] or progression of existing AMD [HR = 1.05 (0.56–1.97)], there was a suggestion of benefit in reducing the risk of developing advanced AMD in persons without AMD at baseline [HR = 0.72 (0.48–1.09); P = 0.12].101 The promising findings for autoimmune disease and AMD warrant further examination in future studies. On the other hand, supplemental n−3 FAs did not reduce the incidence of diagnosed dry eye disease or a combined end point of diagnosed dry eye disease or incident severe dry eye disease symptoms;102 did not alleviate chronic knee pain or improve function or stiffness among participants with a history of such pain;103 did not affect migraine frequency or severity among those with a history of migraine;104 did not confer cognitive benefits over 2–3 years in a substudy of 4218 participants aged 60 years or older;105 and did not prevent the onset of depression or clinically relevant depressive symptoms among those without such symptoms at baseline.106 {Indeed, contrary to expectation, the intervention was associated with an elevated risk of this outcome [HR = 1.13 (1.01–1.26); P = 0.03] although not with higher total mood scores.} Supplemental n−3 FAs also did not improve kidney function (as assessed by change in estimated glomerular filtration rate or urine albumin excretion) in participants with diabetes.107 These findings do not support the use of n−3 FA supplements to prevent these outcomes.
| Results have been reported (see text): |
| Heart failure |
| Atrial fibrillation |
| Post-stroke function |
| Colorectal adenoma |
| Autoimmune disorders |
| Age-related macular degeneration |
| Dry eye disease |
| Chronic knee pain |
| Migraine frequency and severity |
| Cognitive function |
| Depression/mood |
| Diabetic kidney disease |
| Racial/ethnic differences |
| Results have not yet been reported: |
| Diabetes |
| Hypertension |
| 2D echocardiogram |
| Infections |
| Respiratory diseases |
| Fractures/bone imaging |
| Physical performance |
| Anaemia |
| Hypertension-related nephropathy |
| Telomere biology |
| Results have been reported (see text): |
| Heart failure |
| Atrial fibrillation |
| Post-stroke function |
| Colorectal adenoma |
| Autoimmune disorders |
| Age-related macular degeneration |
| Dry eye disease |
| Chronic knee pain |
| Migraine frequency and severity |
| Cognitive function |
| Depression/mood |
| Diabetic kidney disease |
| Racial/ethnic differences |
| Results have not yet been reported: |
| Diabetes |
| Hypertension |
| 2D echocardiogram |
| Infections |
| Respiratory diseases |
| Fractures/bone imaging |
| Physical performance |
| Anaemia |
| Hypertension-related nephropathy |
| Telomere biology |
| Results have been reported (see text): |
| Heart failure |
| Atrial fibrillation |
| Post-stroke function |
| Colorectal adenoma |
| Autoimmune disorders |
| Age-related macular degeneration |
| Dry eye disease |
| Chronic knee pain |
| Migraine frequency and severity |
| Cognitive function |
| Depression/mood |
| Diabetic kidney disease |
| Racial/ethnic differences |
| Results have not yet been reported: |
| Diabetes |
| Hypertension |
| 2D echocardiogram |
| Infections |
| Respiratory diseases |
| Fractures/bone imaging |
| Physical performance |
| Anaemia |
| Hypertension-related nephropathy |
| Telomere biology |
| Results have been reported (see text): |
| Heart failure |
| Atrial fibrillation |
| Post-stroke function |
| Colorectal adenoma |
| Autoimmune disorders |
| Age-related macular degeneration |
| Dry eye disease |
| Chronic knee pain |
| Migraine frequency and severity |
| Cognitive function |
| Depression/mood |
| Diabetic kidney disease |
| Racial/ethnic differences |
| Results have not yet been reported: |
| Diabetes |
| Hypertension |
| 2D echocardiogram |
| Infections |
| Respiratory diseases |
| Fractures/bone imaging |
| Physical performance |
| Anaemia |
| Hypertension-related nephropathy |
| Telomere biology |
6. Conclusion and future directions
In VITAL, supplemental n−3 FAs among initially healthy men and women was associated with a small but statistically non-significant reduction in a composite endpoint of major CVD events, a statistically significant 28% reduction in total MI, and reductions in other coronary outcomes, including recurrent hospitalization for heart failure. There was no reduction in stroke or cardiovascular deaths not related to heart disease. Supplemental n−3 FAs neither decreased nor increased risk of atrial fibrillation. The benefit for total MI supports a possible cardioprotective role for n−3 FAs in a usual-risk setting, particularly in individuals with low dietary fish intake or with cardiovascular risk factors, and in African Americans. Meta-analyses that include VITAL and other recent n−3 FA trials show coronary risk reduction. Additional research is needed to determine which individuals may be most likely to derive a net benefit from such supplementation.
Forthcoming findings of VITAL ancillary studies addressing effects of n−3 FAs on cardiac structure and function (2D-echocardiograms), hypertension, and diabetes will augment the assessment of the impact of supplementation on cardiovascular health and may help elucidate mechanisms for protective coronary effects. Moreover, forthcoming results of ancillary studies of additional non-cardiovascular outcomes, including infections, pulmonary health, fractures, falls, and physical performance will allow a fuller assessment of the overall benefit–risk balance of supplementation. Post-intervention follow-up of the study population is ongoing to determine if there are potential latent and long-term treatment effects and to maximize statistical power, particularly for the examination of secondary endpoints and subgroup effects.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Acknowledgements
The authors thank the VITAL investigators, staff, and study participants for their commitment to the trial. A list of VITAL Research Group members is in Supplement A, online.
Funding
VITAL is an investigator-initiated trial supported by grants U01 CA138962, R01 CA138962, and R01 AT011729, which include support from the National Cancer Institute; National Heart, Lung and Blood Institute; Office of Dietary Supplements; National Institute of Neurological Disorders and Stroke; and the National Center for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple Institutes, including the National Heart, Lung and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Mental Health; and others. Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. Quest Diagnostics (San Juan Capistrano, CA) measured the serum 25(OH)D levels and plasma omega-3 index at no cost to the study. The NIH sponsors of VITAL had a role in the design and conduct of the study and interpretation of the data. Final decisions concerning the above, however, as well as data collection, management, analysis, manuscript review or approval, and decision to submit the manuscript for publication resided with VITAL investigators and the VITAL research group. The opinions expressed in the manuscript are those of the study authors and do not necessarily represent the views of the Department of Health and Human Services/National Institutes of Health.
Ethical approval
VITAL was approved by the Institutional Review Board of Partners Healthcare/Brigham and Women's Hospital and was monitored by an external Data and Safety Monitoring Board. The study agents have received Investigational New Drug Approval from the U.S. Food and Drug Administration.
Trial registration
VITAL is registered at clinicaltrials.gov (NCT01169259). The VITAL website is www.vitalstudy.org.
Data availability
As this is a review article, readers should contact the principal investigators of individual trials regarding data-sharing policies.
References
Author notes
A list of the members of the VITAL Research Group is provided in Supplement A, online.
Conflict of interest: VITAL receives grant support from the National Institutes of Health, which helps support the authors’ salaries.



