-
PDF
- Split View
-
Views
-
Cite
Cite
Bianca Rocca, Karlheinz Peter, Platelets, coagulation, and the vascular wall: the quest to better understand and smarten up our therapeutic targeting of this triad in primary and secondary prevention of cardiovascular events, Cardiovascular Research, Volume 117, Issue 9, 1 August 2021, Pages 1998–2000, https://doi.org/10.1093/cvr/cvab121
Close - Share Icon Share
This editorial refers to ‘The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis’ by L. Badimon et al., pp. 2001–2015; ‘Pleiotropic actions of factor Xa inhibition in cardiovascular prevention—mechanistic insights and implications for anti-thrombotic treatment’ by H. ten Cate et al., pp. 2030–2044; ‘From organic and inorganic phosphates to valvular and vascular calcifications’ by M. Back et al., pp. 2016–2029.
Cardiovascular Research aims to publish manuscripts that enhance insights into cardiovascular disease mechanisms and their translational relevance. Primary and secondary prevention of cardiovascular events, e.g. myocardial infarction (MI), stroke and peripheral artery disease, is such an area where understanding the mechanisms of disease and the mechanistic role of therapeutic targets is essential for the development and optimization of therapeutic strategies. A good example for the journal’s mechanistic and translational quest are three excellent review articles in the current issue of Cardiovascular Research, which cover basic mechanisms up to the clinical outcome of therapeutic approaches. These articles provide insights on the antiplatelet drug aspirin, direct oral anticoagulant drugs (DOACs), non-steroidal anti-inflammatory drug (NSAID)-driven endothelial dysfunction, and vessel calcification and its therapeutic modification. These topics cover the triad of therapeutic targets in atherosclerosis and its complications, platelets, coagulation, and the pathology of the artery wall (Figure 1).
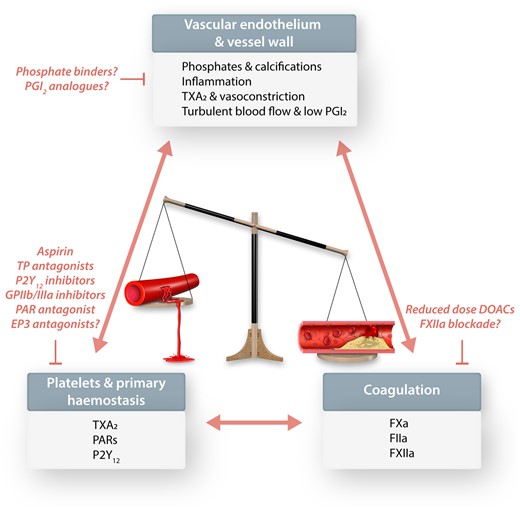
Topics reviewed in this special issue of Cardiovascular Research: vascular endothelium and vessel wall, platelets and primary haemostasis, as well as coagulation. This pathogenic triad involved in promoting the progression of atherosclerosis and its thrombotic complications in arteries and specific therapeutic targets and strategies are discussed. All the currently used antiplatelet and anticoagulant drugs and their combinations come with the inherent link between antithrombotic effects and bleeding risk. The development of newer antithrombotic drugs is focused on shifting the balance towards beneficial antithrombotic effects with less bleeding.
Arachidonic acid (AA) is one of the most abundant and ubiquitous polyunsaturated fatty acid, present in an esterified form in the membrane phospholipids of all mammalian cells, including humans.1 AA released by phospholipase A2 in response to various stimuli is the substrate of numerous enzymes and the precursor of several classes of biologically active compounds including prostanoids, leucotrienes, epoxyeicosatetraenoic acids, endocannabinoids, epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids.2 In the review collection in this edition of Cardiovascular Research, Badimon et al.3 review the role of this complex and finely balanced metabolism and metabolites, not only in preserving the physiological homeostasis of platelet, endothelial, and vascular function, but also in contributing to the pathological processes leading to atherothrombosis, where this homeostasis gets profoundly disrupted. The relevance of the AA-dependent pathways in Science and in human diseases is highlighted by the 1982 Nobel Prize awarded to Sune K. Bergström, Bengt I. Samuelsson, and John R. Vane ‘for their discoveries concerning prostaglandins and related biologically active substances’,4 and by the 2013 GrandPrix Scientifique from the Lefoulon-Delalande Foundation of the Institute of France, conferred to Garret A. FitzGerald and Carlo Patrono (the last author of the review by Badimon et al.3), ‘for their studies in the development of low-dose aspirin for the prevention of cardiovascular disease’.5 The review summarizes data in a truly translational perspective, spanning from studies in vitro and in genetically modified animals, to studies in humans including large randomized clinical trials with low-dose aspirin and NSAIDs, which are the strongest evidence supporting a central role for TXA2 and PGI2 in atherothrombosis and vascular protection, respectively. However, the role of other pathways of AA metabolism in cardiovascular disease and their contribution to atherothrombosis remains to be investigated, thus the game is not over yet.
The discussion about the role of the coagulation system in the pathology of atherosclerosis and particularly its suitability as a therapeutic target in the treatment of atherosclerosis has been dormant for many years. The major reason for this was that warfarin and phenprocuomon, for decades the only clinically available oral anticoagulants, did not deliver convincing outcomes in clinical trials. A significant increase in bleeding complications when warfarin was combined with aspirin dampened the enthusiasm for warfarin to be used for secondary prevention.6 Nevertheless, the finding that in patients who achieved an international normalized ratio of 2–3, a reduction in all-cause death, non-fatal MI, and non-fatal thromboembolic stroke was achieved, implicated that inhibition of the coagulation system can deliver beneficial effects in atherothrombosis. Indeed, with the introduction of DOACs, other promising anticoagulant drugs in the pipeline and a plethora of new preclinical tools and data available (e.g. mouse knockouts of coagulation factors), anticoagulation as therapy for atherosclerotic disease has been awaken from its dormancy.7
In the review collection in this edition of Cardiovascular Research, Ten Cate et al.8 provide a comprehensive review both on basic research of the potential role of coagulation factors in atherothrombosis and clinical data available supporting therapeutic targeting of the coagulation system. Central stage in the new clinical data discussed by the authors are the ATLAS ACS 2-TIMI 51, VOYAGER PAD and in particular the COMPASS trial, investigating a low dose of the activated Factor Xa (FXa) inhibitor rivaroxaban and concomitant antiplatelet therapy with aspirin. Most importantly this lower dose of rivaroxaban in association with low-dose aspirin was not associated with an increased rate of bleeding complications as seen with the higher doses of DOACs combined with antiplatelet agents. Nevertheless, rivaroxaban, given in addition to aspirin e.g. in COMPASS, reduced the cardiovascular event rate, including cardiovascular death, stroke and MI. The promising results from COMPASS and other trials can be interpreted either by arguing that low anticoagulant effects are sufficient to deliver secondary prevention or by arguing that FXa inhibition has a pleiotropic effect independent of anticoagulation.
Ten Cate et al. take a clear side of the dispute, strongly arguing for pleiotropic effects of FXa inhibition. The authors reason that low concentrations of thrombin and FXa, which are expected to be preserved at low doses of FXa inhibitors, are sufficient to maintain both haemostasis as well as endothelial function, e.g. endothelial barrier integrity. The latter is mediated via protease-activated receptor (PAR)1/PAR2 signalling as well as thrombomodulin and the endothelial protein C receptor (EPCR). However, whether this classifies as a pleiotropic effect is a rather semantic dispute and the term pleiotropic might distract from the various cross-reactivities of the coagulation system e.g. with cellular (platelet) activation pathways, the complement system and inflammatory processes. This is obvious as several coagulation factors have inflammatory properties as well and thereby any degree of inhibition of the coagulation system, even with a low dose FXa inhibition could result in reduced atherogenesis and plaque stabilization. The same is true for low-dose inhibition of the coagulation pathway as it is obviously involved in microthrombosis (increasing plaque instability) and macrothrombosis (precipitating occlusive events such as MI and stroke) and thus can clearly contribute to the reduction of cardiovascular events.
The combination of low-dose rivaroxaban (2.5 mg b.i.d.) is recommended in patients with high to moderate risk of a cardiovascular event and without high bleeding risk.9,10 No doubt, the combination of antiplatelet and anticoagulant drugs for secondary prevention will become even more interesting with the clinical arrival of additional potent anticoagulant drugs targeting various factors of the coagulation system, some of which such as the FXIIa inhibitor promise to deliver antithrombotic and anti-inflammatory effects without perturbing haemostasis.11
In both manuscripts, Badimon et al.3 and Ten Cate et al.,8 the understanding and clinical relevance of bleeding complications takes central stage. In fact, avoiding bleeding complications via reducing doses of anticoagulants, such as rivaroxaban,8 or the development of drugs that maintain normal haemostasis has become the major focus in the development of novel antiplatelet and anticoagulant drugs.12 Inhibition of FXIIa11 or activation-specific inhibition of GPIIb/IIIa are such approaches, which promise sufficient anti-thrombotic effects while maintaining normal haemostasis.7,12 In fact, drugs that have antiplatelet or anticoagulant effects without disturbing haemostasis are highly attractive and provide a strong rationale to be tested in secondary or even primary prevention of cardiovascular events.
Together with platelet and coagulation activation, calcification of the arterial wall is an important mechanism involved in the development of atherosclerosis and its thrombotic complications, especially in large arteries. Back and Michel13 in this edition of Cardiovascular Research review the role of inorganic and organic phosphates as essential components of both physiologic mineralization of bones as well as of pathologic calcification of soft tissues, including vessel wall and heart valves, with a parallelism between the two processes. While mineralization of bones progresses through highly regulated and coordinated pathways,14 mineralization of atherosclerotic lesions in the arteries progresses in a dysregulated and uncontrolled fashion, similarly to platelet and coagulation activation.14 Calcification of the vessels includes different types of calcium phosphates (apatite, beta-tri- or octa-calcium phosphates), can be localized in the intima and/or media, involves many different cell types (macrophages, vascular smooth muscle cells, endothelial cells), extracellular matrix components (different types of collagen), and diverse pathological processes (apoptosis, cell death, ageing, inflammation). The interplays between these components are rather complex and only partially known, as extensively reviewed by Back and Michel13 Independently of the underlying mechanisms, there is convincing evidence that coronary artery calcium (CAC) content is associated with coronary artery diseases and can predict major atherothrombotic complications, especially in patients with cardiovascular risk factors even in the absence of symptomatic atherothrombotic events, such as MI and stroke.13 Consistently, the most recent guidelines identify CAC as a ‘risk modifier’, especially useful in patients at apparent low-to-moderate cardiovascular risk and no thrombotic history.9,10,14 Moreover, CAC may have value in the decision to recommend primary prevention with prophylactic daily aspirin.9,10,15,16 It remains unknown whether calcifications are only by-products or active triggers of atherosclerotic disease progression and complications, which is important for the understanding whether calcification can be a suitable therapeutic target. The authors discuss also a possible therapeutic role for phosphate binders and inorganic pyrophosphate analogues (i.e. bisphosphonates), which may limit the release of endogenous inorganic phosphate from the bones, although evidence from randomized clinical trials of bisphosphonates in non-cardiovascular settings are overall inconclusive so far to support an extension of their clinical indication towards secondary prevention of cardiovascular events.
In conclusion, the current issue of Cardiovascular Research extensively revises pillars of the pathophysiology of atherothrombosis, i.e. mechanisms related to platelets, coagulation and the vessel wall with a truly translational perspective, spanning from molecules (AA derivatives, FXa, phosphates) to animal models to clinical trials and guidelines.
Conflict of interest: none declared.
Funding
K.P. is supported by a National Health and Medical Reserach Council of Australia L3 Investigator Fellowship.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References



