-
PDF
- Split View
-
Views
-
Cite
Cite
Tina Blažević, Anja M. Schaible, Katharina Weinhäupl, Daniel Schachner, Felix Nikels, Christina Weinigel, Dagmar Barz, Atanas G. Atanasov, Carlo Pergola, Oliver Werz, Verena M. Dirsch, Elke H. Heiss, Indirubin-3′-monoxime exerts a dual mode of inhibition towards leukotriene-mediated vascular smooth muscle cell migration, Cardiovascular Research, Volume 101, Issue 3, 1 March 2014, Pages 522–532, https://doi.org/10.1093/cvr/cvt339
Close - Share Icon Share
Abstract
The small molecule indirubin-3′-monoxime (I3MO) has been shown to inhibit vascular smooth muscle cell (VSMC) proliferation and neointima formation in vivo. The influence of I3MO on VSMC migration and vascular inflammation, two additional key players during the onset of atherosclerosis and restenosis, should be investigated.
We examined the influence of I3MO on VSMC migration, with focus on monocyte-derived leukotrienes (LTs) and platelet-derived growth factors (PDGFs) as elicitors. Exogenous LTB4 and cysteinyl leukotrienes as well as LT-enriched conditioned medium of activated primary human monocytes induced VSMC migration, which was inhibited by I3MO. I3MO also blunted migration of VSMC stimulated with the PDGF, the strongest motogen tested in this study. Induction of haem oxygenase 1 accounted for this anti-migratory activity of I3MO in VSMC. Notably, I3MO not only interfered with the migratory response in VSMC, but also suppressed the production of pro-migratory LT in monocytes. Conditioned media from monocytes that were activated in the presence of I3MO failed to induce VSMC migration. In cell-based and cell-free assays, I3MO selectively inhibited 5-lipoxygenase (5-LO), the key enzyme in LT biosynthesis, with an IC50 in the low micromolar range.
Our study reveals a novel dual inhibitory mode of I3MO on LT-mediated VSMC migration: (i) I3MO interferes with pro-migratory signalling in VSMC and (ii) I3MO suppresses LT biosynthesis in monocytes by direct inhibition of 5-LO. These inhibitory actions on both migratory stimulus and response complement the previously demonstrated anti-proliferative properties of I3MO and may further promote I3MO as promising vasoprotective compound.
1. Introduction
Indirubin-3′-monoxime (I3MO) is a derivative of indirubin, a naturally occurring alkaloid which was recognized as an active ingredient of the traditional Chinese anti-leukemic recipe, Danggui Longhui Wan. In a previous study, we have demonstrated that I3MO is a potent inhibitor of vascular smooth muscle cell (VSMC) proliferation (IC50 ∼2 µM) and successfully blocks neointima formation in vivo, in a murine femoral cuff model.1 Neointima formation, also referred to as intimal hyperplasia, causally contributes to the reduction of the vessel lumen during atherosclerosis and restenosis. In addition to cell proliferation, intimal hyperplasia also involves migration of VSMCs.2 During the onset of atherosclerosis, monocytes adhere to sites of endothelial dysfunction and migrate into the subendothelial layer, where they contribute to early lesion development by secreting cytokines, growth factors, and leukotrienes (LTs). Those mediators facilitate further recruitment of immune cells and stimulate migration of VSMCs from the medial into the intimal layer and finally their proliferation.3,4
LTs, formed by lipoxygenases (LO) from arachidonic acid (AA), were initially recognized as important lipid-derived bronchoconstrictors, but are meanwhile known as mediators of immune reactions and inflammation in diverse diseases, such as asthma, rhinitis, cancer, and atherosclerosis.5 Based on the accumulated data, two LOs are the prime candidates implicated in atherosclerosis, i.e. 5-LO and 15-LO Type 1 (15-LO-1) in humans and its closely related orthologue in mice, 12-LO.6 15-LO-1 catalyses the synthesis of 12(S)- and 15(S)- hydroxyeicosatetraenoic acid (HETE), and several studies have suggested a role of 15-LO in the process of low density lipoproteins (LDL) oxidation, foam cell, and fatty streak formation.7–9 A more recent study, however, demonstrated 5-LO as the main LO type expressed within atherosclerotic plaques.10 The pro-atherogenic role of 5-LO does not involve the oxidative modification of LDL,11,12 but rather its ability to synthesize LTs [LTB4 and cysteinyl leukotrienes (cysLTs)], which exert their pro-inflammatory actions on other cell types, such as VSMC, through seven transmembrane G-protein coupled receptors.3
In this study, we addressed the question whether I3MO can—next to inhibition of proliferation—also interfere with LT-mediated migratory processes in VSMC, and whether or not I3MO interferes with LT biosynthesis.
2. Methods
2.1 Materials and reagents
I3MO and tin protoporphyrin IX chloride (SnPP) were purchased from Enzo Life Sciences (Lausen, Switzerland). 8BWA4C, PGB1 Ca2+-ionophore A23187, and other common chemicals were from Sigma-Aldrich (Deisenhofen, Germany). LTB4 was obtained from Calbiochem (Darmstadt, Germany) and from Cayman Chemical (Ann Arbor, MI, USA). AA and cysLT mixture I, containing equal amounts of leukotriene C4, leukotriene D4, leukotriene E4, leukotriene F4, and N-acetyl leukotriene E4, were purchased from Cayman Chemical. Platelet-derived growth factor (PDGF)-BB was from Bachem (Weil am Rhein, Germany). The antibody against haem oxygenase (HO)-1 was from Stressgen (Plymouth Meeting, PN, USA) and the anti-actin antibody came from MP Biomedicals (Solon, OH, USA). Secondary horseradish-peroxidase coupled with antibodies were obtained from Cell Signalling (Danvers, MA, USA). All the sterile cell culture material was from Greiner (Frickenhausen, Germany), unless stated otherwise. For the isolation of human monocytes by positive and negative selection, the EasySep™ Human CD14-Positive Selection Kit and the EasySep™ Human Monocyte Enrichment Kit (Stemcell Technologies, Grenoble, France) were used, respectively.
2.2 Cells and cell isolation
For the analysis of LO activities, neutrophils and monocytes were routinely isolated from human peripheral blood of healthy adult male and female donors according to reported methods13,14 (for details of monocyte isolation via adherence, see Supplementary Materials online) or by positive and negative immunomagnetic selection, respectively (applicable for Figure 5E and F). For positive selection we followed the provided protocol within the EasySep™ Human CD14 Positive Selection Kit (Stemcell Technologies), which is designed to isolate CD14+ cells from peripheral blood mononuclear cells (PBMCs). Desired cells are hereby labelled with a bispecific tetrameric complex composed of monoclonal anti-CD14 and anti-dextran antibodies. By addition of dextran-coated magnetic particles, target cells get magnetically tagged and remain in the tube when this is placed into the purple EasySep™ magnet while unwanted cells are poured off. For the negative selection of monocytes, we used the EasySep™ Human Monocyte Enrichment Kit (also from Stemcell Technologies). Unwanted cells are labelled for removal with bispecific tetrameric antibody complexes recognizing the respective target antigen (CD2, CD3, CD16, CD19, CD20, CD56, CD66b, CD123, or glycophorin A) and dextran-coated magnetic particles. The antibody cocktail also contains an antibody to the human Fc receptor to prevent non-specific binding of monocytes. Labelled cells are separated using the purple EasySep™ magnet and remain in the tube while monocytes can be poured off into a new vial. The isolated neutrophils (purity >96–97%) and monocytes (purity >85%) were finally resuspended in PBS pH 7.4 containing 1 mg/mL of glucose and 1 mM CaCl2 (PGC buffer).
Isolation of monocytes for the experiments using conditioned medium was performed as follows: fresh buffy coats from healthy donors were obtained from the Austrian Red Cross, Vienna (for detailed information on donors please refer to Supplementary Material online). Isolation of peripheral blood PBMC was performed by density gradient isolation out of buffy coat using Ficoll-Paque™ Plus (GE Healthcare, Uppsala, Sweden) as recommended by the manufacturer. Monocytes were isolated by the positive selection method using CD14 Microbeads, MACS® LS columns, and MACS® Separator (all from Miltenyi Biotec, Gladbach, Germany) as recommended by the manufacturer. The obtained purity of isolated monocyte population using this technique was >95% as determined by CD14 staining.
Primary VSMCs were isolated from rat thoracic aortas of Sprague-Dawley rats using a digestion method and were kindly provided by Kathy Griendling, Emory University.15 Murine WT and Nrf2−/− embryonic fibroblast were kindly provided by Thomas Kensler, University of Pittsburgh.16 Rats and mice were kept and handled in accordance with the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health.
Cells were passaged twice a week and cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 2 mM l-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% newborn calf serum for VSMC and 10% foetal calf serum for fibroblasts (all from Lonza, Basel, Switzerland) at 37°C and 5% CO2. Passages 5–14 of VSMCs were used in this study. These VSMC show a proliferative phenotype which was not changed by treatment with I3MO as evident in comparable expression levels of osteopontin, a marker for the proliferative-synthetic VSMC phenotype, and of smooth muscle 22α, a marker for the contractile phenotype, in DMSO- and I3MO-treated cells (data not shown).
All described investigations conformed to the principles outlined in the Declaration of Helsinki and were approved by the respective university ethics review board.
2.3 Preparation of LT-enriched conditioned media from activated monocytes
1 × 107 of monocytes were resuspended in 2 mL of PGC buffer at 37°C, pre-treated with I3MO or BWA4C for 15 min, and subsequently stimulated for 10 min with Ca2+- ionophore, A23187 (2.5 µM), and 20 µM AA as 5-LO substrate to induce eicosanoid production. Cells were then removed by centrifugation and supernatants were frozen immediately at −80°C until further handling. Thawed supernatants were acidified with 2 M HCl to pH 3.5 and left to incubate at 4°C for 15 min. A centrifugation step at 370 × g for 4 min followed to remove any precipitate. HyperSep C18 SPE columns (200 mg/3 mL, Thermo Scientific, Runcorn, UK) were washed with 10 mL of ethanol and 10 mL of deionized water. Samples were applied to the columns with a flow rate of about 0.5 mL/min. Columns were washed with 10 mL of deionized water, 10 mL of 15% (v/v) ethanol and 10 mL of hexane. Eicosanoids were eluted using 10 mL of ethyl acetate and stored at −80°C. Ethyl acetate was evaporated in a rotor vacuum pump GeneVac EZ-2 Plus (Genevac, Ipswich, UK) immediately before the treatment of VSMCs (method: low boiling point, temperature <30°C). Dried residual material was resuspended in 2 mL of DMEM supplemented with 0.1% calf serum. Samples were incubated at 37°C for 15 min, sterile filtered, and applied on VSMCs in the course of a wound healing assay. For each monocyte treatment condition, a cell-free control condition was prepared in parallel.
2.4 Wound healing assay (scratch assay)
VSMCs were seeded in a six-well plate at a density of 800 000 cells/well, grown to confluence, and starved for 24 h. The cell monolayer was scratched using a sterile 1000 µl pipette tip (width of the scratch ∼1 mm) and left to recover for the next 24 h in freshly exchanged starvation medium (0.1% serum-supplemented DMEM). VSMCs were then treated with compounds or conditioned media for the next 21 h and their influence on migration was monitored: images of the scratch under the light microscope (magnification ×100) were taken at 0 h and 21 h after the treatment (four different sites per scratch were monitored and evaluated to give an average value for each experimental condition). To ensure that at 0 and 21 h the very same area of each scratch is captured perpendicular lines were drawn at the bottom side of the plates before cell seeding. The cell re-colonization rate was recorded by measuring the cell-free area of each scratch, using the Cell Profiler software (www.cellprofiler.org, Broad Institute, Cambridge, MA, USA).
2.5 Immunoblot
Extraction of proteins, electrophoresis, transfer, immunodetection, and densitometric evaluation were performed as previously described.1,17
2.6 Determination of LO products in intact cells
Induction of LO product formation in isolated human neutrophils or monocytes, and analysis of the LO products, was performed according to reported methods.13,14 In brief, cells were resuspended in PGC buffer, pre-incubated (15 min, 37°C) with the test compounds (e.g. I3MO) or DMSO (vehicle), and the respective stimuli were added. After 10 min, the LO products were isolated by solid phase extraction (RP-18) and quantified by RP-HPLC as described.13 Experimental details can also be found in the supporting material together with an exemplary HPLC chromatogram (see Supplementary material online, Figure S1).
2.7 Determination of cysLT formation in monocytes
Freshly isolated monocytes (2 × 106 cells) were resuspended in 1 mL of PGC buffer, primed with 1 µg/mL of LPS for 5 min at 37°C, and incubated with the test compounds or vehicle (0.1% DMSO) for 15 min at 37°C, and then stimulated with 1 µM formyl-methionyl-leucyl-phenylalanin (fMLP) for 10 min at 37°C. The reaction was stopped on ice, and the supernatants were collected after centrifugation at 600 × g for 10 min at 4°C. CysLT levels were measured by an ELISA which detects LTC4, LTD4, and LTE4 according to the manufacturer's (Enzo Life Sciences International Inc., Lörrach, Germany) instructions.
2.8 Expression and purification of human recombinant 5-LO
E.coli BL21 was transformed with pT3-5-LO plasmid, and recombinant 5-LO protein was expressed at 30°C as described.17 Cells were then lysed, the lysates were centrifuged at 40 000 × g for 20 min, and 5-LO was partially purified from the 40 000 × g supernatant using an ATP-agarose affinity column as described.18
2.9 Determination of 5-LO activity in cell-free assays
Analysis of 5-LO activity in cell-free assays was performed as described.18 Briefly, aliquots of semi-purified 5-LO (0.5 µg) were diluted in PBS containing 1 mM EDTA. For the determination in cell homogenates, neutrophils were resuspended in PBS containing 1 mM EDTA and sonicated on ice. After addition of 1 mM ATP, the samples were pre-incubated with the test compounds for 15 min at 4°C, pre-warmed for 30 s at 37°C, and 2 mM CaCl2 and 20 µM AA were added. After 10 min at 37°C, the formed metabolites were analysed by HPLC. 5-LO products include the all trans-isomers of LTB4 and 5(S)-hydro(pero)xyeicosatetraenoic acid (H(P)ETE).
2.10 Analysis of cytotoxicity
Cytotoxicity of I3MO in monocytes was analysed by MTT assay in a 96-well format using a multi-well scanning spectrophotometer (Multiskan Spectrum Reader, Thermo Fisher Scientific Oy, Vantaa, Finland) as described before.19 Neutrophils (5 × 106 cells/mL) or monocytes (2 × 106 cells/mL) were incubated for 30 min with I3MO, and the viability of the cells was analysed by MTT assay. Compared with vehicle (0.3% DMSO), no significant acute cytotoxicity was observed (neutrophils: 103.9 ± 4.4%; monocytes: 129.4 ± 5.4%; n = 3, each).
2.11 Statistics
Data are expressed as mean ± SE. IC50 values were graphically calculated from averaged measurements at three different concentrations of the compounds using the GraphPad Prism (GraphPad Software, Inc.). Statistical evaluation of the data was performed by one-way analysis of variance followed by a Bonferroni or Tukey-Kramer post hoc test for multiple comparisons, respectively. A P-value <0.05 (*) was considered significant with a confidence interval of 95%.
3. Results
3.1 LTs or LT-enriched conditioned medium from activated monocytes induce VSMC migration
Monocytes are major players within the inflammatory processes of the vessel wall during the onset of atherosclerosis.20 They express LO enzymes and produce LTs, which are assumed to contribute to VSMC migration and proliferation in vitro and in vivo.3,4 We first confirmed migration of our VSMC population and stimulated VSMCs with increasing concentrations of LTB4 (1–1000 nM), the major pro-migratory LT. In a scratch assay, LTB4 stimulated VSMC migration concentration dependently, with a significant effect at 100 nM (Figure 1A). A mixture of cysLT also induced VSMC migration being significant at 100 nM (Figure 1B). Using a BrdU assay, we could show that neither LTB4 nor the cysLT mixture induced VSMC proliferation in our experimental setting (see Supplementary material online, Figure S2), ruling out a proliferative component of the observed scratch closure upon LT treatment. Compared with the PDGF, LTs were less potent motogens, an effect that was corroborated by matching results from the complementary Boyden chamber chemotaxis assay (see Supplementary material online, Figure S3). Prostaglandin E2 (PGE2), 5-HETE or 5-oxo-ETE, and other AA-derived eisosanoids did not stimulate VSMC migration up to a concentration of 1 µM (data not shown).
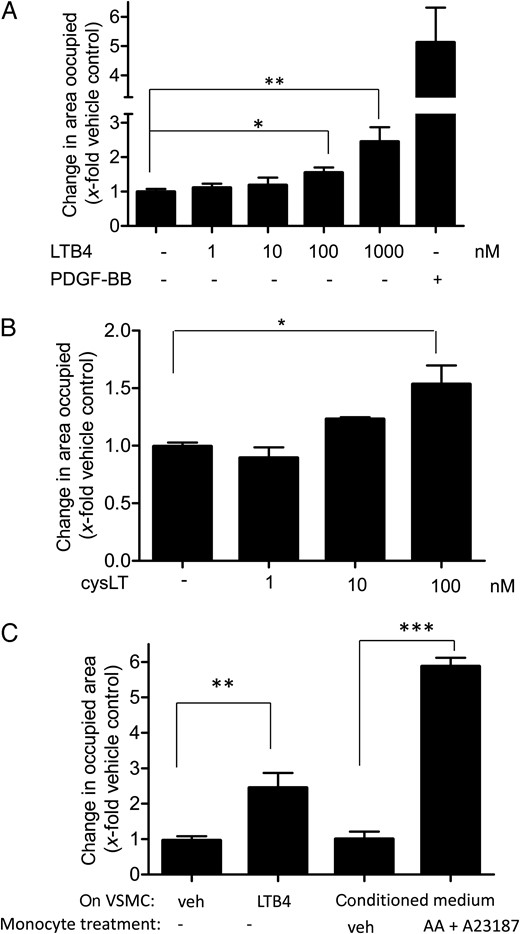
LTB4, CysLT, and LT-enriched conditioned medium from activated monocytes induce VSMC migration. VSMCs were stimulated with vehicle (0.33% ethanol; –), increasing concentrations of LTB4 (A) or cys LT (B) as indicated, or with 10 ng/mL of PDGF-BB as a positive control for 21 h in a wound healing assay. (C) VSMCs were incubated for 21 h in a wound healing assay with either vehicle (0.33% ethanol; veh), LTB4 (1 µM), or conditioned medium from monocytes treated for 10 min with either vehicle (0.33% ethanol + 0.1% DMSO; veh), or AA (20 µM) + A23187 (2.5 µM). (A–C) Graphs indicate the change in area occupied by cells as fold induction of VSMC migration from 4 to 6 (A) and 3 (B and C) biological replicates with four technical replicates each; mean + SE; *P < 0.05; **P < 0.01; ***P < 0.001.
Next, we investigated whether also LT-enriched conditioned medium from freshly isolated and activated monocytes was capable of inducing VSMC migration in our model. For this purpose, we used primary human monocytes isolated from buffy coats obtained from freshly donated blood, containing >95% CD14-positive cells (see Supplementary material online, Figure S4). Monocytes were stimulated to produce eicosanoids by Ca2+-ionophore A23187 (2.5 µM) in the presence of 20 µM AA as a substrate. LT-enriched conditioned medium was prepared as described in the Methods sections. This medium triggered a five-fold increase in migration when administered to VSMC (Figure 1C), a response even stronger than obtained by 1 µM LTB4 (about 2.5-fold). Medium that was prepared in parallel in the absence of monocytes did not exert any significant effect on VSMC migration (see Supplementary material online, Figure S5), indicating that the observed induction of VSMC migration is due to monocyte-derived products and not due to the residual presence of A23187 and/or AA in the prepared medium.
3.2 I3MO inhibits LT-induced VSMC migration
Next, we wanted to investigate whether I3MO can inhibit LT-induced VSMC migration and performed a scratch assay in the presence of I3MO. Upon stimulation with LTB4, I3MO concentration-dependently inhibited VSMC migration, with significance at 1 µM (Figure 2A). I3MO also inhibited cysLT-induced VSMC migration at 3 µM (Figure 2B). These data show that I3MO can interfere with LT-mediated migration of VSMCs, in a concentration range, that was also effective in inhibition of VSMC proliferation.1 Thus, I3MO possesses not only anti-proliferative but also anti-motogenic properties against VSMC. I3MO also inhibited PDGF-induced migration in a concentration-dependent manner (Figure 2C), suggesting that the anti-migratory property of I3MO is not restricted to LT-induced migration but stimulus-independent, and that I3MO is unlikely to directly interfere with LT receptors.
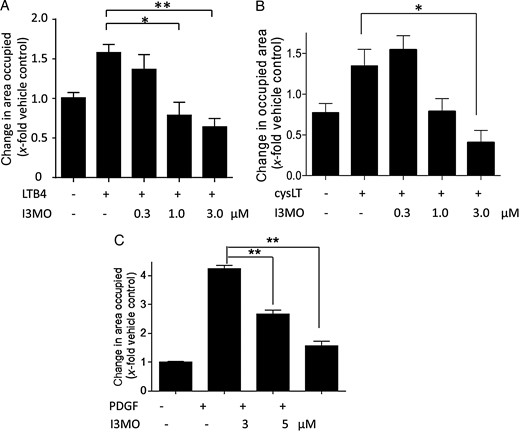
I3MO inhibits LTB4-, cysLT-, and PDGF-induced VSMC migration. VSMCs were pre-treated with either vehicle (0.1% DMSO; –) or the indicated concentrations of I3MO for 15 min and stimulated with (A) LTB4 (1 µM), (B) cysLT mixture (100 nM), or (C) PDGF (10 ng/mL) for 21 h in the course of the wound healing assay. (A–C) Graphs indicate the change in area occupied by cells as fold induction of VSMC migration from 3 (A and C) and 4 (B) biological replicates with four technical replicates each; mean + SE; *P < 0.05; **P < 0.01.
3.3 I3MO inhibits VSMC migration by induction of HO-1
Induction of HO-1 interferes with VSMC migration, as reported previously.21–23 We therefore examined whether I3MO can trigger expression of HO-1, and whether this could account for its anti-migratory potential. Treatment with I3MO led to a significant and time-dependent induction of HO-1 in quiescent and activated VSMC (Figure 3A and B). Co-treatment of VSMC with I3MO and different concentrations of the HO-1 inhibitor tin protoporphyrine IX (SnPP IX)24 diminished the anti-migratory activity of I3MO in a concentration-dependent manner, as exemplified in PDGF-stimulated VSMC. This finding clearly demonstrated causality between increased HO-1 activity and inhibition of VSMC migration (Figure 3C). Supporting this finding, an indirubin derivative (E847) that failed to induce HO-1 in VSMC also lacked anti-migratory activity (see Supplementary material online Figure S6). Making use of wild-type mouse embryonic fibroblasts and isogenic cells lacking the transcription factor nuclear factor E2-related factor 2 (Nrf2), known as one of the major transcription factors governing HO-1 induction,25 we revealed that I3MO induces HO-1 in a Nrf2-dependent manner (Figure 3D). Overall, I3MO inhibits VSMC migration by induction of HO-1 and leads to HO-1 expression in an Nrf2-dependent fashion.
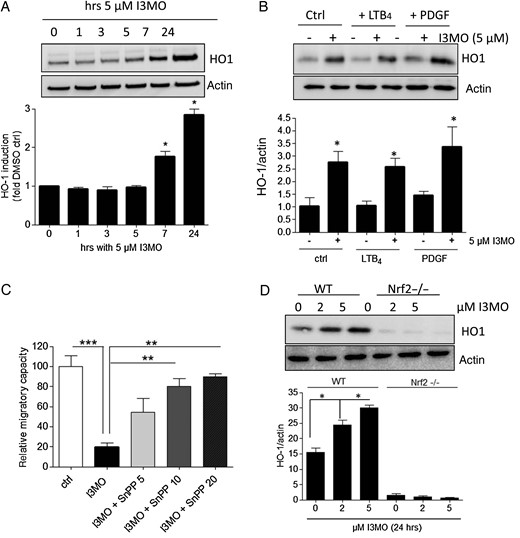
I3MO inhibits migration by induction of HO-1. Quiescent VSMCs were treated with I3MO (5 µM) for the indicated periods of time (A) or with LTB4 (1 µM), PDGF (10 ng/mL) and 5 µM I3MO as indicated for 24 h (B) before their lysates were subjected to immunoblot analysis for HO-1 and actin as a loading control. Representative blots of three independent biological experiments (with one technical replicate) with consistent results are depicted. The bar graph shows compiled densitometric data of all performed experiments (mean + SE, *P < 0.05). (C) The PDGF (10 ng/mL)-induced migratory potential of VSMC was investigated via a scratch assay in cells treated with vehicle (ctrl), I3MO (5 µM), or I3MO + the HO-1 inhibitor SnPP (5, 10, or 20 µM). The migratory potential was defined as the change in the occupied area after 21 h treatment with the PDGF. The migration observed in vehicle treated cells was considered 100%. The bar graph depicts compiled data of three independent biological replicates with four technical replicates each. (D) WT and Nrf2−/− mouse embryonic fibroblasts were treated with I3MO (2 or 5 µM) for 24 h before their lysates were subjected to immunoblot analysis for HO-1 and actin as a loading control. Representative blots are shown, the bar graph depicts compiled densitometric analyses from three biological replicates with one technical replicate each (mean + SE, *P < 0.05).
3.4 I3MO abrogates the pro-migratory effect of conditioned medium derived from activated monocytes
As I3MO has been reported to also act as anti-inflammatory agent,26 we investigated whether I3MO is able to interfere with the production of the pro-inflammatory LTs in monocytes and, thus, not only to inhibit migration in VSMC but also the biosynthesis of the pro-migratory stimuli. Therefore, we pre-treated monocytes with I3MO (5 µM) prior to activation with A23187 and AA. Conditioned medium from these cells failed to induce migration in the performed wound healing assay (Figure 4). The same was true for medium from activated monocytes pre-treated with the well-known 5-LO inhibitor BWA4C.27 5-LO products seem to be responsible for the induction of VSMC migration by conditioned medium, and 5-LO inhibition by I3MO may account for the failure of VSMC to migrate. The residual presence of I3MO or BWA4C in the conditioned medium is unlikely to account for the observed inhibitory impact on VSMC migration since exogenous addition of LTB4 to conditioned medium, which was prepared in parallel in the absence of monocytes, resulted in a similar migratory response as addition to fresh naïve medium (data not shown).
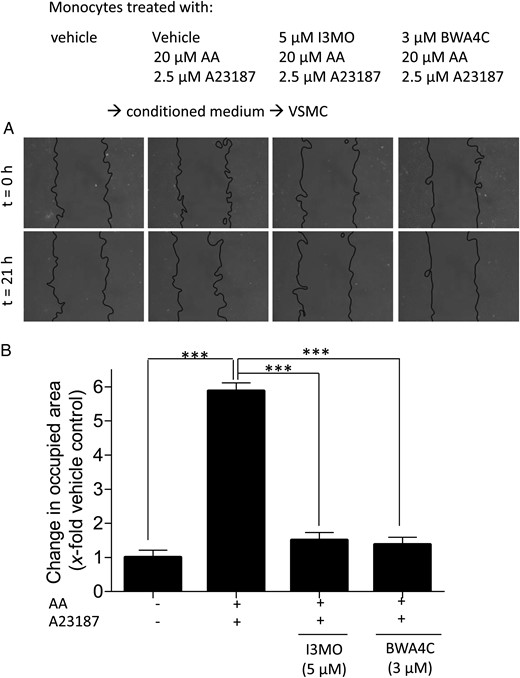
Incubation of monocytes with I3MO or BWA4C prevents VSMC migration induced by conditioned medium from activated monocytes. VSMCs were stimulated for 21 h in a wound healing assay with conditioned medium from monocytes that were pre-incubated with vehicle, I3MO, and BWA4C for 15 min and then stimulated for 10 min with AA (20 µM) + A23187, as indicated. (A) Photos of the ‘wound’ in the VSMC monolayer at the beginning (t = 0 h) and the end (t = 21 h) of stimulation period (light microscopy, magnification ×100). (B) Graph indicating the change in area occupied by cells as fold induction of VSMC migration from three independent biological replicates with four technical replicates each; mean + SE; ***P < 0.001.
3.5 I3MO inhibits the synthesis of 5-LO products in monocytes and neutrophils
The data from above suggest that I3MO may act as an inhibitor of LT biosynthesis. Therefore, we investigated whether I3MO inhibits LT biosynthesis in monocytes. Besides LTB4 and cysLTs, monocytes also form the trans-isomers of LTB4 and 5-H(P)ETE via the 5-LO pathway. Isolated human monocytes were pre-treated with I3MO and stimulated to form 5-LO products by the addition of A23187 or alternatively by priming the cells with LPS (20 min) and the addition of fMLP.14 BWA4C was used as a reference compound. I3MO caused a concentration-dependent inhibition of all analysed products formed via the 5-LO pathway [i.e. LTB4 and its trans-isomers, and 5-H(P)ETE] independent of the stimulus with IC50 values of 4.1 ± 1.1 and 4.3 ± 1.1 µM (Figure 5A). Similarly, the synthesis of cysLTs in LPS/fMLP-stimulated monocytes was reduced by I3MO with even slightly higher potency (IC50 = 2.6 ± 1.1 µM, Figure 5B). BWA4C inhibited 5-LO product synthesis as expected. A comparable inhibitory effect of I3MO was observed when neutrophils, another prominent source of LT synthesis,13 were used. Here, I3MO suppressed the synthesis of all analysed 5-LO products upon stimulation with A23187 with an IC50 of 3.2 ± 1.1 µM (Figure 5C).
![I3MO inhibits the formation of 5-LO products from monocytes and neutrophils. (A) Isolated human monocytes (5 × 106 cells/mL PGC buffer) were pre-treated with I3MO (or 0.1% DMSO as vehicle) for 15 min at 37°C and then induced to form 5-LO products [i.e. LTB4 and its trans-isomers, and 5-H(P)ETE) by addition of 2.5 µM A23187 or alternatively by priming with LPS (20 min) and addition of 0.1 µM fMLP]. After 10 min at 37°C, reactions were terminated and 5-LO products were analysed by HPLC. 5-LO products of 100% controls: A23187: 123.3 ± 25.45 ng/5 × 106 cells, LPS/fMLP: 34.1 ± 15.9 ng/5 × 106 cells. Data are expressed as the percentage of control (100%), means ± SE, n = 3. LPS/fMLP: *P < 0.05; A23187:+P < 0.05; +++P < 0.001 vs. vehicle control. (B) Isolated human monocytes (2 × 106 cells/mL PGC buffer) were primed with LPS (20 min), after 5 min I3MO (or 0.1% DMSO) was added for 15 min at 37°C, and followed by 0.1 µM fMLP. After 10 min at 37°C, reaction was terminated and cysLTs were analysed by ELISA. CysLT formation of 100% controls: 588.5 ± 144.2 pg/2 × 106 cells. Data are expressed as the percentage of control (100%), means ± SE, n = 3. *P < 0.05; **P < 0.01 vs. vehicle control. (C) Isolated human neutrophils (5 × 106 cells/mL PGC buffer) were pre-treated with I3MO (or 0.1% DMSO) for 15 min at 37°C. Then, 2.5 µM A23187 was added and the reaction stopped after 10 min. 5-LO products [i.e. LTB4 and its trans-isomers, and 5-H(P)ETE] were analysed by HPLC. 5-LO products of 100% controls: A23187: 84.0 ± 20.9 ng/5 × 106 cells. Data are expressed as the percentage of control (100%), means ± SE, n = 3. **P < 0.01 vs. vehicle control. (D) Isolated human monocytes or neutrophils (5 × 106 cells/mL PGC buffer) were pre-treated with I3MO (or 0.1% DMSO) for 15 min at 37°C. Then, 2.5 μM A23187 plus 20 μM AA were added and the reaction stopped after 10 min. 5-LO products [i.e. LTB4 and its trans-isomers, and 5-H(P)ETE] were analysed by HPLC. 5-LO products of 100% controls: neutrophils: 375.8 ± 44.1 ng/5 × 106 cells, monocytes: 169.7 ± 58.0 ng/5 × 106 cells. Data are expressed as the percentage of control (100%), means ± SE, n = 3. (E and F) Monocytes were isolated side by side via adherence, positive or negative selection, and tested for their production of 5-LO products (E) and their susceptibility to the inhibitory action of I3MO (F) as described in (D), means ± SE, n = 3, *P < 0.05, ***P < 0.001 vs. vehicle control.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cardiovascres/101/3/10.1093_cvr_cvt339/3/m_cvt33905.jpeg?Expires=1747890471&Signature=Gqog1laIB~2cGHGrnG-YXU2VnHICwWkelH3xGxE9c2~Ezk5eodSe1w74NuDGgDDEBeJDd--bGxBCQGSf2RDNSIPPnGHBgDaBU60vePc7GQ~hGx5csJbxNwJegbMY55FIPn52r31rgOtCGHYRsBC1hEIZNuLvuTyiKxHAHoPTvQ2evuWVVQJiEIQOkXk-Rnz-mR9ahjqvqdr9jLIVRLT-IbzQitEn3hm1SnHIIf5TsSibx8~YkO8J4La2wKa-wyYup3VaXa1EGi7ktH~xqUtNS-T55XMcT9tOULWaloMhkwvXQPc-bYqsmx3UuOkJiZXexZIJo0V61a3m1FJ5pY5~7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
I3MO inhibits the formation of 5-LO products from monocytes and neutrophils. (A) Isolated human monocytes (5 × 106 cells/mL PGC buffer) were pre-treated with I3MO (or 0.1% DMSO as vehicle) for 15 min at 37°C and then induced to form 5-LO products [i.e. LTB4 and its trans-isomers, and 5-H(P)ETE) by addition of 2.5 µM A23187 or alternatively by priming with LPS (20 min) and addition of 0.1 µM fMLP]. After 10 min at 37°C, reactions were terminated and 5-LO products were analysed by HPLC. 5-LO products of 100% controls: A23187: 123.3 ± 25.45 ng/5 × 106 cells, LPS/fMLP: 34.1 ± 15.9 ng/5 × 106 cells. Data are expressed as the percentage of control (100%), means ± SE, n = 3. LPS/fMLP: *P < 0.05; A23187:+P < 0.05; +++P < 0.001 vs. vehicle control. (B) Isolated human monocytes (2 × 106 cells/mL PGC buffer) were primed with LPS (20 min), after 5 min I3MO (or 0.1% DMSO) was added for 15 min at 37°C, and followed by 0.1 µM fMLP. After 10 min at 37°C, reaction was terminated and cysLTs were analysed by ELISA. CysLT formation of 100% controls: 588.5 ± 144.2 pg/2 × 106 cells. Data are expressed as the percentage of control (100%), means ± SE, n = 3. *P < 0.05; **P < 0.01 vs. vehicle control. (C) Isolated human neutrophils (5 × 106 cells/mL PGC buffer) were pre-treated with I3MO (or 0.1% DMSO) for 15 min at 37°C. Then, 2.5 µM A23187 was added and the reaction stopped after 10 min. 5-LO products [i.e. LTB4 and its trans-isomers, and 5-H(P)ETE] were analysed by HPLC. 5-LO products of 100% controls: A23187: 84.0 ± 20.9 ng/5 × 106 cells. Data are expressed as the percentage of control (100%), means ± SE, n = 3. **P < 0.01 vs. vehicle control. (D) Isolated human monocytes or neutrophils (5 × 106 cells/mL PGC buffer) were pre-treated with I3MO (or 0.1% DMSO) for 15 min at 37°C. Then, 2.5 μM A23187 plus 20 μM AA were added and the reaction stopped after 10 min. 5-LO products [i.e. LTB4 and its trans-isomers, and 5-H(P)ETE] were analysed by HPLC. 5-LO products of 100% controls: neutrophils: 375.8 ± 44.1 ng/5 × 106 cells, monocytes: 169.7 ± 58.0 ng/5 × 106 cells. Data are expressed as the percentage of control (100%), means ± SE, n = 3. (E and F) Monocytes were isolated side by side via adherence, positive or negative selection, and tested for their production of 5-LO products (E) and their susceptibility to the inhibitory action of I3MO (F) as described in (D), means ± SE, n = 3, *P < 0.05, ***P < 0.001 vs. vehicle control.
3.6 I3MO is a direct and selective 5-LO inhibitor
The observed inhibition of LT production could be due to reduced liberation of AA from membranes and limited availability of 5-LO substrate in the presence of I3MO. To test this possibility, cells were stimulated with A23187 in the presence of exogenous 20 µM AA, in order to circumvent the required supply of endogenous AA mediated by cytosolic phospholipase A2 (cPLA2). As shown in Figure 5D, I3MO was still able to inhibit 5-LO product synthesis in monocytes and neutrophils, with the same potency (IC50 = 5.0 ± 1.1 and 3.7 ± 1.2 µM, respectively), indicating that I3MO does not act on the level of AA release. Along these lines, I3MO (up to 10 µM) failed to inhibit the activity of human recombinant cPLA2 in a cell-free assay.28 Although previous studies suggested that differences in selection procedure could affect gene expression and activation status of monocytes, no such differences were noted in this study. Comparable 5-LO activity was observed in monocytes obtained by different isolation procedures (i.e. adherence, positive selection, or negative selection; Figure 5E), and I3MO showed similar inhibitory efficiencies (Figure 5F).
To further investigate whether I3MO is a direct inhibitor of 5-LO, we performed cell-free 5-LO activity assays using homogenates of neutrophils or of monocytes or isolated human recombinant 5-LO as an enzyme source. I3MO inhibited 5-LO activity in these cell-free assays in a concentration-dependent fashion regardless of the enzyme source (Figure 6). Even though the IC50 values in the cell-free assays were somewhat higher (7.8–10 µM) than that from the cell-based test system, these data identify I3MO as a direct 5-LO inhibitor.
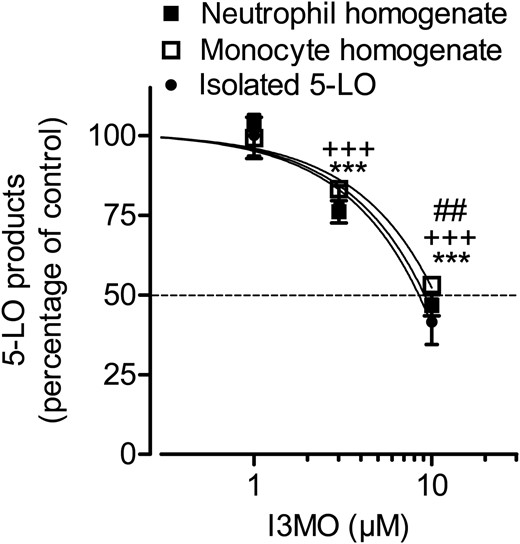
I3MO inhibits 5-LO product formation in cell-free assays. (A) Partially purified recombinant 5-LO (0.5 µg/mL) or homogenates of neutrophils or monocytes (corresponding to 5 × 106 cells/mL) were incubated with I3MO or vehicle (DMSO, 0.1%) at 4°C for 15 min. Samples were pre-warmed for 30 s at 37°C after addition of 1 mM ATP, 2 mM CaCl2, and 20 µM AA were added and 5-LO product formation was determined after 10 min. 5-LO products of 100% controls: isolated 5-LO: 1083.5 ± 345.9 ng/mL, homogenates of neutrophils: 574.9 ± 67.8 ng/mL, homogenates of monocytes: 363.7 ± 32.5 ng/mL. Data are expressed as the percentage of control (100%), means ± SE, n = 3. Neutrophil homogenates: ***P < 0.001; monocyte homogenates: +++P < 0.001; purified 5-LO: ##P < 0.01 vs. vehicle control.
Next, we analysed the selectivity of LO inhibition by I3MO. Besides 5-LO, human monocytes also express the related 15-LO enzyme that converts AA to 12-H(P)ETE and 15-H(P)ETE. Pre-incubation of monocytes with I3MO up to 10 µM failed to significantly inhibit 12-/15-H(P)ETE formation upon stimulation with A23187 plus 20 µM AA (Figure 7A). Isolated neutrophils are often associated with adherent platelets that express the platelet-type 12-LO isoform and therefore with the generation of 12-H(P)ETE upon stimulation. I3MO failed to repress 12-H(P)ETE formation in neutrophils stimulated by A23187 plus 20 µM AA (Figure 7A). Moreover, I3MO could not repress the synthesis of 12-/15-H(P)ETE in homogenates of monocytes or neutrophils, respectively, in a cell-free assay (Figure 7B). These data indicate that I3MO selectively inhibits 5-LO but not the platelet type 12-LO or the 15-LO in human monocytes and neutrophils.
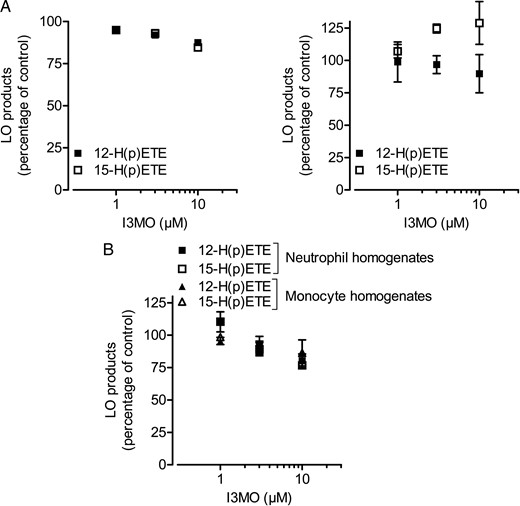
I3MO fails to inhibit 12- and 15-LO product formation. (A) Isolated human monocytes (left panel) or human neutrophils (right panel) were pre-treated with I3MO (or 0.1% DMSO as vehicle) for 15 min at 37°C and stimulated with 2.5 µM A23187 plus 20 µM AA. After 10 min, the formation of 12-H(P)ETE and 15-H(P)ETE was analysed by HPLC. 12-H(P)ETE formation of 100% controls: monocytes: 679.7 ± 248.1 ng/5 × 106 cells, neutrophils: 37.4 ± 9.1 ng/5 × 106 cells. 15-H(P)ETE formation of 100% controls: monocytes: 48.5 ± 8.2 ng/5 × 106 cells, neutrophils: 375.8 ± 44.1 ng/5 × 106 cells. (B) Homogenates of human monocytes or neutrophils were pre-incubated with I3MO or vehicle (DMSO, 0.1%) at 4°C for 15 min. Samples were pre-warmed for 30 s at 37°C, 2 mM CaCl2 and 20 µM AA were added, and 12-H(P)ETE and 15-H(P)ETE formation was determined after 10 min. 12-H(P)ETE formation of 100% controls: monocytes: 446.1 ± 180.1 ng/mL, neutrophils: 59.6 ± 10.3 ng/mL. 15-H(P)ETE formation of 100% controls: monocytes: 37.8 ± 7.2 ng/mL, neutrophils: 272.5 ± 149.1 ng/mL. Data are expressed as the percentage of control (100%), means ± SE, n = 3.
4. Discussion
In this study, we reveal that I3MO dually protects against LT-induced VSMC migration. I3MO inhibits migratory signalling in VSMC on one hand and LT production in activated monocytes by direct selective inhibition of 5-LO on the other, implying additional vasoprotective features of this compound besides its known anti-mitogenic activity.1
I3MO potently and concentration-dependently inhibited LTB4 and cysLT-induced as well as PDGF-elicited VSMC migration. In our system, LTB4 and cysLT induced VSMC migration without exhibiting a stimulatory effect on proliferation as determined using the BrdU incorporation assay. This is in contrast to a report showing an LTB4-mediated increase in both VSMC migration and proliferation.4 However, the stronger effect of cysLTs on VSMC migration than on proliferation was also observed in a previous study.29
The interference with pro-migratory signalling might contribute to the reduced neointima formation in the presence of I3MO observed in our previous study.1 Regarding the underlying anti-migratory mechanism, we demonstrate that induction of HO-1 by I3MO accounts for the observed abrogated migration. HO-1 is known as atheroprotective protein.30 We are the first to show that I3MO significantly triggers HO-1 induction and hereby inhibits migration in VSMC. As shown in WT and Nrf2−/− fibroblasts, I3MO induces HO-1 expression in an Nrf2-dependent manner. Inhibition of glycogen synthase kinase (GSK) 3β or Src-family kinases positively influences Nrf2 activity,31,32 and I3MO knowingly interferes with both kinase pathways.33–34 It is therefore conceivable that I3MO obtains HO-1 induction via the GSK3β/Src inhibition → Nrf2 axis. This hypothesis of Nrf2 activation by I3MO needs to be thoroughly studied in VSMC in the future. Notably, the indirubin derivative E847 failing to induce HO-1 lacks Src inhibition (own unpublished data) and further prompts such investigations.
We furthermore demonstrate in this study for the first time that I3MO is a direct inhibitor of 5-LO, as it inhibited the enzymatic formation of all 5-LO-derived products in a cell-free assay. 5-LO transforms AA in two steps: it first catalyses the incorporation of oxygen into AA yielding 5-HPETE (oxygenase activity) that can be reduced to 5-HETE.35 Then, in a second step, 5-LO catalyses incorporation of another oxygen into 5-HPETE and after dehydration yields LTA4 (synthase activity). I3MO clearly blocked 5-HPETE formation upstream of LTA4. Therefore, it blocks the first 5-LO reaction step, and hence it is a direct 5-LO inhibitor. Current ongoing studies address structure–activity relationships and the exact molecular mechanisms of I3MO interaction with 5-LO in detail, including analysis of modulation of 5-LO subcellular localization, 5-LO phosphorylation, and interaction with 5-LO activating protein and coactosin-like protein by I3MO.
Inhibition of 5-LO product formation suggests anti-inflammatory features in the pharmacological profile of I3MO. Of interest, I3MO has recently been reported to also inhibit Jun and NF-κB signalling26 complementing its anti-inflammatory potential. Here, we observed that I3MO interferes with LT production in activated monocytes and neutrophils with an IC50 value of around 4 µM. Suppression of LT synthesis also explains the abrogated pro-migratory property of medium from I3MO- or BWA4C-treated activated monocytes. Our data furthermore show that I3MO is a direct and selective (over 15-LO and 12-LO) 5-LO inhibitor.
The feature of I3MO as a 5-LO inhibitor not only suggests I3MO as promising possible lead compound for the treatment of asthmatic diseases, but also of atherosclerosis. In fact, 5-LO and its products have gained increasing attention in recent years as important players in atherogenic diseases. Thus, products of the 5-LO pathway were found as potent vasoconstrictors36,37 and to promote leukocyte adhesion in post-capillary venules in vivo.38 Moreover, LTs induced DNA synthesis and proliferation as well as migration in cultured smooth muscle cells.4,39 However, as mentioned above, we could only reproduce the migratory effect of LTs in the present study and with the chosen experimental parameters. The expression of 5-LO is augmented in carotid and coronary arteries and aorta specimens of patients with clinically significant atherosclerosis and localized within immune cells such as macrophages, dendritic, and foam cells.10 In a study using ApoE−/− and LDL receptor−/− mice, LTB4 antagonism resulted in reduced lesion progression and monocyte recruitment.40 All these data support a positive impact of 5-LO inhibition for vascular health.
Overall, our findings show that I3MO has the potential to interfere with proliferation, migration, and seemingly also with the inflammatory processes at the site of vascular injury and thus, to combat the three main features contributing to vascular dysfunction. Future studies are warranted to confirm this triple action in vivo.
Authors' contribution
T.B., A.M.S., C.P., O.W., V.M.D., and E.H.H. conceived and designed the study. T.B., A.M.S., K.W., D.S., F.N., C.P., and E.H.H. performed experiments and analysed data. D.B. and C.W. have taken blood from patients and prepared leucocyte concentrates. A.G.A., C.P., O.W., V.M.D., and E.H.H. supervised the execution of experiments and their interpretation. T.B., A.M.S., C.P., O.W., V.M.D., and E.H.H. wrote the manuscript. All authors have read the manuscript and approved the content.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Austrian Science Fund (FWF, P23317 to E.H.H.). Funding to pay the Open Access publication charges for this article was provided by the FWF (Austrian Science Fund).
Acknowledgements
The authors thank the expert technical assistance of H. Beres and Dr I. Sroka for pilot data on the anti-migratory potential of I3MO.
Conflict of interest: none declared.



