-
PDF
- Split View
-
Views
-
Cite
Cite
Alice Tisserand, Frédéric Blanc, Candice Muller, Hélène Durand, Catherine Demuynck, Alix Ravier, Léa Sanna, Paulo Loureiro de Sousa, Anne Botzung, Mary Mondino, Nathalie Philippi, Neuroimaging of autobiographical memory in dementia with Lewy bodies: a story of insula, Brain Communications, Volume 6, Issue 4, 2024, fcae272, https://doi.org/10.1093/braincomms/fcae272
Close - Share Icon Share
Abstract
Although deficits in learning and retrieving new information are well characterized in dementia with Lewy bodies, autobiographical memory has never been explored in this disease. Yet, autobiographical memory impairments are a pervasive feature of dementia, well characterized in other neurodegenerative diseases. Moreover, autobiographical memory corresponds to an extension over time of the self, which we hypothesize is altered in dementia with Lewy bodies and impairment of which could be linked to the insular atrophy occurring from an early stage of the disease. In this study, we sought to characterize autobiographical memory impairments and explore their neural correlates in dementia with Lewy bodies, on the assumption that insular damage could impact the self, including its most elaborate components, such as autobiographical memory. Twenty patients with prodromal to mild dementia with Lewy bodies were selected to participate in this exploratory study along with 20 healthy control subjects. The Autobiographical Interview was used to assess autobiographical memory. Performances were compared between patients and control subjects, and an analysis across life periods and recall conditions was performed. 3D magnetic resonance images were acquired for all participants, and correlational analyses were performed in the patient group using voxel-based morphometry. The behavioural results of the Autobiographical Interview showed that autobiographical memory performances were significantly impaired in dementia with Lewy body patients compared to control subjects in a temporally ungraded manner, for both the free recall and the specific probe conditions (P < 0.0001), though with greater improvement after probing in the patient group. Furthermore, autobiographical memory impairments were correlated with grey matter volume within right insular cortex, temporoparietal junction, precuneus, putamen, left temporal cortex, bilateral parahippocampus and cerebellum, using a threshold of P = 0.005 uncorrected. The behavioural results confirm the existence of temporally ungraded autobiographical memory impairments in dementia with Lewy bodies, from the early stage of the disease. As we expected, neuroimaging analysis revealed a role for the insula and the precuneus in autobiographical memory retrieval, two regions associated with elementary aspects of the self, among other brain regions classically associated with autobiographical memory, such as medial temporal lobe and temporoparietal junction. Our findings provide important insights regarding the involvement of the insula in the self and suggest that insular damage could lead to a global collapse of the self, including its more elaborated components, such as autobiographical memory.
Introduction
While pervasive deficits in learning and retrieving new information are well characterized in dementia with Lewy bodies,1,2 autobiographical memory (ABM) has never been explored in this disease. Yet, in dementia with Lewy bodies, there seem to be obvious alterations of the present self,3-7 which has been proposed as a prerequisite for the self extended in time, namely ABM.8-10 The present self pertains to the aspects of the sense of self that are related to and accessible in the present moment. According to Prebble et al.,9 the present self comprises two components: the subjective sense of self and the self-concept. The subjective sense of self refers to the subjective living experience that implies the construction of a mental representation,11-13 whereas the self-concept contains the object of this representation, including knowledge about ourselves.12,14 Recently, a study in dementia with Lewy body patients has shown deficits in awareness, such as anosognosia3 that could reflect alteration of the subjective sense of self, while other studies demonstrated that dementia with Lewy body patients display changes in their personal tastes and impairments in representing who they are,5,15 revealing alteration of the self-concept. In these latter studies, we were able to link the alteration of the self to the insula, which is damaged early in the course of dementia with Lewy bodies16-19 and is a core region for the self.15 Indeed, the insula is involved in multiple processes related to the present self, ranging from internal bodily states, such as interoception,20,21 to high-order processes, such as knowledge about oneself,7,22 but little is known about its contribution to the self extended in time.
ABM refers to the capacity to recollect experiences personally lived and to autobiographical knowledge, supporting the construction of the representation of our identity over time.23 ABM is a complex construct that can be dissected into two distinct memory systems: an episodic one and a semantic one.10,24 The episodic component refers to the subjective re-experiencing of events from the past, with emotions and perceptual details, within a particular place and time, whereas the semantic components consist of knowledge, facts and conceptual information related to personal life, devoid of the spatio-temporal context in which this information was acquired.25 However, an obvious interconnection between the self and ABM is recognized by expert researchers in the field.8,9,21,26,27 A growing number of studies have investigated the neurobiological basis of this complex form of cognition. In a meta-analysis conducted using haemodynamic imaging in healthy adults,28 researchers identified a core ABM network, comprising medial temporal lobe structures, including the hippocampus and parahippocampal cortices, the lateral temporal cortices, the medial prefrontal, posterior parietal region and the cerebellum, whereas the lateral prefrontal and temporal cortices, insula, precuneus and basal ganglia appeared less frequently involved. To our knowledge, only two studies found a correlation between the insula and ABM. In the first study, Fink et al.29 found the insula to be a secondary region of ABM in a healthy population. In the second study, Descamps et al.30 found a diminished ABM richness in patients with insular resection compared to healthy controls. Moreover, some behavioural studies found impaired ABM in disorders associated with insular dysfunction, such as schizophrenia and autism spectrum disorders,31,32 though no imaging study to confirm potential insular involvement was included.
Impaired ability to remember events from the past is considered a transdiagnostic feature of dementia and is often among the earliest symptoms reported by patients. ABM impairments can take different forms arising from the dysfunction of neurocognitive processes, depending on the distribution of neural atrophy in the brain.33 In Alzheimer’s disease, episodic memory dysfunction caused by neuronal degeneration in the medial temporal lobe leads to an extended retrieval deficit due to a global degradation of specific ABM,34,35 more pronounced for the recent period due to an additional encoding/consolidation deficit for recent memories.36,37 Meanwhile, semanticized memories from remote periods of life are better preserved given that they rely on the relative integrity of the temporal neocortex.38 Conversely, semantic dementia is characterized by damage to the anterior temporal neocortex39 and by a ‘reversed’ ABM profile, with spared recent episodic memories40 contrasting with the loss of remote semanticized memories.35,41 In the behavioural variant of frontotemporal dementia, which is characterized by damage to the frontoinsular and ventromedial prefrontal cortices,42,43 the ABM profile appears flat due to multiple mechanisms, including strategic retrieval difficulties.33,35,40,44 A similar ABM flat profile is described in posterior cortical atrophy and would be related to disruption of access to the visual contextual information integral to the ABM trace, in connection with atrophy of posterior parietal regions, including the right précuneus.45
Despite the evidence of altered ABM in the aforementioned dementia syndromes, no studies have yet explored this type of memory in dementia with Lewy bodies, which is, however, the second most common form of cognitive neurodegenerative disease after Alzheimer's disease.46,47 Thus, in this paper, we report the first behavioural and neuroimaging study of ABM in dementia with Lewy body patients. Our purpose was to explore the potential ABM deficit in dementia with Lewy bodies along with its anatomical substrates, with a particular interest in the insula. Based on the premise that the insula is damaged at an early stage in dementia with Lewy body patients and based on the assumption that the insula plays a central role in the self, we predicted that ABM would be impaired in an ungraded manner due to a global alteration of the self. Moreover, given that memory disorders in dementia with Lewy bodies are characterized by deficits in learning and retrieving the information, we expected that retrieval support would have beneficial effects on the richness of memories.
Materials and methods
Study population
The current study was conducted using the same cohort of patients as in our previous research on the self.6 Specifically, it included 20 patients with early-stage dementia with Lewy bodies and 20 healthy control subjects matched for age, gender and level of education (with a minimum of 9 years) were enrolled in the present study between January 2021 and February 2023. Patients were recruited from the tertiary memory clinic of Strasbourg University Hospital (CM2R of Strasbourg), France, including the geriatrics and neurology departments. Control subjects were recruited in three ways: among friends and relatives of the patients, via the listing of controls of the local clinical investigation centre and from the control group of the AlphaLewyMA cohort (http://clinicaltrials.gov/ct2/show/NCT01876459). Diagnosis of prodromal and mild dementia with Lewy bodies was based on core clinical features.48,49 Some of the patients had also benefited from biomarkers during their clinical follow-up. Indeed, a dopamine transporter (DAT) imaging was performed when parkinsonism was doubtful, and a CSF analysis was performed when an amnestic syndrome of hippocampal type suggested possible Alzheimer’s disease, to ensure that there was no co-pathology. Thus, a DAT scan was available to support the diagnosis in approximately a quarter of the patients, and CSF analysis was available to confirm the absence of associated Alzheimer’s disease in approximately half of the patients. Patients with prodromal dementia with Lewy bodies were defined as having mild cognitive impairment if they had a Mini-Mental State Examination (MMSE) score of ≥26, had preservation of independence as assessed by the Instrumental Activities of Daily Living50 and fulfilled both the DSM-5 criteria of mild neurocognitive disorder51 and McKeith’s criteria for the diagnosis of prodromal dementia with Lewy bodies.48 Patients were defined as having mild dementia with Lewy bodies if they had an MMSE score between 20 and 25 and were diagnosed as having probable dementia with Lewy bodies according to the current dementia with Lewy body criteria.49 All participants benefited from a classic medical examination, which notably included evaluation of the features of parkinsonism using the Unified Parkinson’s Disease Rating Scale (Part 3): akinesia, rigidity and resting tremor (rated from 0 for no symptoms to 4 for serious symptoms). The dementia with Lewy bodies group underwent further clinical examination of dementia with Lewy body core criteria, among which fluctuations were assessed with the Mayo Clinic Fluctuations Scale52 and the Newcastle-upon-Tyne Clinician Assessment of Fluctuation Scale.53 The Parkinson’s disease-associated psychotic symptoms questionnaire54 was used to evaluate the presence of hallucinations. Rapid eye movement sleep behaviour disorder (RBD) was evaluated using a sleep questionnaire on RBD,55 simplified into four questions, two each for the patient and for the caregiver: one concerning movements during sleep and the second concerning vivid dreams and nightmares.
Subjects with a history of alcohol/substance abuse, significant visual or auditory disabilities, relevant neurological of psychiatric comorbidities or the presence of other severe or unstable medical illnesses were not enrolled in the study. Subjects with an abnormal neurological examination—except for parkinsonism in the case of patients—depression symptoms (mini-Geriatric Depression Scale56) or a significant cerebral vascular burden (Modified Hachinski Ischemic Score of >757) were not enrolled. Participants with CSF biomarkers suggestive of Alzheimer’s disease (i.e. abnormal Aβ42/Aβ40 ratio, t-Tau, phospho-Tau181) were not enrolled. Finally, participants with claustrophobia or contraindications to MRI were not enrolled. All participants provided written informed consent for the study, in accordance with the Declaration of Helsinki, and the study was approved by the ethics committee Sud Méditerranée III.
Behavioural study
Baseline
General cognitive assessment was performed in the patients, including an evaluation of anterograde memory with the RL/RI-16 (‘Rappel libre/Rappel indicé à 16 items’, i.e. 16-item free recall/probed recall),58 a French test similar to the FCSRT (Free and Cued Selective Reminding Test),59 verbal fluencies,60 Frontal Assessment Battery,61 TMTA and TMTB,62 digit span of the WAIS (Wechsler Adult Intelligence Scale)63 and Rey-Osterrieth Complex Figure.64 The results are displayed in Supplementary Table 1.
Autobiographical Interview
The Autobiographical Interview25 was administered and rated according to Levine’s methodology,65 for an analysis of the participants ability to relate, under different levels of retrieval support, autobiographical memories established at different times in their lives. Participants were instructed to provide detailed descriptions of two events that were personally experienced and that occurred at a specific time and place from each of five life periods (childhood, age 0–15 years; young adulthood, age 16–30; middle adulthood, age 31–50; late adulthood, age 51–the past year; during the past year). In the original version of the Autobiographical Interview,25 only one event per life period was required, but in the present study, participants were asked to describe two events, so as to increase the quantity of material to be analysed. Life periods were randomly assigned during the session, and a list of 20 word cues (e.g. car, piano, apple) was presented to assist in event retrieval.
To examine facilitative effects of retrieval support on memory, we manipulated the level of structure available to participants across three conditions: free recall, general probe and specific probe. At free recall, participants spoke about the event without any interruption from the examiner, continuing until they had reached a natural ending point. After an event had been recalled, the examiner prompted the participant once for a greater recall of details (general probe). At the specific probe phase, a structured interview was administered to elicit additional contextual details encompassing four categories: time, place, perceptual details and emotion/thought.
Descriptions were recorded and transcribed. Following administration, event descriptions were segmented into details and classified as ‘internal’ and assigned to one of five categories (event, place, time, perceptual and emotion/thought) if they related directly to the main event described, were specific to time and place and conveyed a sense of episodic re-experiencing. Otherwise, details were considered ‘external’, consisting of satellite autobiographical events or unrelated to the main event, semantic facts, repetitions or other metacognitive statements. Details from each category were summed separately for each condition (i.e. free recall, general probe, specific probe). As the effect of general probing on general performance is minimal in comparison to that of specific probing,25 free recall and general probe scores were combined. Quantitative ratings were accompanied by qualitative ratings assigned for time, place, perception and emotion/thought, with the possibility of attaining a maximum rating of 3 points for each category (time, place, perceptual, emotion/thought). A rating of 3 points was assigned when the description was rich, highly specific and appeared to emerge from a feeling of re-experiencing. A rating of 2 points was assigned to detailed descriptions falling short of a 3-point description. A rating of 1 point was assigned to descriptions containing general, non-specific information but still episodic in nature. A rating of 0 point was assigned when there was no information pertaining to the specified category, or for responses based on semantic knowledge rather than episodic memory. Episodic richness (the overall degree to which a feeling of re-experiencing was conveyed) was rated on a similar scale that was extended to 6 points to provide a more accurate rating and to take into account the greater importance of this category relative to the others. Although ratings in the first four categories were mutually exclusive (i.e. aspects of a memory could not be counted in more than one category), the episodic richness was based on an overall assessment of the event. To investigate potential differences across time periods, the episodic richness ratings of the two memories were added for each period for both the free recall (i.e. free recall plus general probe) and the probed recall (i.e. total score following probing) (maximum = 36). To analyse the possible differences between the two recall conditions, the scores of each period were added up to obtain a general free recall score and probed recall score (maximum = 180).
Participants’ memories were scored by one trained rater (A.T.). To assess interrater reliability, 10% of the memories were selected at random (with the constraint that age, groups and life periods were equally represented) and scored by another trained rater (N.P.) who was blind to subject status, in accordance with established scoring procedures from previous research.25,66 Interrater reliability was high for free recall score and probed recall score (all Cronbach’s α ≥ 0.94).
Statistical analysis for the behavioural study
Student’s t-tests were used to compare intergroup differences between dementia with Lewy body patients and control subjects for demographic quantitative data. A χ2 test was used to compare the sex ratio between groups. For the behavioural data, Mann–Whitney U-tests were used to compare the free recall score and probed recall score,25 for both dementia with Lewy body patients and control subjects, as we hypothesized that scores would be lower in the free recall condition compared to the specific probe. We also compared the percentage of improvement after specific probe ((probed recall score-free recall score/free recall score) × 100) between dementia with Lewy body patients and control subjects, using Mann–Whitney U-tests. Two-way repeated measures ANOVAs, including age as nuisance covariate, were conducted to examine the effect of life period across the different recall conditions, for both dementia with Lewy body patients and control subjects. Bonferroni correction was applied for multiple comparisons.
Neuroimaging study
As described previously,6 each participant underwent a high-resolution anatomical MRI scan within a maximum of 12 weeks after taking the Autobiographical Interview. T1-weighted 3D anatomical images were obtained using a 3T MRI scanner (Verio 32-channel Tim Siemens scanner; Siemens, Erlangen, Germany) using a volumetric magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) sequence (field of view = 256 × 256 mm, image matrix = 256 × 256, slice thickness = 1 mm, repetition time = 1900 ms, echo time = 2.52 ms, flip angle = 9°).
Voxel-based morphometry analyses
We used voxel-based morphometry (VBM) to investigate differences in grey matter (GM) volume between the healthy controls and the dementia with Lewy body patients and to examine the neuroanatomical correlates of ABM in dementia with Lewy body patients. VBM analyses included image preprocessing and statistical analyses. These steps were carried out using the SPM12 software package (Wellcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/) running on Matlab R2017b (MathWorks, Natick, MA, USA). Anatomical MRI images were spatially preprocessed using standard procedures.67 All T1-weighted structural images were first segmented, bias corrected and spatially normalized to the Montreal Neurological Institute space using an extension of the unified segmentation procedure that includes six classes of tissue.68 The DARTEL registration toolbox was then used to build a study-specific template and to bring into alignment all of the segmentation images. The VBM analysis was done on modulated GM images; that is, the GM value in each voxel was multiplied by the Jacobian determinant derived from the spatial normalization. This procedure preserves the total amount of GM from the original images. These modulated GM images were smoothed with a Gaussian kernel (full width at half maximum: 8 mm). Between-group voxel-based comparisons were displayed after correcting for multiple comparisons with false discovery rate (FDR; P < 0.05). A voxel-wise general linear model was employed to investigate the regions of atrophy related to disorders of the episodic ABM, in dementia with Lewy body patients. To analyse the potential different regions involved in the free recall and in the probed recall, we tested the correlation between the GM volume and both the free recall score and the probed recall score, using a threshold of P = 0.005 uncorrected, including age, gender and total intracranial volume (TIV) as nuisance covariates. MMSE was also considered as an additional covariate to investigate the potential impact of disease severity. Moreover, to obtain a control measure of memory not involving the self, we tested the correlations between GM volume and the 1–3 free and total recall conditions of the RL/RI-16,58 using the same threshold, including the same nuisance covariates as for the Autobiographical Interview. A cluster spatial extent of 50 voxels was used to avoid irrelevant and isolated detections. The software xjView (http://www.alivelearn.net/xjview8/) enabled us to characterize each cluster.
Results
Clinical characteristics
Twenty patients with early-stage dementia with Lewy bodies and 20 healthy control subjects were enrolled in the present study. A description of the study population is presented in Table 1. Groups were well matched for age (t = 0.64, P = 0.526), years of education (t = −1.61, P = 0.116) and sex (χ2 = 0.44, P = 0.507). The dementia with Lewy bodies group comprised 13 patients at the prodromal stage of dementia with Lewy bodies and 7 patients at the mild stage of the disease. Among the 20 dementia with Lewy body patients, 85% presented fluctuations, 60% presented hallucinations or illusions and 60% presented RBD. Concerning parkinsonism features, akinesia was observed in 80% of dementia with Lewy body patients, rigidity in 70% of dementia with Lewy body patients and resting tremor in only one patient (5%).
Demographic and clinical characteristics of the dementia with Lewy bodies group and the control group
| Characteristic . | Dementia with Lewy bodies group (n = 20) . | Control group (n = 20) . | Student’s t-test or χ2 test . |
|---|---|---|---|
| Mean age in years (+SD) | 71.9 (7.9) | 70.4 (7.3) | t = 0.64, P = 0.526 |
| Years of education (+SD) | 12.7 (2.5) | 14.05 (2.6) | t = −1.61, P = 0.116 |
| Sex, M/F | 14/6 | 12/8 | χ2 = 0.44, P = 0.507 |
| MMSE score (/30) | 26.3 (2.39) | 28.8 (1.0) | t = −4.32, P < 0.001 |
| Handedness, R/L | 19/1 | 20/0 | χ2 = 1.03, P = 0.311 |
| Mean of disease duration (+SD) | 3.19 (2.12) | N/A | |
| Fluctuations (%) | 17/20 (85) | N/A | |
| Hallucinations/illusions (%) | 12/20 (60) | N/A | |
| RBD (%) | 12/20 (60) | N/A | |
| Parkinsonism | |||
| Akinesia (%) | 16/20 (80) | N/A | |
| Rigidity (%) | 14/20 (70) | N/A | |
| Tremor at rest (%) | 1/20 (5) | N/A |
| Characteristic . | Dementia with Lewy bodies group (n = 20) . | Control group (n = 20) . | Student’s t-test or χ2 test . |
|---|---|---|---|
| Mean age in years (+SD) | 71.9 (7.9) | 70.4 (7.3) | t = 0.64, P = 0.526 |
| Years of education (+SD) | 12.7 (2.5) | 14.05 (2.6) | t = −1.61, P = 0.116 |
| Sex, M/F | 14/6 | 12/8 | χ2 = 0.44, P = 0.507 |
| MMSE score (/30) | 26.3 (2.39) | 28.8 (1.0) | t = −4.32, P < 0.001 |
| Handedness, R/L | 19/1 | 20/0 | χ2 = 1.03, P = 0.311 |
| Mean of disease duration (+SD) | 3.19 (2.12) | N/A | |
| Fluctuations (%) | 17/20 (85) | N/A | |
| Hallucinations/illusions (%) | 12/20 (60) | N/A | |
| RBD (%) | 12/20 (60) | N/A | |
| Parkinsonism | |||
| Akinesia (%) | 16/20 (80) | N/A | |
| Rigidity (%) | 14/20 (70) | N/A | |
| Tremor at rest (%) | 1/20 (5) | N/A |
Significant P-value and χ2 value are in italics. SD, standard deviation; M, male; F, female; MMSE, Mini-Mental State Examination; R, right; L, left; RBD, rapid eye movement sleep behaviour disorder; N/A, not applicable.
Demographic and clinical characteristics of the dementia with Lewy bodies group and the control group
| Characteristic . | Dementia with Lewy bodies group (n = 20) . | Control group (n = 20) . | Student’s t-test or χ2 test . |
|---|---|---|---|
| Mean age in years (+SD) | 71.9 (7.9) | 70.4 (7.3) | t = 0.64, P = 0.526 |
| Years of education (+SD) | 12.7 (2.5) | 14.05 (2.6) | t = −1.61, P = 0.116 |
| Sex, M/F | 14/6 | 12/8 | χ2 = 0.44, P = 0.507 |
| MMSE score (/30) | 26.3 (2.39) | 28.8 (1.0) | t = −4.32, P < 0.001 |
| Handedness, R/L | 19/1 | 20/0 | χ2 = 1.03, P = 0.311 |
| Mean of disease duration (+SD) | 3.19 (2.12) | N/A | |
| Fluctuations (%) | 17/20 (85) | N/A | |
| Hallucinations/illusions (%) | 12/20 (60) | N/A | |
| RBD (%) | 12/20 (60) | N/A | |
| Parkinsonism | |||
| Akinesia (%) | 16/20 (80) | N/A | |
| Rigidity (%) | 14/20 (70) | N/A | |
| Tremor at rest (%) | 1/20 (5) | N/A |
| Characteristic . | Dementia with Lewy bodies group (n = 20) . | Control group (n = 20) . | Student’s t-test or χ2 test . |
|---|---|---|---|
| Mean age in years (+SD) | 71.9 (7.9) | 70.4 (7.3) | t = 0.64, P = 0.526 |
| Years of education (+SD) | 12.7 (2.5) | 14.05 (2.6) | t = −1.61, P = 0.116 |
| Sex, M/F | 14/6 | 12/8 | χ2 = 0.44, P = 0.507 |
| MMSE score (/30) | 26.3 (2.39) | 28.8 (1.0) | t = −4.32, P < 0.001 |
| Handedness, R/L | 19/1 | 20/0 | χ2 = 1.03, P = 0.311 |
| Mean of disease duration (+SD) | 3.19 (2.12) | N/A | |
| Fluctuations (%) | 17/20 (85) | N/A | |
| Hallucinations/illusions (%) | 12/20 (60) | N/A | |
| RBD (%) | 12/20 (60) | N/A | |
| Parkinsonism | |||
| Akinesia (%) | 16/20 (80) | N/A | |
| Rigidity (%) | 14/20 (70) | N/A | |
| Tremor at rest (%) | 1/20 (5) | N/A |
Significant P-value and χ2 value are in italics. SD, standard deviation; M, male; F, female; MMSE, Mini-Mental State Examination; R, right; L, left; RBD, rapid eye movement sleep behaviour disorder; N/A, not applicable.
Behavioural study
Overall performance on the Autobiographical Interview
Figure 1 shows the total scores of episodic richness summed across all time periods in the free and probed retrieval conditions of the Autobiographical Interview. Mann–Whitney U-test revealed that controls displayed higher episodic richness across both retrieval conditions in comparison to dementia with Lewy body patients (P < 0.0001). The mean score obtained in free recall was significantly inferior in the dementia with Lewy bodies group (46.9, SD 21.02) than in the control group (103.95 SD 11.17) (U = 2.5, P < 0.0001). Similarly, the mean score obtained in probed recall was significantly inferior in the dementia with Lewy bodies group (82.3, SD 31.68) than in the control group (152.1, SD 12.36) (U = 2.5, P < 0.0001). The episodic richness evolution rate between the free recall score and the probed recall score was higher in dementia with Lewy body patients (gain of 75.48% of episodic richness in the probed recall) compared to controls (gain of 46.32% of episodic richness in the probed recall) (U = 341, P < 0.0001).
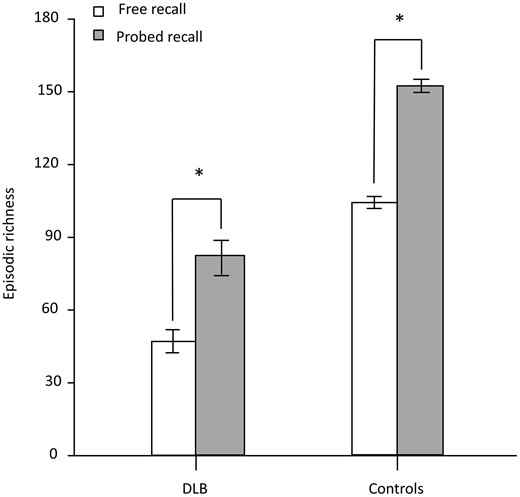
Total scores of episodic richness for the free recall and probed recall on the Autobiographical Interview, for all participants. Mann–Whitney U-tests were used to compare the free recall score and probed recall score for both dementia with Lewy body patients (n = 20) and control subjects (n = 20). *P < 0.0001 (error bars represent standard deviation). DLB, dementia with Lewy bodies.
ABM retrieval across life periods
Figure 2 shows the profile of free recall and probed recall across life periods. A two-way repeated measures ANOVA revealed significant main effect for group [F(1,37) = 98.93, P < 0.0001], with controls displaying overall higher episodic richness than dementia with Lewy body patients (t = 9.95, P < 0.001). Another significant main effect was found for recall condition [F(1,37) = 9.83, P = 0.0034], with significantly higher episodic richness in the probed recall condition for both dementia with Lewy body patients and controls (t = −23.23, P < 0.001). There was also a significant interaction between group and recall condition [F(1,37) = 11.16, P = 0.0019], with significantly lower scores in the dementia with Lewy bodies group compared to the control group for both free recall (t = 8.62, P < 0.001) and probed recall (t = 10.48, P < 0.001) conditions. No significant main effect was found for life periods for both controls and dementia with Lewy body patients, in either the free recall condition or the probed recall condition. Other comparisons did not reveal significant differences between the scores.
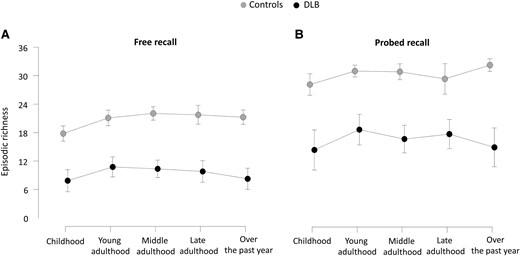
Profile of free recall (A) and probed recall (B) across life periods on the Autobiographical Interview for all participants. Two-way repeated measures ANOVAs, including age as nuisance covariate, were conducted to examine the effect of life period across the different recall conditions, for both dementia with Lewy body patients (n = 20) and control subjects (n = 20). Bonferroni correction was applied for multiple comparisons (error bars represent standard deviation). DLB, dementia with Lewy bodies.
Neuroimaging study
A voxel-based analysis comparing GM volume in dementia with Lewy body patients versus control subjects is presented in Supplementary Fig. 1. The analysis included TIV and age as nuisance covariates and revealed patterns of cerebral atrophy typically reported in dementia with Lewy body patients,17,69 with reduction in GM volume in the insular, temporal, occipital, frontal and cingular cortices, and to a lesser extent the parietal cortex, and also in the cerebellum and subcortical regions such as the putamen (P < 0.05, FDR corrected), when compared to control subjects.
The free recall score and the probed recall score were analysed separately to examine facilitative effects of retrieval support on memory. VBM analyses revealed a positive correlation between both the free recall score (Fig. 3) and the probed recall score (Fig. 4) and GM volumes within a total of seven clusters, four of which were common to both Autobiographical Interview conditions (see Table 2). The first cluster included the right posterior insular cortex and the temporoparietal junction, the second cluster included the right precuneus, the third cluster included the right parahippocampal gyrus and the fourth cluster included the left parahippocampal gyrus and the left cerebellum. In the free recall condition, other clusters were found in the right cerebellum and in the right putamen, whereas in the probed recall condition, another cluster was found in the left inferior temporal gyrus.
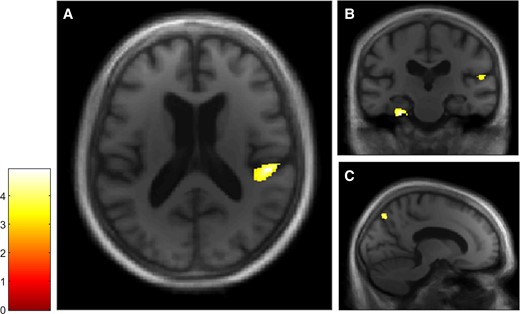
VBM analyses for the Autobiographical Interview free recall condition in the dementia with Lewy bodies group. Multiple linear regression was used to obtain correlation for Autobiographical Interview free recall score and GM volume in patients with dementia with Lewy bodies (n = 20). GM volumes within right posterior insula (A and B), left parahippocampal gyrus and right temporoparietal junction (B) and right precuneus (C) are positively correlated with the free recall score on the Autobiographical Interview, using a threshold of P = 0.005 uncorrected, including age, gender, TIV and MMSE score as nuisance covariates (k = 50).
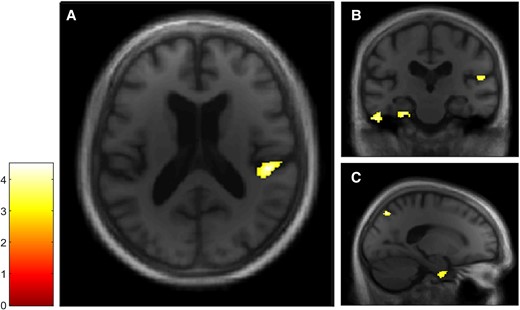
VBM analyses for the Autobiographical Interview probed recall condition in the dementia with Lewy bodies group. Multiple linear regression was used to obtain correlation for Autobiographical Interview probed recall score and GM volume in patients with dementia with Lewy bodies (n = 20). GM volumes within right posterior insula (A and B), left inferior temporal and parahippocampal gyri (B) and right precuneus and parahippocampal gyrus (C) are positively correlated with the probed recall score on the Autobiographical Interview, using a threshold of P = 0.005 uncorrected, including age, gender, TIV and MMSE score as nuisance covariates (k = 50).
VBM results for the free recall and probed recall conditions on the Autobiographical Interview in the dementia with Lewy bodies group
| . | . | . | . | K . | |
|---|---|---|---|---|---|
| Cluster . | VBM . | Side . | BA . | FRS . | PRS . |
| 1. | Posterior insular cortex | R | 13 | 216/813 | 226/765 |
| Temporoparietal junction | R | 41 | 361/813 | 305/765 | |
| 2. | Precuneus | R | 7 | 97/112 | 184/194 |
| 3. | Parahippocampal gyrus | R | 28/36 | 120/126 | 509/544 |
| 4. | Parahippocampal gyrus | L | 35/36 | 393/701 | 423/709 |
| Cerebellum | L | NA | 109/701 | 104/709 | |
| 5. | Cerebellum | R | NA | 93/100 | |
| 6. | Putamen | R | NA | 92/195 | |
| 7. | Inferior temporal gyrus | L | 20 | 77/137 | |
| . | . | . | . | K . | |
|---|---|---|---|---|---|
| Cluster . | VBM . | Side . | BA . | FRS . | PRS . |
| 1. | Posterior insular cortex | R | 13 | 216/813 | 226/765 |
| Temporoparietal junction | R | 41 | 361/813 | 305/765 | |
| 2. | Precuneus | R | 7 | 97/112 | 184/194 |
| 3. | Parahippocampal gyrus | R | 28/36 | 120/126 | 509/544 |
| 4. | Parahippocampal gyrus | L | 35/36 | 393/701 | 423/709 |
| Cerebellum | L | NA | 109/701 | 104/709 | |
| 5. | Cerebellum | R | NA | 93/100 | |
| 6. | Putamen | R | NA | 92/195 | |
| 7. | Inferior temporal gyrus | L | 20 | 77/137 | |
Please note that the entire table with exact Talairach coordinates is provided in Supplementary Table 2. FRS, free recall score; PRS, probed recall score; L, left; R, right; BA, Brodmann area; K, cluster size in voxels (specific region’s volume/cluster’s global volume).
VBM results for the free recall and probed recall conditions on the Autobiographical Interview in the dementia with Lewy bodies group
| . | . | . | . | K . | |
|---|---|---|---|---|---|
| Cluster . | VBM . | Side . | BA . | FRS . | PRS . |
| 1. | Posterior insular cortex | R | 13 | 216/813 | 226/765 |
| Temporoparietal junction | R | 41 | 361/813 | 305/765 | |
| 2. | Precuneus | R | 7 | 97/112 | 184/194 |
| 3. | Parahippocampal gyrus | R | 28/36 | 120/126 | 509/544 |
| 4. | Parahippocampal gyrus | L | 35/36 | 393/701 | 423/709 |
| Cerebellum | L | NA | 109/701 | 104/709 | |
| 5. | Cerebellum | R | NA | 93/100 | |
| 6. | Putamen | R | NA | 92/195 | |
| 7. | Inferior temporal gyrus | L | 20 | 77/137 | |
| . | . | . | . | K . | |
|---|---|---|---|---|---|
| Cluster . | VBM . | Side . | BA . | FRS . | PRS . |
| 1. | Posterior insular cortex | R | 13 | 216/813 | 226/765 |
| Temporoparietal junction | R | 41 | 361/813 | 305/765 | |
| 2. | Precuneus | R | 7 | 97/112 | 184/194 |
| 3. | Parahippocampal gyrus | R | 28/36 | 120/126 | 509/544 |
| 4. | Parahippocampal gyrus | L | 35/36 | 393/701 | 423/709 |
| Cerebellum | L | NA | 109/701 | 104/709 | |
| 5. | Cerebellum | R | NA | 93/100 | |
| 6. | Putamen | R | NA | 92/195 | |
| 7. | Inferior temporal gyrus | L | 20 | 77/137 | |
Please note that the entire table with exact Talairach coordinates is provided in Supplementary Table 2. FRS, free recall score; PRS, probed recall score; L, left; R, right; BA, Brodmann area; K, cluster size in voxels (specific region’s volume/cluster’s global volume).
VBM analyses for both the 1–3 free and total recall at the RL/RI-16 did not reveal any association with the insula. The results are presented in Supplementary Figs 2 and 3.
Discussion
This study explored the anatomical correlates and the profile of episodic ABM impairments in patients with early-stage dementia with Lewy bodies. The main aim was to determine whether the insula, as a core region of the self,15 is involved in the self extended in time in dementia with Lewy body patients. In summary, in the behavioural study, our findings confirm that ABM is impaired in patients with early-stage dementia with Lewy bodies, in an ungraded manner impacting all time periods, partially linked to strategic retrieval deficits, but not exclusively. Neuroimaging analyses confirm that the insular cortex is associated with ABM, namely in the self extended in time, aside from its well-known role in the present self. Additionally, we found that another crucial region of the self, the precuneus, is also associated with ABM. Other well-known regions of ABM retrieval networks, such as the parahippocampal gyri and the parietotemporal junction, were also correlated with ABM richness. Finally, the cerebellum and the putamen were correlated with ABM free recall, whereas the temporal lobe was only correlated with ABM probed recall. These results bring new insights into the role of the insular cortex in ABM, into the reliance of ABM on more elementary aspects of the self and into the behavioural characteristics of dementia with Lewy body patients.
Empirical evidence suggests that ABM impairments are a hallmark feature of dementia, such as in Alzheimer’s disease, as well as in other dementia syndromes such as semantic dementia and frontotemporal lobar degeneration.33,44 However, our study is the first to demonstrate that ABM is impaired in dementia with Lewy bodies. The present findings show an improvement in the episodic richness of memories through the provision of structured probing, notably for the dementia with Lewy body patients who displayed higher evolution rate of episodic richness, which grew by 75% in the probed recall versus the free recall, while the controls grew by 46%. These results reinforce the idea that ABM impairments could partially pertain to a failure of the strategic retrieval process, due to the executive dysfunction characterizing dementia with Lewy bodies.2 However, despite the provision of structured probing, patients continued to display reduced episodic re-experiencing when compared to control subjects, suggesting inability to access previously stored memories entirely. Importantly, we found that ABM impairments were temporally ungraded in dementia with Lewy body patients, with a flat performance of episodic re-experiencing across life epochs, including performance for the recent period contrary to what has been documented in Alzheimer's disease or in semantic dementia. Indeed, the neuroanatomical signature of Alzheimer's disease affects the encoding of new information and the storage of the episodic memory trace, which affects the recent period to a greater extent,36,37 whereas recent episodic memories are relatively spared in semantic dementia.40
In dementia with Lewy bodies, this finding of a flat retrieval profile without greater impairment for the recent period could be related to a breakdown in research strategies,33 whose probing would allow access to stored memories up to a limit. This flat profile could also be partially explained by a storage deficit; however, we would expect to find greater impairment for the recent period if such a deficit were to be at the forefront. Alternatively, as ABM and the subjective experience are closely linked,9 another hypothesis could be that the subjective sense of self might be impaired, thus leading to alteration of autonoetic consciousness and disturbing access to memories, regardless of the period,9,70 as it has already highlighted in pathology with flat profile of ABM.35 This postulate could be reinforced by the correlation between ABM and insular atrophy that is the central finding to emerge from our study.
Indeed, concordant with our hypothesis, imaging analyses confirmed that the insular cortex, and more precisely its right posterior part, is specifically associated with ABM, whereas it was not involved in anterograde verbal memory on the RL/RI-16, which performance reflects general memory processes. The insular cortex is commonly reported to be associated with interoceptive awareness71 and socio-emotional processes,21 but also with social recognition memory,72 taste memory73 and recognition memory formation and consolidation.74-76 Regarding dementia with Lewy bodies, our study is the first to suggest an impoverished ABM in relation to the insular atrophy occurring early in the course of the disease,16-19 whereas the insula has been mentioned in only a few studies on ABM and is often considered as a region of secondary importance. Yet, the role of the insula is already well known in both the subjective and objective components of the present self.5,7,20-22,77-79 As mentioned above, the subjective sense of self can readily be assessed by measuring interoceptive awareness, sense of body ownership and sense of agency and metacognition. Yet, numerous studies found that these different levels of the subjective sense of self are indeed associated with the right insula.80-83 Specifically, the most elementary aspects of the subjective sense of self, namely interoception, sense of agency and sense of body ownership, involve the posterior insular cortex.71,84-88 The self-concept, however, encompasses a body of autobiographical knowledge, self-esteem and self-image and has also been related to the insula in several studies.22,83,89 Our team recently highlighted a correlation between poorer knowledge about oneself and diminished GM volume in the right insula in dementia with Lewy bodies.6 The subjective sense of self and the self-concept are respectively elementary preconditions for episodic and semantic ABM,10 which in turn is central for the formation and maintenance of identity in the present moment and a continuous mental representation of the self over time.8-10,24,27,90,91 Thus, our findings suggest that the insula is a key region of the network of the self extended in time, its atrophy leading to an ABM deficit, likely through an alteration of the self at the present moment. Moreover, the fact that the posterior part of the insular cortex is involved suggests that the most elementary aspects of the self could be involved in ABM.71,84-88 This in in line with our hypothesis whereby alteration of the most elementary component of the self, namely the subjective sense of self, would lead to impairments of higher order self-components such as ABM. However, the insula is not commonly found as a core region in other studies on ABM. An explanation could be that ABM studies in healthy subjects show a prominent involvement of brain regions involved in more elaborated aspects of the self, as in the case of the cortical midline structures, in addition to regions involved in episodic recollection. In dementia with Lewy bodies, we can speculate that it is the insular damage that prevents the grounding of the networks of the self, such as the ABM network, and leads to a global collapse of the self. Conversely, when the insula and the subjective sense of self are preserved, we can surmise that their activation is in the background, compared to activation in regions involved in episodic recollection and in more elaborated aspects of the self.
Our second main finding concerns the relationship between the right precuneus and autobiographical memories. The precuneus is considered part of the cortical midline structures,92 which are a complex of brain regions involved in the self networks. Thus, it is already known to be a crucial component of the self, notably for processing of bodily self, namely the subjective sense of self,93-95 but also in self-referential processing, namely the self-concept.83,93,96-99 Beyond its role in the present self, the precuneus has also been reported to have a role in ABM, though not as a core region.28 Interestingly, some researchers found that the precuneus was specifically involved in the retrieving of specific autobiographical events, as opposed to general past memories100 and personal semantic information,101 which is consistent with the notion of the precuneus being more likely related to the self and personal aspects, rather than directly to the memory aspects. Moreover, the precuneus is involved in visuo-spatial processes102 and visual imagery processes occurring in conscious memory recall.103,104 Our results in dementia with Lewy body patients are in line with a study on patients with posterior cortical atrophy45 who displayed a disruption of visual and perceptual processing, as reported in dementia with Lewy body patients.2 Ahmed et al. found a specific correlation between perceptual details in ABM and GM density in the precuneus; thus, impairments in perception processing and the deprivation of visual imagery could impact recollection in ABM, acting like a barrier to mental travel in memories. Furthermore, another finding of our study, which has extensively been explored and associated with specific autobiographical memories, is the temporoparietal junction,28 which is also known to be specifically involved in retrieval of the spatial context of events.105
In line with previous studies exploring ABM, we found an involvement of the medial temporal lobe, which is part of the ‘core network’ of ABM28 and has already been described in healthy subjects25,106 and in Alzheimer's disease.37,107 Indeed, aside from its well-known role in the formation and consolidation of newly learned material,106,108,109 the hippocampus plays a central role in autobiographical recollection, serving as an index pointing to details of the memories stored in parts of the neocortex.28 Nevertheless, our study found no involvement of the hippocampus, but rather an involvement of bilateral parahippocampal structures, with clusters also encompassing areas of the perirhinal and entorhinal cortices, namely Brodmann area (BA) 35/36 and BA 28. This is likely due to the fact that dementia with Lewy bodies would relatively spare CA1, the larger subregion of the hippocampus, whereas neuropathological lesions are rather found in the more restricted CA2 subfield and the entorhinal cortex.110 Yet, it is through the parahippocampal region (i.e. the perirhinal, parahippocampal and entorhinal cortices) that the hippocampus receives inputs and sends output to neocortical area.111,112 Moreover, a large body of literature has explained the parahippocampus activity as dedicated to associative memory, that is the memory that links different items together (e.g. objects, relations, places and sounds) to make it a single composite construction, namely an episodic memory.113-116 Thus, a parahippocampal lesion would prevent binding of the details that constitute a specific memory and thus lead to a deficit in the episodic component of ABM.
Besides the medial temporal lobe, we found, in line with other studies,117-119 that autobiographical memories involve the lateral temporal cortex and, particularly, the left inferior temporal gyrus (BA 20). The implication of the lateral temporal lobe would be associated with autobiographical knowledge and personal semantic memory, which feed substantially autobiographical recollection.28 Indeed, as proposed by Svoboda, most episodic memories contain semantic representations of what composes our environment, such as relatives, personal objects or even home. Moreover, life periods and life chapters constitute a frame for memories and provide a gateway to access more specific events.8 This finding in our study is particularly relevant, since the inferior temporal gyrus was correlated only in the probed recall condition, suggesting a role in the storage of information independent of retrieval abilities, as if a semanticized version of the memory was stored in these regions. The concomitant involvement of the medial temporal lobe and the lateral temporal cortex could be accounted for by the ‘transformation’ theory,120 which predicts that strictly episodic memories remain dependent on the medial temporal lobe and that a schematic version with fewer contextual details would develop in the neocortex as time goes by.
Among the other regions described in ABM imaging studies, we found a role for the right cerebellum, as highlighted in numerous studies that report medial and right lateralized involvement as a core neural signature of ABM, notably for its role in conscious retrieval of episodic memories by means of its connections to the dorsolateral prefrontal cortex.101,121,122 These results are more convincing as the cerebellum appeared to be correlated only with the free recall condition in our study, rather suggesting an involvement as an executive process. This is concordant with the existence of a strategic retrieval deficit in dementia with Lewy body patients, as attested by a higher improvement of ABM scores after probing, compared to the controls. Interestingly, we also found a correlation between the free recall condition and the putamen, which belongs to the striatum and is part of the reward circuit and has been associated with learning and episodic memory.123,124 Finally, our study did not reveal any correlation with the prefrontal cortex, while we might have expected it to play a role in accord with the hypothesis of an impairment in strategic retrieval. Nevertheless, we found a role for subcortical regions such as the cerebellum and the putamen, as if these regions rather than the prefrontal cortex were responsible for the executive impairments in the early stages of dementia with Lewy bodies, as is the case for attention.125
Overall, our study has some limitations. In particular, the sample sizes were relatively small (n = 20 in each group) and the results for the VBM analyses were uncorrected. Consequently, further studies involving a larger cohort of dementia with Lewy body patients will be needed to confirm our findings. Therefore, our future work aims to investigate the relationship between the subjective sense of self, the self-concept and ABM in dementia with Lewy bodies, with a special focus on the insula’s role within the different components. Moreover, we will plan to analyse the self networks with a functional neuroimaging approach.
Conclusion
While evidence suggests that ABM impairments are a transdiagnostic feature of dementia, this study is the first to document them in dementia with Lewy bodies, from the early stage of the disease. We found that ABM impairments are temporally ungraded in dementia with Lewy body patients and are partially related to deficits in strategic retrieval processes, similar to what is described in frontotemporal lobar degeneration. The flat performance of episodic re-experiencing across life epochs, including the recent period, is also concordant with a global alteration of the self. The volumetric study suggests the involvement of posterior insular atrophy to account for the ABM deficit, as well as the involvement of the precuneus, two regions linked to elementary aspects of the self. This finding reinforces our hypothesis whereby insular damage would lead to a global collapse of the self, with consequences for autobiographical memories. Additionally, this study appears to be reliable since our results also revealed regions known to be part of the ABM network, such as the medial temporal lobe, which would bind the details of the memory, the lateral temporal lobe involved in the semantic component, the temporoparietal junction associated with the spatial context and the cerebellum, a subcortical component of strategic retrieval. Finally, the different regions identified in our study draw an ABM pattern in which the insula fits nicely, as the grounding element of the phenomenological experience.
Supplementary material
Supplementary material is available at Brain Communications online.
Acknowledgements
We thank the Clinical Investigation Centre of Strasbourg for its support in the recruitment of participants. We owe special thanks to all the patients, the controls and their relatives for their selfless participation and support of the study. We also warmly thank Cindy Schmitz for transcription of the memories and Nick Barton for language editing.
Funding
This work was supported by the Association France Alzheimer, the Fondation Vaincre Alzheimer (FR-22061T) and the Université de Strasbourg.
Competing interests
The authors report no competing interests.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


