 Placebo Effects Through the Lens of Translational Research
Placebo Effects Through the Lens of Translational Research
Contents
-
-
-
-
-
-
-
-
-
-
-
-
-
Introduction Introduction
-
Placebo effects in antidepressant trials Placebo effects in antidepressant trials
-
Doubts about the effectiveness of antidepressants Doubts about the effectiveness of antidepressants
-
Experimental modulation of expectation in the affective system Experimental modulation of expectation in the affective system
-
How to make use of these placebo mechanisms? How to make use of these placebo mechanisms?
-
Nocebo effects in clinical trials of antidepressants Nocebo effects in clinical trials of antidepressants
-
Modulating expectations to reduce side effects in antidepressant trials Modulating expectations to reduce side effects in antidepressant trials
-
Summary and conclusions Summary and conclusions
-
References References
-
-
-
-
-
-
-
5.1 Placebo responses and effects: Processes, potential, and ethical considerations in clinical care
-
-
-
-
-
-
-
-
4.2 C4.2Placebo and nocebo effects in depression: Implications for treatment and clinical trial designs
-
Published:October 2023
Cite
Abstract
This chapter reviews evidence for placebo and nocebo effects in depression, including their mechanisms of action. The authors then consider how these effects could be used clinically. Meta-analyses indicate that antidepressants provide only marginal benefits over placebos, which suggests that placebos could benefit patients nearly as much as antidepressants do, but without medication side effects. Open-label placebos are a promising avenue for evoking these effects. It is also presented evidence for expectancy as an important mechanism underlying placebo and nocebo effects. It is discussed how expectancies regarding treatment efficacy and side effects could be manipulated to reduce side effects and improve clinical outcomes. In particular, the authors discuss how communication strategies such as contextualizing informed consent and framing treatment information can optimize treatment expectations, improve clinical outcomes, and reduce nocebo-related side effects. Finally, implications that such expectancy manipulations might have for clinical trial design are covered.
C4.2S1Introduction
C4.2P1In this chapter, we examine evidence for placebo and nocebo effects in depression, including their mechanisms of action, and then consider how these effects might be used clinically to benefit patients. Although meta-analyses of clinical trial data indicate that the efficacy of antidepressants is statistically greater than placebos, the effect sizes are small, which suggests that antidepressants provide only marginal clinical benefits over placebos. In addition, placebos appear to duplicate about 80% of the effects of antidepressants, and the available data from natural course controls suggests that regression toward the mean and natural history account for a relatively small fraction of placebo responses. Taken together, the negative side-effect profile of antidepressants and their small clinical benefit compared to placebo, along with the robust effect of placebos compared to natural course controls suggest that placebos could benefit patients nearly as much as antidepressants, but without the negative side effects that accompany pharmaceutical treatment. We discuss ways that this might be achieved, most prominently by the use of open-label placebos. In addition, we present evidence for the role of expectations as an important mechanism underlying placebo efficacy, the experience of nocebo effects, and the exacerbation of genuine negative side effects, as well as the augmentation of genuine drug effects. Finally, we discuss how expectations for treatment improvement, on the one hand, and expectations about side effects, on the other, might be manipulated to improve clinical outcomes and reduce the frequency and severity of side effects, as well as the implications that such expectancy manipulations might have for clinical trial design.
C4.2S2Placebo effects in antidepressant trials
C4.2P2Antidepressant medications are included in all evidence-based treatment guidelines for depression, and they are especially recommended as treatment for severe cases of the disorder. These treatment guidelines have relied upon decades of randomized controlled trials (RCTs) of the efficacy of antidepressants that have reported statistically significant effects, and they have led to widespread adoption of antidepressants as a first-line treatment for depression. Moreover, the introduction of the selective serotonin reuptake inhibitors (SSRIs) in the late 1980s, with their reported lower levels of side effects, led to enthusiastic single-case reports in the public media, and these reports, along with Peter Kramer’s bestselling popular book, Listening to Prozac,1 led to a tremendous increase in prescriptions for antidepressants, which has continued unabated until the present. For example, in 2017, about 13% of the US population took antidepressants within the past month. In addition, 20% of adults aged 60 years and older reported that they were currently being treated with antidepressants.2
C4.2S3Doubts about the effectiveness of antidepressants
C4.2P3However, over the past 2 decades, this enthusiastic endorsement of the efficacy of antidepressants has been increasingly challenged, leading some experts to even question whether antidepressants actually confer any clinically meaningful benefits at all as compared to placebos.3 For example, after carefully reviewing previously published meta-analyses, Jakobsen and colleagues concluded that antidepressants seem to have minimal beneficial effects on depressive symptoms, and, problematically, they also increase the risk of adverse events, including serious ones (e.g., suicide attempt and/or ideation) albeit with a smaller absolute risk.4 Importantly, the US Food and Drug Administration’s focus on statistically significant effects, as opposed to clinically meaningful ones, as the standard for approval, has blurred the line between marginally beneficial treatments and treatments that provide robust benefits to patients.
C4.2P4Cipriani and colleagues have conducted perhaps the most comprehensive and highest quality meta-analysis of antidepressant drug trials to date.5 Their network meta-analysis included 522 trials with 116,477 patients, and they calculated that the overall effect size for the benefit of antidepressants as compared to placebos was only a standardized mean difference (SMD) of .30. According to Cohen’s widely-accepted conventions, an SMD = .30 is considered a small effect.6 From a clinical perspective, an SMD = .30 can be converted to the number needed to treat to achieve a minimal therapeutic benefit, which in this case is 10. In other words, 10 persons would need to be treated with antidepressants in order for one to benefit, which means that 9 persons treated with antidepressants—who might, of course, also experience noxious side effects—would not show an advantage compared to placebo treatments. Similarly small effect sizes have been found for antidepressants in children with mental and behavioral disorders.7
C4.2P5The problem with such a small effect size is not only that it indicates a lack of robustness of the medication’s effects, but it also raises the possibility that some other difference between the drug and placebo groups may account for the small difference in efficacy. For example, antidepressants often produce side effects that can reduce the effectiveness of patient and physician blinding and thus trigger more positive treatment expectations in the drug group.8,9 Not surprisingly, the drug-specific effect of antidepressants can vanish if participants do not expect to receive the drug, suggesting that expectation is a major mediator of antidepressants’ efficacy.10,11 Indeed, when patients are led to believe that they are receiving an inert placebo, the effect of the SSRI escitalopram, for example, is reduced to a level that is not clinically meaningful.11
C4.2P6It has been increasingly recognized that the problem in demonstrating a benefit of antidepressants over placebos is mainly caused by strong effects in the placebo arms of clinical trials. Not infrequently, the placebo arms of antidepressant trials show improvements of more than 10 points on standard screening tools, such as the Hamilton Depression Scale.12 Some authors report that the effect sizes of improvements in the placebo group have even increased over the last several decades,13,14 although this conclusion has been questioned in a more recent publication.3 These changes over time are likely caused in part by a steady reduction in the heterogeneity of clinical trials, which also reduces the generalizability of the results.14 Further, the methodological quality of antidepressant studies has increased over time, which may have also contributed to smaller differences in efficacy between drugs and placebos.
C4.2P7Several meta-analyses have also shown that an advantage of antidepressants over placebos is only found if the study design limits the induction of placebo mechanisms. If patients are seen once a week, the improvements in the placebo group are much higher than in studies where patients are only seen every other week or less.15 Social contact with study physicians or study nurses seems to have the potential to boost positive effects in placebo arms to the same effect size that was found in the drug arms.
C4.2P8A general question when considering these effects is the influence of statistical artifacts, instead of genuine clinical changes in the symptomatology of patients. One of these statistical effects is regression toward the mean, in which patients in the extreme areas of a distribution (e.g., very strong depressive symptoms) have a greater probability to move in the direction of average scores (e.g., moderate depression), while the probability of becoming even more depressed is much lower. In clinical trials, patients who are typically included have serious symptoms or are in a serious crisis. Therefore, the natural course (without treatment) is more likely to develop in the direction of lower depression scores rather than higher. These effects can only be investigated if the drug and placebo arms of clinical trials are compared to natural course groups. Unfortunately, however, this is rarely done. One of the few studies including an arm with patients who received only low intensity medical care confirmed that the substantial improvements found in the placebo arms of antidepressant trials are indeed much greater than any changes in the natural course group (see Figure 4.2.1).16 Over the 10-week course of treatment, both the active drug group and the placebo group showed substantial improvements, whereas the supportive care group showed only minor changes. These results suggest that the strong effects in placebo arms of antidepressant trials are a real effect of placebo mechanisms, and not simply attributable to the effects of regression toward the mean and natural course.
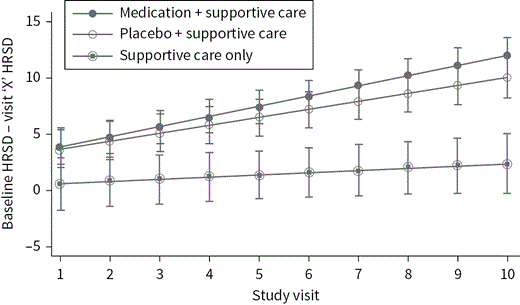
C4.2F1 RCT on antidepressants including a supportive care–only arm.
While improvements in pill taking arms were similar, they were higher than improvements in natural course.16 This indicates that regression to the mean did not explain the improvements (e.g., in the placebo pill group).
C4.2P9The ascertainment strategy used to assess change can also contribute to larger improvements in outcome variables over time. In antidepressant trials, it has been shown that effect sizes are much higher when improvement is derived from expert ratings as compared to self-ratings.14,17 One possible explanation for this finding is that many experts can correctly guess the group allocation of patients (i.e., drug vs. placebo) despite blinding, presumably because patients with more side effects are more likely to be taking an active drug.18 Thus, the expectation of study physicians that a person with side effects is more likely allocated to the drug arm, and should also show more improvement, can contribute to larger drug-placebo differences when the effects are assessed by expert ratings.
C4.2P10To summarize, clinical trials investigating antidepressants suggest that strong placebo effects are involved, and they also indicate that expectations both on the part of the patients as well as the study personnel could contribute to treatment success. This is further supported by findings that the number of arms of the study design also affects improvement rates of placebo arms. If the study only uses two arms (i.e., drug vs. placebo), the likelihood of being in the placebo arm is 50%. If two or more active arms are compared to one placebo arm, the likelihood (and presumably the expectation) of being in the placebo arm is much lower. It has been shown that the response rate in the placebo arm is significantly lower in two-arm designs compared to three- or four-arm designs.19 Again, this can be interpreted as meaning that a higher expectation on the part of both patient and physician that the patients may be in the placebo arm leads to lower response rates.
C4.2S4Experimental modulation of expectation in the affective system
C4.2P11The problem of the clinical trials cited above is that they are typically not designed to evaluate expectation effects, and therefore our conclusions come more from an indirect analysis of the results. Most clinical trials do not systematically modulate expectations, and, therefore, any conclusions about expectation effects are necessarily tentative. The conclusions would be much firmer if clinical trials used balanced-placebo designs and/or more trials also included natural course groups.
C4.2P12Although there is a paucity of high-quality, prospective clinical trial data on expectations, several experimental studies have systematically manipulated expectations and investigated the effects on affective outcomes. In one study, participants were exposed to movie clips designed to elicit sadness, but prior to viewing the clips, a placebo nasal spray was administered that participants were led to believe would either (a) protect them from experiencing sadness or (b) would exacerbate their feelings of sadness. Depending on the differing explanations of the expected effects of the “drug,” the video clips either induced sadness, or the experience of sadness was substantially blocked.20 While this effect was first shown in healthy volunteers, subsequent studies using similar designs have included patients who were diagnosed with major depression. These studies with depressed patients showed even stronger expectation effects.21 The same research group was also able to demonstrate that these expectation modulations are not only effective in changing symptoms such as sadness, but they can also modulate other symptoms of depression such as rumination.22 Finally, expectations can also influence the formation of intrusive memories.23
C4.2P13In other studies, the investigation of the role of expectations in depression was extended to examine not only outcome expectations for treatments, but also the processes and mechanisms that maintain depression in general.24,25 Depression is characterized by expectations for social rejection and expectations for failure.26,27 A depressed mood blocks the update of these negative expectations such that when positive experiences occur, the negative expectations are maintained.28 This effect is crucially important for understanding why depressed patients remain depressed, even when positive events occur that contradict their negative view of themselves and their world. Further, many patients with depression use cognitive immunization strategies to prevent a positive update of negative expectations, which further explains the persistence of depression, especially in chronic depression. Given these findings, a prediction-processing perspective might offer new pathways to better understand the mechanisms that maintain depression, and to improve treatments for patients with depressive disorders.24
C4.2S5How to make use of these placebo mechanisms?
C4.2P14Given how strong placebo effects appear to be in clinical trials of antidepressants and given our increasing knowledge of the mechanisms that underlie these effects, the question arises whether we can make use of these insights to improve patient care for depressed patients. Deceptive administration of placebos is, of course, in conflict with medical ethics in most countries. However, administering placebos openly and without deception (i.e., open-label placebo (OLP)) could circumvent this problem. But given the fact that placebo effects seem to be driven, at least in part, by expectancies, could open administration of placebos be effective, even when a patient knows that she is receiving an inert treatment? Over the past decade, researchers have conducted clinical trials of open-label placebos that, importantly, were administered with a persuasive rationale for efficacy. A recent meta-analysis of 11 of these trials in a variety of disorders including low back pain, cancer-related fatigue, migraine headaches, depression, menopausal hot flashes, and irritable bowel syndrome found a significant overall effect favoring open-label placebo over treatment as usual (SMD = 0.72, p < 0.0001).29 Regarding depression specifically, a pilot trial of open-label placebo administered in the context of a rationale for efficacy provided encouraging results, and suggested that OLP could be effective in alleviating major depression.30 A larger trial investigating OLP in depression followed, which suggested that openly-administered placebos could be a treatment option, at least for some subgroups.31 In particular, patients aged younger than 65 years seemed to respond better to OLP than patients aged 65 and older. It should be noted, however, that the sample size in this trial was small and the analysis was post hoc, so these findings regarding the moderating influence of age on OLP effects in depression should be considered tentative. To date, these are the only two clinical trials of OLP in depression; and in the absence of larger clinical trials, the evidence for the efficacy of OLP for the treatment of depression needs to be considered tentative.
C4.2P15Even if OLPs have positive effects in depression, there is some evidence that the effects of deceptive placebos are even larger.32,33 Interestingly, this is also in accordance with a survey asking about the acceptability of the use of placebos that showed that double-blind placebo administration leads to more positive outcome expectations than OLP administration; and acceptability in the lay public for deceptive placebo applications is high.34 These findings suggest that there is further potential to boost expectation effects to improve clinical outcomes, but the ethical issues associated with the use of deception in routine clinical practice would need to be carefully considered.
C4.2P16Another approach is to consider expectations in depression in a broader sense, with expectations being a core mechanism maintaining depressive states. Here, interventions can be further developed and improved that aim to counter dysfunctional expectations, while reducing the impact of cognitive immunization. Details can be found elsewhere.25
C4.2S6Nocebo effects in clinical trials of antidepressants
C4.2P17The influence of treatment expectations on antidepressant efficacy has been shown in numerous clinical trials and meta-analyses.14 In particular, participants in placebo groups frequently report improvement of depressive symptoms that is comparable to the drug response (40% vs. 50% response rate, respectively).5 At the same time, participants in the placebo arms of clinical trials often report debilitating side effects, known as nocebo effects.35 Interestingly, these adverse events in the placebo groups often mimic the actual adverse events in the verum groups, suggesting that information about expected side effects that is provided to all patients creates negative expectations that in turn leads to placebo-treated patients then reporting those side effects.
C4.2P18A meta-analysis that included 143 antidepressant RCTs with over 12,000 patients documented nocebo rates that were two to six times higher in the placebo groups for trials of tricyclic antidepressants (TCAs) as compared to the placebo groups for trials of SSRIs, including adverse events such as blurred vision, fatigue, and constipation (see Table 4.2.1).36 Since SSRIs have a well-known reputation for producing fewer side effects as compared to TCAs, these findings emphasize the importance of individual expectations for the efficacy and safety of antidepressants. Negative expectations regarding potential adverse effects of antidepressants might arise from treatment information provided to patients, through prior negative experiences with side effects to antidepressants, or by social observation of side effects experienced by friends or family.37,38
| Adverse Events . | TCA-Placebo . | SSRI-Placebo . | Odds Ratio . |
|---|---|---|---|
Blurred vision | 7 % | 1 % | OR = 6.1 (95%CI 2.6–14.5) |
Fatigue | 17 % | 6 % | OR = 3.6 (95%CI 2.7–4.7) |
Dry mouth | 19 % | 6 % | OR = 3.5 (95%CI 2.9–4.2) |
Dizziness | 14 % | 7 % | OR = 2.7 (95%CI 2.2– 3.4) |
Constipation | 11 % | 4 % | OR = 2.7 (95%CI 2.1–3.6) |
Sexual dysfunction | 5 % | 2 % | OR = 2.3 (95%CI 1.5–3.5) |
Tremor | 4 % | 2 % | OR = 1.7 (95%CI 1.1–2.7) |
| Adverse Events . | TCA-Placebo . | SSRI-Placebo . | Odds Ratio . |
|---|---|---|---|
Blurred vision | 7 % | 1 % | OR = 6.1 (95%CI 2.6–14.5) |
Fatigue | 17 % | 6 % | OR = 3.6 (95%CI 2.7–4.7) |
Dry mouth | 19 % | 6 % | OR = 3.5 (95%CI 2.9–4.2) |
Dizziness | 14 % | 7 % | OR = 2.7 (95%CI 2.2– 3.4) |
Constipation | 11 % | 4 % | OR = 2.7 (95%CI 2.1–3.6) |
Sexual dysfunction | 5 % | 2 % | OR = 2.3 (95%CI 1.5–3.5) |
Tremor | 4 % | 2 % | OR = 1.7 (95%CI 1.1–2.7) |
Higher rates of nocebo effects resulted in the placebo arms of TCAs trials compared to the placebo arms of SSRIs trials.36 This indicates that specific nocebo effects resulted depending on the side effects profile of the active drug, suggesting that treatment expectations and treatment information might play a role in explaining these nocebo effects.
C4.2P19The fact that nocebo effects tend to mimic the actual side effects to the medication given in the verum arm has major consequences for the design and conduct of clinical trials. First, nocebo effects of placebo treatments are not a constant, but seem to be variable and specific to the active medication, and, thus, a single placebo control group does not suffice to control for different sources of nocebo effects in trials, in particular, if they compare different drugs. Separate placebo controls would be necessary to depict the nocebo effects of each single active arm. However, it remains unclear how practical this approach might be.
C4.2P20Second, the potential and highly likely interaction effects of placebo and verum groups need to be accounted for. Estimating the drug effect or the drug safety simply by subtracting adverse effects in the placebo arm from those in the verum arm, depends on the assumption that these effects are additive. The balanced-placebo design is a viable option to test these interaction effects while accounting for the potentially missing prerequisite of additivity.39
C4.2P21Third, including a placebo group in a clinical trial might influence participants’ expectations per se. In a recent meta-analysis, adverse events rates were about 20% higher in antidepressant trials including a placebo group than in head-to head trials.40 Thus, future studies should pay close attention to how participants’ expectations are influenced by information about trial design. This can be done by documenting information procedures and assessing resulting treatment expectations.
C4.2P22Fourth and most obvious, adverse effects need to be systematically measured as patient-reported outcomes in order to analyze their occurrence and potential effect on positive treatment outcome. A systematic quantitative review documented that adverse event reporting was insufficient in RCTs of persistent depressive disorder, with some studies using unstructured assessment methods such as spontaneous recall.41 This was also true for 46% of the included studies in the meta-analysis comparing nocebo effects in SSRI versus TCA trials.36 Validated and structured tools to assess treatment side effects in clinical depression trials on pharmacological as well as psychological treatments such as the general assessment of side effects scale (GASE) are available.42 Utilizing such structured tools to assess adverse events in randomized controlled depression trials enables researchers to compile risk-benefit information and thus use safety data to improve clinical decision-making and help patients optimally navigate the options available for treating depression.43
C4.2S7Modulating expectations to reduce side effects in antidepressant trials
C4.2P23Nocebo effects can significantly increase nonspecific symptoms and complaints in patient populations, resulting in psychological distress and reducing medication adherence. For example, over 30% of the patients who dropped out of the RCTs included in the meta-analysis on antidepressant nocebo effects actually came from the placebo groups.36 Patient beliefs and expectations about medicines are some of the major mechanisms of nocebo effects, and they are shaped by personal and witnessed prior experiences, as well as by information provided by health care providers and the media. For example, headaches and other common side effects of antidepressants can result simply from the mention of headaches in the informed consent process as a potential side effect.44
C4.2P24Informed consent procedures have great potential to modify patients’ treatment expectations in order to optimize treatment outcome by maximizing placebo effects and minimizing nocebo effects. However, today’s informed consent processes are largely viewed as obligatory procedures to prevent litigation. In routine clinical practice, they are mainly focused on the potential risks and side effects of a given treatment, presented within a one-size-fits-all approach. The downsides of this approach have been critically discussed, particularly regarding the harm it may cause by shaping negative expectations and triggering nocebo-related side effects in routine clinical practice.45
C4.2P25On the positive side, treatment expectations can be influenced by short and economical psychological interventions.46 Effective strategies to optimize treatment expectations within informed consent include framing of side effects information, either by emphasizing the probability of being free from adverse effects,47 by elaborating on the anticipated positive effects and their mechanisms of action,48 or by accompanying side-effect information with specific coping strategies.46 Moreover, counteracting symptom misattribution by explicitly informing patients about the nocebo effect has been shown to be effective in reducing experimentally induced nocebo effects.44
C4.2P26A recent experimental study showed that a short patient-oriented interaction that provided information about the nocebo effect was effective in functionally adapting patient’s informational needs regarding antidepressant medication.49 This was the first empirical test of the feasibility and effectiveness of a contextualized informed consent procedure. Specifically, as compared to patients who were not informed about the nocebo effect, informed patients reported reduced needs for full information about possible side effects of antidepressants and stronger wishes to be informed about the desired effects and the mechanisms of action of antidepressants, including context and expectation effects.49 This type of informational interaction might help healthcare practitioners optimize patient’s treatment expectations via informed consent, while still respecting patient autonomy. Taken together, contextualizing informed consent about antidepressants using framing strategies and information about the nocebo effect may help to functionally adapt patient’s expectations and reduce nocebo-related adverse events.
C4.2S8Summary and conclusions
C4.2P27In this chapter on placebo and nocebo effects in depression, including their mechanisms of action, we reviewed clinical trial results, as well as data from laboratory experiments, and our conclusions can be summarized as follows:
C4.2P28 Although antidepressants show statistically greater efficacy compared to placebos, the effect sizes are small, and the specific effect of these drugs provides only a small clinical benefit to patients.
C4.2P29 Placebo responses in double-blind trials are about 80% as large as the response to antidepressants.
C4.2P30 Regression toward the mean and natural course account for only a small fraction of the antidepressant effect of placebos, which indicates that most of placebo responses are genuine placebo effects.
C4.2P31 Given these large placebo effects, and the small specific effect for antidepressants, as well as their negative side effects, efforts should be made toward harnessing placebo effects to benefit patients.
C4.2P32 OLPs show promise in treating depression, but larger studies of longer duration are needed.
C4.2P33 Nocebo effects are prevalent in antidepressant trials; they are detrimental to patient’s quality of life and can motivate patients to stop taking the medication prematurely.
C4.2P34 Communication strategies such as contextualizing informed consent and framing treatment information are viable options to optimize treatment expectations and reduce nocebo-related adverse effects.
C4.2P35 Patient and physician expectations are an important mechanism underlying placebo and nocebo effects, and they likely also contribute to patient responses to genuine treatment, including both symptom improvement and the development of negative side effects. As such, expectations are an important target for future research aimed at improving our ability to reduce depressive symptoms, minimize the frequency and severity of negative side effects, and improve clinical trial design.
C4.2S9References
C4.2P361. Kramer P.
C4.2P372. Winerman L.
C4.2P383. Kirsch I.
C4.2P394. Jakobsen JC, Gluud C, Kirsch I.
C4.2P405. Cipriani A, Furukawa TA, Salanti G, et al.
C4.2P416. Cohen J.
C4.2P427. Locher C, Koechlin H, Zion SR, et al.
C4.2P438. Rief W, Glombiewski JA.
C4.2P449. Berna C, Kirsch I, Zion SR, et al.
C4.2P4510. Rutherford BR, Wall MM, Brown PJ, et al.
C4.2P4611. Faria V, Gingnell M, Hoppe JM, et al.
C4.2P4712. Stahl SM, Fava M, Trivedi MH, Caputo A, Shah A, Post A.
C4.2P4813. Walsh BT, Seidman SN, Sysko R, Gould M.
C4.2P4914. Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG.
C4.2P5015. Rutherford BR, Tandler J, Brown PJ, Sneed JR, Roose SP.
C4.2P5116. Leuchter AF, Hunter AM, Tartter M, Cook IA.
C4.2P5217. Cuijpers P, Li J, Hofmann SG, Andersson G.
C4.2P5318. Margraf J, Ehlers A, Roth WT, et al.
C4.2P5419. Sinyor M, Levitt AJ, Cheung AH, et al.
C4.2P5520. Glombiewski JA, Rheker J, Wittkowski J, Rebstock L, Rief W.
C4.2P5621. Haas JW, Rief W, Glombiewski JA, Winkler A, Doering BK.
C4.2P5722. Rebstock L, Schäfer LN, Kube T, Ehmke V, Rief W.
C4.2P5823. Herzog P, Barth C, Rief W, Brakemeier EL, Kube T.
C4.2P5924. Kube T, Schwarting R, Rozenkrantz L, Glombiewski JA, Rief W.
C4.2P6025. Rief W, Joormann J.
C4.2P6126. Kube T, D’Astolfo L, Glombiewski JA, Doering BK, Rief W.
C4.2P6227. Kirchner L, Schummer SE, Krug H, Kube T, Rief W.
C4.2P6328. Kube T, Kirchner L, Gärtner T, Glombiewski JA.
C4.2P6429. von Wernsdorff M, Loef M, Tuschen-Caffier B, Schmidt S.
C4.2P6530. Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M.
C4.2P6631. Nitzan U, Carmeli G, Chalamish Y, et al.
C4.2P6732. Kube T, Rief W, Vivell MB, et al.
C4.2P6833. Gohler AC, Haas JW, Sperl MFJ, Hermann C, Winkler A.
C4.2P6934. Haas JW, Rief W, Doering BK.
C4.2P7035. Meister R, Jansen A, Härter M, Nestoriuc Y, Kriston L.
C4.2P7136. Rief W, Nestoriuc Y, von Lilienfeld-Toal A, et al.
C4.2P7237. Rheker J, Winkler A, Doering BK, Rief W.
C4.2P7338. Zilcha-Mano S, Brown PJ, Roose SP, Cappetta K, Rutherford BR.
C4.2P7439. Enck P, Weimer K, Klosterhalfen S. Balanced placebo design, active placebos, and other design features for identifying, minimizing and characterizing the placebo response. In: Luana C, Magne Arve F, Karin M, eds.
C4.2P7540. Cheung CP, Thiyagarajah MT, Abraha HY, et al.
C4.2P7641. Meister R, von Wolff A, Mohr H, et al.
C4.2P7742. Rief W, Barsky AJ, Glombiewski JA, Nestoriuc Y, Glaesmer H, Braehler E.
C4.2P7843. Meister R, Lanio J, Fangmeier T, et al.
C4.2P7944. Pan Y, Kinitz T, Stapic M, Nestoriuc Y.
C4.2P8045. Wells RE, Kaptchuk TJ.
C4.2P8146. Shedden-Mora M, Pan Y, Heisig S, et al.
C4.2P8247. Faasse K, Huynh A, Pearson S, Geers AL, Helfer SG, Colagiuri B.
C4.2P8348. Heisig SR, Shedden-Mora MC, Hidalgo P, Nestoriuc Y.
C4.2P8449. Nestoriuc Y, Pan Y, Kinitz T, Weik E, Shedden-Mora MC.
| Month: | Total Views: |
|---|---|
| October 2023 | 17 |
| November 2023 | 19 |
| December 2023 | 29 |
| January 2024 | 84 |
| February 2024 | 49 |
| March 2024 | 104 |
| April 2024 | 145 |
| May 2024 | 110 |
| June 2024 | 98 |
| July 2024 | 85 |
| August 2024 | 89 |
| September 2024 | 61 |
| October 2024 | 80 |
| November 2024 | 78 |
| December 2024 | 65 |
| January 2025 | 49 |
| February 2025 | 58 |
| March 2025 | 84 |
| April 2025 | 44 |
| May 2025 | 20 |