 Placebo Effects Through the Lens of Translational Research
Placebo Effects Through the Lens of Translational Research
Contents
-
-
-
-
-
-
-
-
-
-
-
-
Historical background: placebo responses as nuisance, threat to drug development, and potential therapeutically Historical background: placebo responses as nuisance, threat to drug development, and potential therapeutically
-
A model of placebo effects in antidepressant clinical trials A model of placebo effects in antidepressant clinical trials
-
Patient expectancy Patient expectancy
-
Causal evidence for expectancy effects and their neural mechanisms Causal evidence for expectancy effects and their neural mechanisms
-
-
The therapeutic context and doctor-patient interaction as a source of placebo effects The therapeutic context and doctor-patient interaction as a source of placebo effects
-
Causal evidence for therapeutic contact effects and their mechanisms Causal evidence for therapeutic contact effects and their mechanisms
-
-
Patient characteristics bearing on the magnitude of placebo effects Patient characteristics bearing on the magnitude of placebo effects
-
-
Conclusions and directions for future research Conclusions and directions for future research
-
References References
-
-
-
-
-
-
-
-
5.1 Placebo responses and effects: Processes, potential, and ethical considerations in clinical care
-
-
-
-
-
-
-
-
4.1 C4.1A model of placebo responses in antidepressant clinical trials
-
Published:October 2023
Cite
Abstract
A substantial proportion of the observed response to clinical treatment with antidepressants is not attributable to specific effects of the medication but rather to placebo responses. This chapter proposes a conceptual model for understanding placebo responses in antidepressant randomized clinical trials. It specifies factors contributing to placebo responses and focuses on those conceptualized as contributing to the therapeutic effect of placebos: expectancy and contact with the physician. It synthesizes the literature on each factor, with a special focus on whether and how each can be manipulated. A thorough understanding of the factors contributing to placebo responses, their neurobiological underpinning, and the manners in which they can be manipulated may result in the ability to optimize placebo response to the diverging needs of pharmacotherapy research and clinical practice: minimizing it in clinical trials and maximizing it in clinical practice.
C4.1S1Historical background: placebo responses as nuisance, threat to drug development, and potential therapeutically
C4.1P1Major depressive disorder (MDD) is the leading cause of disability worldwide.1 Antidepressant medication (ADM) is a commonly prescribed, effective treatment for MDD2 that remedies dysfunctional activations in brain regions related to MDD. Yet it is also true that a substantial proportion of the response observed to clinical treatment with antidepressants is not due to specific effects of the medication (i.e., serotonin reuptake inhibition) but rather to nonspecific features of the treatment. Meta-analyses of placebo-controlled antidepressant trials show that medication response averages approximately 50%, whereas placebo response rates are typically 30%–35%.3
C4.1P2Substantial placebo responses generally have been viewed as a nuisance to be eliminated in pharmacologic research and clinical practice. Historically, the patient variables explored as predictors of placebo responses were histrionic traits, such as suggestibility, which were in general not considered desirable traits for an individual to possess. In addition, prevailing models construed the therapeutic action of ADM as being incremental, delayed (i.e., after 4 weeks), and persistent,4 as opposed to the “abrupt” and transient pattern of response associated with a placebo.4 This contrast between “true drug” effects and placebo responses conceptualized the latter in pejorative terms that did not lead investigators to take up placebo responses as a phenomenon worthy of serious scientific investigation.
C4.1P3In addition, the high magnitude of placebo responses relative to medication response posed significant challenges to new drug development and led to methodological changes in clinical trials aimed at minimizing placebo response. Increasing numbers of failed trials have made developing psychiatric medications progressively more time-consuming and expensive.5 These considerations led several large pharmaceutical companies to reduce or discontinue research and development on medications for brain disorders. Moreover, media coverage of failed trials has been used as a platform for critiques of the pharmaceutical industry and questioning the efficacy of antidepressants, which may have the dangerous public health consequence of dissuading patients with depression from accessing treatment.
C4.1P4Newer data suggested a need to revisit previous assumptions and question whether placebo responses in fact represented true, durable change in a patient’s depression. Meta-analyses of clinical trial data reported similar time courses of response by medication- and placebo-treated patients.6 Findings suggested that one-third of all patients, irrespective of whether they received a placebo or ADM, showed sudden symptom improvements, which were often sustained over time.7 Data-driven approaches further suggested that most individuals receiving antidepressant medication do not show a more distinct pattern of early treatment symptomatic change than do those receiving a placebo.8 Rather than being restricted to suggestible individuals, it was appreciated that most any individual is capable of experiencing placebo responses.9
C4.1P5Most importantly, insights into the neuroscience of placebo effects led to increased interest in placebo effects as tools to understand both the pathophysiology of specific disorders such as depression and the mechanisms of brain regulatory systems across disorders.10 For example, studies of placebo analgesia11 found that the anticipation of pain relief was associated with activations in orbitofrontal, dorsolateral prefrontal, parietal, and pregenual anterior cingulate cortices, which modulated activity in parts of the insula, thalamus, and cingulate cortex associated with pain,11 possibly by potentiating pain-related opioid release.12 Such top-down modulation underlying expectancy effects was supported by further research in both pain and depression.13 The possibility arose of harnessing these internal self-regulatory capacities as a means of safely optimizing therapeutic outcomes.
C4.1S2A model of placebo effects in antidepressant clinical trials
C4.1P6It is heuristically useful to differentiate placebo responses, which refer to the directly observable treatment responses occurring among individuals randomly assigned to placebo in a clinical trial, from placebo effects, which represent a conceptual cause of placebo responses. Specifically, a placebo effect can be defined as a genuine effect of a substance or procedure upon a target disorder that is not due to the inherent powers of the substance or procedure.14 From that definition it is apparent that clinical trials comprise many sources of placebo responses that are not attributable to placebo effects (see Figure 4.1.1).
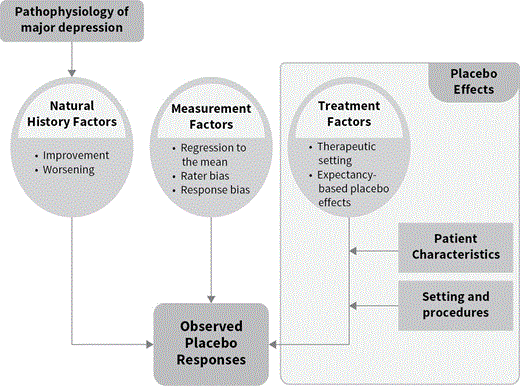
C4.1F1 A model of placebo responses in antidepressant clinical trials.
C4.1P7For example, individuals with MDD may experience spontaneous improvement and worsening unrelated to the study procedures.15 Patients with MDD typically experience symptoms for several months prior to seeking treatment.16 Those who choose to enroll in a clinical trial during a time of peak symptomatology may experience alleviation in the precipitating stressors and a natural waning of symptoms. A meta-analysis of acute symptom changes among participants in wait-list control conditions in psychotherapy studies reported an average improvement of four Hamilton Rating Scale for Depression (HRSD) points over a mean follow-up duration of 10 weeks. This magnitude of change is approximately 33% of the average improvement occurring with medication treatment and 40% of the improvement seen with placebo administration in clinical trials.17
C4.1P8In addition, clinical trials contain sources of bias and error inherent in measuring depressive symptoms. Regression to the mean is a statistical phenomenon occurring when repeated measurements associated with random error are made on the same individual over time.18 Regression to the mean poses a problem at the group level in clinical trials because a threshold depression severity score is set as an inclusion criterion, and some enrolled participants in the study have true means below this threshold. Rater bias occurs when an individual’s rating of symptom severity in an antidepressant clinical trial is influenced by underlying beliefs or motivations with respect to the treatments under study.19 Conversely, response biases on the part of participants occur when respondents choose the responses that they perceive to be the most socially desirable or that is favored by the clinicians in a research study.20
C4.1P9True placebo effects contrast with these sources of placebo responses that either are unrelated to study procedures (i.e., natural history) or do not involve true change in a patient’s depression (i.e., rater bias and regression to the mean). First, taking a pill believed to be an effective treatment for depression may generate an expectancy of improvement in a patient, which may directly influence medication response21,22 or lead to symptom reduction via positive behavioral changes (e.g., improved compliance). Second, medications are provided in the context of therapeutic contacts with doctors and other research staff that possess many elements in common with supportive psychotherapy.23 Patients are given diagnoses and psycho-education to explain their symptoms. They regularly meet with research staff who listen to their experiences and are encouraged to have faith in the potential effectiveness of the treatment. Each clinic visit in an antidepressant randomized controlled trial (RCT) amounts to an additional “dose” of these therapeutic factors that may influence medication response. In the case of patients assigned to a placebo, expectancy of improvement, positive behavioral change, and the therapeutic contacts provided during clinic visits (in combination with natural history factors and measurement error) are hypothesized to be the primary causes of placebo responses.
C4.1P10In the remainder of this chapter, we review recent research on patient expectancy and therapeutic contact with providers as mechanisms of placebo effects in antidepressant trials. We synthesize findings based on meta-analyses indirectly indicating the effects of expectancy and therapeutic contact, as well as RCTs designed to directly investigate their effects. We conclude with implications for clinical practice and future research.
C4.1S3Patient expectancy
C4.1P11A growing body of literature suggests that a significant portion of the improvement observed in clinical trials is due to active neurobiological processes related to expectancy.24 Expectation and expectancy are interchangeable terms referring to an individual’s beliefs about future events. In the context of antidepressant clinical trials, they refer to expectations about the outcome of treatment and to how a patient estimates the probabilities associated with various future scenarios, including anticipated positive or negative effects of treatment.24 Patients entering treatment have an outcome expectation based on their understanding of the treatment offered, their own illness, and experiences with past treatments. Expectancy is a dynamic construct that may change during the course of treatment as a function of many factors, such as the patient’s therapeutic alliance with the physician, initial treatment benefits or side effects, and the severity of the patient’s illness.
C4.1P12Several studies tested the association between expectancy and outcome in antidepressant trials and found that higher expectancy was associated with better treatment outcome.25,26,27 Meta-analyses suggest that antidepressant response rates are higher in open-label studies, where patients are certain of receiving an active drug, compared to placebo-controlled RCTs, in which their chances of receiving an active drug are lower. Rutherford et al.21 conducted a systematic analysis of clinical trials of antidepressants for MDD in adults. In the 48 placebo-controlled and 42 comparator trials examined, the odds of being classified as a responder to medication in comparator trials were 1.8 times higher than the odds of being classified as a responder in placebo-controlled trials, and the odds of being classified as a remitter to medication in comparator trials were 1.5 times higher than the odds of being classified as a remitter in placebo-controlled trials. In a sample of patients with late life depression, Sneed et al.28 found the odds of being classified as responder in comparator trials were nearly two times higher than the odds of responding in the placebo-controlled trials.
C4.1P13Interestingly, whereas patient expectancy strongly influences response rates to medication and placebos in depressed adults, it appears to be less important in the treatment of children and adolescents with depression. Specifically, a meta-analysis based on data derived from nine open, four active comparator, and 18 placebo-controlled studies of antidepressants for children and adolescents with depressive disorders suggests that no significant difference in medication response emerged between comparator and placebo-controlled studies.29 One explanation of these findings is that generating treatment expectancies requires relatively advanced cognitive capacities, as well as receiving a detailed information disclosure, neither of which may be the case among children in pediatric depression trials.
C4.1P14Just as increasing patient expectancy of benefit may contribute to symptom improvement, decreasing expectancy of benefit may result in a corresponding symptom worsening. A nocebo effect of expectancy in antidepressant trials has been documented as well.30 Specifically, among adults with MDD responding to a 12-week open treatment, randomization to continued fluoxetine or a placebo for an additional year, resulted in a nocebo effect. The possibility of receiving a placebo following 12 weeks of open fluoxetine was associated with significant symptoms worsening. These results suggest that treatment changes may have influenced patients’ expectancies of improvement, which in turn affect their depressive symptoms.
C4.1S4Causal evidence for expectancy effects and their neural mechanisms
C4.1P15Expectancy must be experimentally manipulated to determine whether it causally influences treatment outcome. Searching for a method to effectively manipulate expectancy in an ethical manner, researchers have sought to manipulate therapist style (e.g., optimistic, neutral, and pessimistic), but this approach was not found to be successful in manipulating patients’ expectancy and outcome.31 Our group has developed a methodology to experimentally manipulate expectancy effects prospectively by randomizing individuals to a high-expectancy group (open trial with a 100% chance of receiving an antidepressant medication) versus a low-expectancy group (placebo-controlled trial, where the chances of receiving medication are lower).32 Using this approach, it is feasible to manipulate expectancy in young adults. Depressed individuals randomly assigned to high-expectancy conditions experience more symptomatic improvement than those in the low-expectancy condition.33 This finding indicates that despite receiving the identical antidepressant medication, being treated by the same study clinicians, and visiting the same treatment site, depressed individuals who knew they were receiving antidepressant medication improved on average six points more on the HRSD (indicative of a large and clinically significant change) than individuals receiving the exact same drug who were aware they had a chance of receiving a placebo. The difference between antidepressant medication outcomes under high- versus low-expectancy conditions was greater in magnitude than the typically observed differences between drug and placebo responses in antidepressant trials, testifying to the powerful influence of expectancy manipulation.
C4.1P16To understand the neural mechanisms by which expectancy affects MDD, a recent study employed an antidepressant trial design capable of manipulating outcome expectancies with integrated functional magnetic resonance imaging (fMRI).34 Following the expectancy manipulation, significant differences between the high- versus low-expectancy conditions were found in neural activation changes in the amygdala as well as in superior temporal gyrus, insula, and thalamus. The findings support a mediation model according to which activation in the left amygdala decreased significantly in the high- versus low-expectancy condition in response to sad emotional faces. The reduced left amygdala activation, in turn, was a significant predictor of decreased depressive symptoms, and the mediation model was significant. These findings suggest that therapeutic modulation of amygdala activity may be an important pathway by which patient outcome expectancy influences depressive symptoms. The findings are consistent with another study investigating the neural correlates of response to a 1-week placebo lead-in phase and the association of placebo responses during lead-in with response to brief antidepressant treatment and pointing to the important role of the amygdala.35
C4.1P17Integrating such findings with studies of placebo analgesia, it appears that treatment expectancies, instantiated in activations within prefrontal cortex regions (PFC) critical for the cognitive regulation of emotion, may function by modulating the activity subcortical areas such as the amygdala, nucleus accumbens (NAcc), and insula, which are important for appraising the aversive or rewarding properties of stimuli.36 There is now evidence to suggest that a PFC-amygdala pathway linked to negative emotional experience and a PFC-NAcc/ventral striatum pathway linked to positive emotional experience may constitute top-down modulatory pathways by which expectancies exert their effects.
C4.1P18As an indirect test of this model, a recent study evaluated neurocognitive predictors of expectancy effects in depressed older adults. Older adults with MDD are a population of great interest in identifying moderators of expectancy-based placebo effects because by virtue of brain aging, they exhibit variability in cognitive (e.g., memory and executive function) and neural (e.g., integrity of frontostriatal tracts, white matter hyperintensities) markers that may be highly relevant to expectancy. Consistent with this possibility, we recently used a combined moderators approach in a population of older adults.37 We were able to identify a subpopulation of older adults with MDD who benefited from expectancy manipulation: those individuals with intact executive functioning (enabling reappraising responses based on the new expectancy-related information arriving) as well as less reduced integrity of the frontostriatal tract (enabling the modulation of limbic and striatal structures).
C4.1S5The therapeutic context and doctor-patient interaction as a source of placebo effects
C4.1P19Meta-analyses suggest that, irrespective of assignment to a medication or a placebo, more visits with the treating physician may result in significantly greater symptom reduction. Posternak and Zimmerman6 investigated the influence of therapeutic contact frequency on antidepressant and placebo responses in 41 RCTs of antidepressants for MDD. They calculated the change in HRSD scores observed over the first 6 weeks of treatment in patients assigned to either antidepressant medication or a placebo, comparing studies having six weekly assessments to those having five and four assessments. A cumulative therapeutic effect of additional follow-up visits on placebo responses was found: between weeks 2 and 6, patients with weekly visits improved 4.24 HRSD points, while those with one fewer visit improved 3.33 points, and those with two fewer visits improved 2.49 points. The presence of additional visits explained approximately 50% of the symptom change observed between weeks 2 and 6 among patients receiving a placebo. This was true to a lesser extent for participants receiving active medication, in whom the effect of this increased therapeutic contact was approximately 50% less than in the placebo group. Subsequent meta-analyses have reported convergent findings and extended these results to both children and adolescents with depression,29 depressed older adults,38 and individuals with anxiety disorders.39
C4.1P20The quantity of therapeutic contact with health care staff provided in a clinical trial has major implications for detecting a signal of effect between antidepressant medication and a placebo. Rutherford et al.38 reported in antidepressant trials for late life depression a significant treatment assignment by visits interaction, whereby increased visit frequency dramatically decreased the average difference between the medication and placebo arms. For a 12-week placebo-controlled study, providing six clinic visits resulted in an average medication response rate of 51.7% and placebo response rate of 39.5%. Intensifying supportive care from 6 to 10 visits over 12 weeks resulted in a reduction of the average medication/placebo difference from 12.2% to 0.4%. While these analyses are suggestive of a relationship between visit frequency and treatment outcome, they leave unclear whether it is the amount of contact with health care staff itself that is the therapeutic factor leading to increased medication and placebo responses or whether visit frequency is a marker for more specific aspects of the doctor-patient interaction.
C4.1P21Direct support of the importance of a strong therapeutic doctor-patient relationship in antidepressant trials comes from studies assessing the associations between measures of the therapeutic relationship, most commonly defined as the working alliance, and treatment outcome.40 The working alliance is commonly defined as the emotional bond established in the therapeutic dyad, and the agreement between the two about the goals of therapy and the tasks necessary to achieve them.41 Downing and Rickels42 were among the first to speculate that nonpharmacologic factors, such as the doctor-patient bond, might affect medication and placebo responses. The first data were reported in Krupnick et al.’s43 analysis of the Treatment of Depression Collaborative Research Program (TDCRP), in which early and mean therapeutic alliance ratings were found to predict imipramine and placebo responses. More recent findings suggest that the alliance served as a common factor in the antidepressant medication condition but functioned as an active specific ingredient mostly in the placebo condition.44
C4.1S6Causal evidence for therapeutic contact effects and their mechanisms
C4.1P22To infer causality regarding its effect, visit frequency must be manipulated. An ongoing study randomly assigned patients to research frequency management (weekly study visits) versus community frequency management (monthly study visits), and double-blind escitalopram versus placebo. To the best of our knowledge, this is the first study to directly examine the effect of the manipulation of visit frequency on medication and placebo responses. Preliminary findings suggest that increasing visit frequency results in greater treatment response across drug and placebo conditions but that the effect of more frequent study visits is particularly important for placebo responses. The average difference in HRSD between research frequency management and community frequency management for those receiving medication was 3.8 points, while the average difference for those receiving placebo 11.7 points. Based on behavioral coding of the sessions, as well as automatic coding of patient and physician movement and acoustic, further analyses test whether the therapeutic alliance and supportive techniques mediate visit frequency effects, and whether these nonpharmacologic factors have stronger effects in placebo responses than in medication responses. The study has the potential to improve clinical recommendations regarding best-practice clinical management techniques. At present, clinical management techniques follow the Fawcett et al.45 manual, which is based mainly on clinical intuition. The study has the potential to provide up-to-date, empirically established guidelines for the use of each technique.
C4.1S7Patient characteristics bearing on the magnitude of placebo effects
C4.1P23Identifying clinical and demographic characteristics of placebo versus medication responders has been one of the main aims of placebo research in the last decades. Brown et al.46 initially identified short episode duration, few previous episodes, good response to previous antidepressant treatment, and low overall symptom severity as key determinants of increased placebo responses. Weimer et al.9 conducted a comprehensive review of 31 meta-analyses and systematic reviews of more than 500 randomized placebo-controlled trials in various areas of psychiatry to identify consistent moderators across studies. Based on their review, only one patient disease characteristic was found to be consistently linked to increased placebo responses: low baseline severity of symptoms.
C4.1P24Going beyond a single moderator, which is expected to explain the heterogeneity between individuals who respond to a placebo and those that do not, researchers have combined moderators. By combining multiple weak moderators into a single stronger one of the expectancy effect a clinically useful index emerges. A combined moderator can amplify the effects of weaker, individual moderators. Moreover, each individual moderator alone may provide conflicting treatment indications for a given individual.
C4.1P25Our group has recently demonstrated the benefits of combining different moderators for the purpose of identifying older adults with MDD who may respond to a placebo.46 Using a machine-learning approach capable of evaluating the contributions of multiple predictor variables, we found that the greatest signal detection between the medication and the placebo in favor of medication was in patients with fewer years of education (≤ 12) who suffered from a longer duration of depression since their first episode (>3.47 years). Compared with medication, the placebo had the greatest response for those who were more educated (>12 years), to the point where the placebo almost outperformed medication.
C4.1S8Conclusions and directions for future research
C4.1P26This chapter discusses a conceptual model for understanding placebo responses in clinical trials for MDD and the empirical findings supporting the proposed model. Consistent with the proposed model, empirical findings support the potential to manipulate expectancy effects to maximize placebo responses in clinical practice and minimize it in clinical trials to allow a more valid testing of antidepressant drugs. The empirical literature suggests that some of the most promising approaches to manipulating placebo effects is manipulating expectancy and the therapeutic relationship.
C4.1P27Several methods have been suggested for manipulating the level of expectancy. One is to provide instructions to patients before the start of treatment regarding their chances of receiving the active drug. As both meta-analyses and RCTs have shown, this is a potent approach to manipulating expectancy. In clinical practice, a similar approach can be implemented through psycho-education of the patients about findings showing the positive effects of the drugs prescribed to them. Another approach is to restore mechanisms underpinning expectancy effects when these may be dysfunctional, for example, by enhancing processing speed in older adults with cerebrovascular lesions and executive dysfunction. We are aware of a current study to develop and test computerized cognitive training designed to increase processing speed and restore appropriate treatment expectancies, thereby enhancing antidepressant treatment response in older adults with MDD.
C4.1P28Several methods may be suggested to strengthen the therapeutic relationship. One is to manipulate the number of contacts between the patients and clinicians. Based on meta-analyses conducted by our group and on our pilot study, which manipulated the number of visits provided, we recommend increasing visits in clinical practice and reducing them to the minimum needed to ensure safety in clinical trials. The state of the art of clinical practice and research is based on exactly the opposite pattern. Currently, patients receive many more visits in clinical research than in clinical practice. Another approach is to personalize the number of visits and the techniques used by therapists to match patient characteristics (e.g., level of loneliness, social support). Empirical findings from the field of psychotherapy research identify specific techniques that are especially effective in strengthening the therapeutic alliance, such as alliance strengthening supportive work and rupture resolution strategies.47 Additional research points to the ways in which such techniques can be matched to the patient’s characteristics and needs to achieve maximum efficacy.47,48,49
C4.1P29Future studies on the factors contributing to placebo effects and their underlying mechanisms are crucial for manipulating the placebo effect. Such studies will be instrumental in establishing clear guidelines on how to minimize placebo responses in clinical research and contribute to the accurate evaluation of new antidepressant medications. Studies crystalizing the factors contributing to placebo effects can also be instrumental in harnessing it in clinical practice to benefit patients.
C4.1S9References
C4.1P301. Friedrich MJ.
C4.1P312. Hollon SD, Thase ME, Markowitz JC.
C4.1P323. Kirsch I, Sapirstein G. Listening to Prozac but hearing placebo: A meta-analysis of antidepressant medications. In: Kirsch I, ed.
C4.1P334. Quitkin FM, Rabkin JG.
C4.1P345. Nutt D, Goodwin G.
C4.1P356. Posternak MA, Zimmerman M.
C4.1P367. Zilcha-Mano S, Roose SP, Brown PJ, Rutherford BR.
C4.1P378. Zilcha-Mano S, Roose SP, Brown PJ, Rutherford BR.
C4.1P389. Weimer K, Colloca L, Enck P.
C4.1P3910. Petrie KJ, Rief W.
C4.1P4011. Wager TD, Rilling JK, Smith EE, et al.
C4.1P4112. Wager TD, Scott DJ, Zubieta JK. Placebo effects on human μ-opioid activity during pain.
C4.1P4213. Schafer SM, Geuter S, Wager TD.
C4.1P4314. Stewart-Williams S, Podd J.
C4.1P4415. Wright AGC, Woods WC.
C4.1P4516. Kisely S, Scott A, Denney J, Simon G. Duration of untreated symptoms in common mental disorders: Association with outcomes. British Journal of Psychology.
C4.1P4617. Rutherford BR, Mori S, Sneed JR, Pimontel MA, Roose SP.
C4.1P4718. Barnett AG, van der Pols JC, Dobson AJ.
C4.1P4819. Marcus SM, Gorman JM, Tu X.
C4.1P4920. Furnham A.
C4.1P5021. Rutherford BR, Sneed JR, Roose SP. Does study design affect outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychotherapy and Psychosomatics.
C4.1P5122. Rutherford BR, Marcus SM, Wang P, et al.
C4.1P5223. Miller MD, Frank E, Reynolds CF.
C4.1P5324. Rutherford BR, Wager TD, Roose SP.
C4.1P5425. Krell HV, Leuchter AF, Morgan M, Cook IA, Abrams M.
C4.1P5526. Meyer B, Pilkonis PA, Krupnick JL, Egan MK, Simmens SJ, Sotsky SM.
C4.1P5627. Zilcha-Mano S, Brown PJ, Roose SP, Cappetta K, Rutherford BR.
C4.1P5728. Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP.
C4.1P5829. Rutherford BR, Sneed JR, Tandler JM, Rindskopf D, Peterson BS, Roose SP.
C4.1P5930. Rutherford BR, Wall MM, Glass A, Stewart JW.
C4.1P6031. Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose R., Berg-Smith SM, Kline JN.
C4.1P6132. Rutherford BR, Roose SP.
C4.1P6233. Rutherford BR, Wall MM, Brown PJ, et al.
C4.1P6334. Zilcha-Mano S, Brown PJ, Roose SP, Cappetta K, Rutherford BR.
C4.1P6435. Peciña M, Bohnert AS, Sikora M, et al.
C4.1P6536. O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ.
C4.1P6637. Zilcha-Mano S, Wallace ML, Brown PJ, Sneed J, Roose SP, Rutherford BR.
C4.1P6738. Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA.
C4.1P6839. Rutherford BR, Bailey VS, Schneier FR, Pott E, Brown PJ, Roose SP.
C4.1P6940. Zilcha-Mano S, Roose SP, Brown PJ, Rutherford BR.
C4.1P7041. Bordin ES.
C4.1P7142. Rickels K, Downing RW, Winokur A. Antianxiety drugs: Clinical use in psychiatry. In: Iversen LL, Iversen SD, Snyder SH, eds.
C4.1P7243. Krupnick JL, Sotsky SM, Simmens S, et al.
C4.1P7344. Zilcha-Mano S, Roose SP, Barber J, Rutherford BR. Therapeutic alliance in antidepressant trials: Cause or effect of symptomatic levels? Psychotherapy and Psychosomatics.
C4.1P7445. Fawcett JPSI., Epstein P, Fiester SJ, Elkin I, Autry JH.
C4.1P7546. Brown WA, Johnson MF, Chen MG.
C4.1P7647. Zilcha-Mano S, Fisher H.
C4.1P7748. Zilcha-Mano S.
C4.1P7849. Zilcha-Mano S.
| Month: | Total Views: |
|---|---|
| October 2023 | 14 |
| November 2023 | 12 |
| December 2023 | 4 |
| January 2024 | 12 |
| February 2024 | 7 |
| March 2024 | 24 |
| April 2024 | 20 |
| May 2024 | 14 |
| June 2024 | 23 |
| July 2024 | 12 |
| August 2024 | 12 |
| September 2024 | 11 |
| October 2024 | 14 |
| November 2024 | 14 |
| December 2024 | 11 |
| January 2025 | 14 |
| February 2025 | 10 |
| March 2025 | 10 |
| April 2025 | 10 |
| May 2025 | 8 |