-
PDF
- Split View
-
Views
-
Cite
Cite
Linda Adwall, Irma Fredriksson, Hella Hultin, Maria Mani, Olov Norlén, Postoperative complications after breast cancer surgery and effect on recurrence and survival: population-based cohort study, BJS Open, Volume 8, Issue 6, December 2024, zrae137, https://doi.org/10.1093/bjsopen/zrae137
Close - Share Icon Share
Abstract
There is conflicting evidence regarding whether postoperative complications after breast cancer surgery are associated with worse oncological outcome. This study aimed to assess the risk of systemic breast cancer recurrence after surgical site infection and also the impact of surgical site infection on locoregional recurrence, breast cancer-specific survival and overall survival.
This nationwide cohort study included patients who underwent surgery for primary breast cancer in Sweden between January 2008 and September 2019. The study cohort was identified in the Breast Cancer Database Sweden 3.0, a database linking the National Breast Cancer Quality Register to national population-based healthcare registers held by the National Board of Health and Welfare and Statistics Sweden. The primary exposure was surgical site infection within 90 days from surgery, and the primary outcome was systemic recurrence of breast cancer. Secondary outcomes included locoregional recurrence, overall survival and breast cancer-specific survival. Multivariable Cox regression analysis was performed to assess the association between exposure, predictors and outcomes.
Of 82 102 patients included in the study, 15.7% experienced a surgical site infection within 90 days of surgery. Surgical site infection was not significantly associated with systemic recurrence, locoregional recurrence or breast cancer-specific survival after adjustment for confounding variables. Surgical site infection was significantly associated with worse overall survival, but the significant association disappeared in a sensitivity analysis excluding all patients with any kind of malignancy before breast cancer diagnosis.
Surgical site infection after breast cancer surgery does not significantly increase the risk of systemic recurrence. All possible actions should nevertheless be taken to reduce complication rates.
Introduction
As early as 1863, Rudolf Virchow discovered white blood cells in malignant tissue and made the conclusion that there is a connection between inflammation and cancer1, a notion that is now widely accepted2. For example, infectious complications after colorectal, head and neck, and gastric cancer surgery have been shown to correlate with worse survival outcomes3–5. There is conflicting evidence regarding whether postoperative complications after breast cancer surgery are associated with worse oncological outcome6–16. In a systematic review and meta-analysis, postoperative wound complications and pyrexia were associated with decreased recurrence-free survival in half of the included retrospective cohort studies, but not in the other half17. After breast cancer surgery, seroma is the most common complication. Haematoma, surgical site infection (SSI) and chronic neuropathic postoperative pain are other well known complications18–20. The reported postoperative SSI rate after breast cancer surgery varies considerably between 0 and 19%8,19–21.
Breast cancer recurrence can develop with latency intervals ranging from years to decades. One theory of these latency intervals is cancer dormancy, a stage in cancer progression where residual disease is present but remains asymptomatic22–24. The perioperative interval has been suggested to be critical for the risk of recurrence25. A postoperative complication with its inflammatory response could theoretically stimulate subclinical micrometastases and promote recurrence. Adjuvant therapy aimed at eradicating residual microinvasive disease is usually initiated no earlier than 1 month after surgery and might, therefore, have limited effect on the potentially stimulated micrometastases caused by the inflammatory response due to complications within the first month.
The primary aim of this study was to assess whether SSI increases the risk of systemic breast cancer recurrence. Secondary aims were to assess the influence of SSI on the risk of locoregional recurrence (LRR), breast cancer-specific survival (BCSS) and overall survival (OS).
Methods
Data source
The study is based on data from Breast Cancer Database Sweden 3.0 (BCBase 3.0), which is a population-based nationwide database including individuals diagnosed with breast cancer in Sweden between 2008 and 2019, created for the purpose of facilitating population-based epidemiological breast cancer research. BCBaSe 3.0 is based on individual-level record linkages between information in the Swedish National Breast Cancer Quality Register (NKBC) and national demographic and population-based healthcare registers held by the Swedish National Board of Health and Welfare (the National Cancer Register, the National Cause of Death Register, the National Patient Register, the National Prescribed Drug Register), by Statistics Sweden (the Total Population Register, the Multi-generation Register, the Longitudinal integration database for health insurance and labour market studies (LISA)) and by the Swedish Social Insurance Agency (the Micro Data for Analysis of Social insurance (MIDAS)).
The NKBC contains detailed clinical data on patient and tumour characteristics, treatment and follow-up. The completeness of the NKBC is high, greater than 99%, assessed by cross-linkage to the National Cancer Register to which reporting is mandatory by law. The proportion of missing values is less than 5% for most variables and reported information generally has high exact concordance26. The National Cancer Registry records data on all cancer diagnoses including site and date, ICD code, morphological SNOMED (Systematized Nomenclature of Medicine – Clinical Terms) code and base for diagnosis. The register is estimated to cover more than 96–98% of all incident malignant tumours in Sweden, and with 98% of the diagnoses being morphologically verified27,28. The National Cause of Death register records information on date of death and underlying and contributing cause(s) of death according to ICD29. Overall, 96% of individuals in the Cause of Death Register have a specific cause of death recorded. For breast cancer the accuracy of death certificates is estimated to be 93.1%30. The National Patient Register includes information on in- and outpatient care with up to eight discharge diagnoses classified according to ICD, data on surgical procedures, dates of admission and discharge. The register is estimated to capture about 99% of all hospitalizations31. The National Prescribed Drug Register comprises information on all prescribed drugs dispensed in Swedish pharmacies classified according to the Anatomic Therapeutic Chemical (ATC) classification system, including dates of dispensing and number of defined daily doses (DDD)32,33. The Total Population Register includes information on vital status (alive/dead), place of residence, country of birth, immigration and emigration34. The LISA database contains individual-level information on socioeconomic variables such as marital status, highest achieved educational level, disposable income, profession, housing type, country of birth and parents’ country of birth35,36. Data from the Multi-generation Register and the MIDAS database were not used for the present study.
Follow-up for systemic recurrence and survival analysis was set as 90 days after primary surgery and as 1 year after primary surgery for LRR, and continued until death or to the end of follow-up on 31 December 2019.
The article was written in accordance with the STROBE guidelines37.
Patients
The study cohort was identified within BCBaSe 3.0, including all patients who underwent surgery for primary invasive or intraductal breast cancer (DCIS) between 1 January 2008 and 30 September 2019. Patients with invasive breast cancer or DCIS before January 2008, with distant metastases at the time of or within 3 months of primary surgery were excluded. Patients with distant metastases originating from other types of malignancies were also excluded. A sensitivity analysis was performed, excluding all patients with any kind of malignancy before breast cancer diagnosis. Patients with synchronous bilateral breast cancer were included once, with the most advanced cancer recorded as the index tumour (based on: highest T-stage, highest N-stage, subtype with more aggressive biology, highest grade or highest Ki67 (proliferation index) level).
Exposures, outcomes and predictors
Exposures
The exposure was defined as SSI or no SSI within 90 days of surgery. SSI was defined as a diagnostic or intervention ICD-10 code T857, T814, HWB00, HWC00 or defined as a dispensing of antibiotic (Flucloxacillin J01CF05 or Clindamycin J01FF01) within 4–90 days of surgery. SSI were divided into early (within 30 days of surgery) and late (31–90 days after surgery). If the patient needed readmission or surgery because of the SSI, it was defined as a major SSI; otherwise it was defined as a minor SSI.
Outcomes
The primary outcome was systemic recurrence of breast cancer. Systemic recurrence was defined as the presence of ICD codes C780-C788, C790-C791, C793-C799, C771, C772, C778 and/or breast cancer death more than 3 months after primary surgery. In the analysis of systemic recurrence only patients with invasive breast cancer were included.
The secondary outcomes were LRR, OS and BCSS. LRR was defined as recurrence in the ipsilateral breast or regional lymph nodes using ICD diagnostic codes C50, D05 except D05.0 (lobular carcinoma in situ (LCIS)) or C792, C770, C773, C778, C779 in combination with Z853 and/or breast radiotherapy more than 1 year after primary surgery or ICD intervention codes for ipsilateral breast cancer surgery in the breast and/or axilla (HAB00, HAB40, HAB99, HAC10, HAC15, HAC20, HAC22, HAC99, PJA10, VXA20, PJA42, VXK21, HAF00, HAF99) performed more than 1 year after primary surgery. Secondary reconstructive procedures (without diagnosis code C50 or D05) were thus not included in the LRR definition.
Predictors
Predictors were age at surgery, country of birth, highest level of education (9 years or less (primary), 10–13 years (secondary) or more than 13 years (tertiary)), family income (low (Q1: 0–25%), middle (Q2–Q3: more than 25–75%) or high (Q4: more than 75%)), menstrual status, hypertension, obesity, diabetes, autoimmune disease, immunodeficiency, Charlson Co-morbidity Index (CCI), breast cancer detection mode, breast cancer laterality, year of breast cancer surgery, region of residence at surgery, type of primary treatment, type of final breast surgery, type of final axillary surgery, number of surgeries, radiotherapy, time to radiotherapy, chemotherapy, endocrine therapy, antihuman epidermal growth factor receptor 2 (anti-HER2) therapy, invasiveness (invasive/in situ), tumour stage, histological tumour type, Nottingham histological grade (NHG), oestrogen receptor (ER), progesterone receptor (PR), HER2, Ki67, subtype and nodal stage.
Co-morbidities were classified according to CCI and also defined as ICD code and/or drug prescription (ATC code) hypertension (I109, C02), obesity (E660-E662, E668-E669, A08), diabetes (E10, E11, A10), autoimmune disease (M05-M08, M32-M35, K50-K51, G35, E10, L40, E06, E27, G61, L04), immunodeficiency (D80-D84, D89) based on ICD codes within 7 years before primary surgery according to coding algorithms for defining co-morbidities38. CCI is a reliable, highly sensitive and valid index and a window of around 6 years before diagnosis has previously been suggested as an optimal interval for the assessment of co-morbidities39,40.
Primary treatment was defined as primary surgery or neoadjuvant therapy (NAT). Type of final breast surgery was categorized as breast conserving surgery (BCS), mastectomy with or without immediate breast reconstruction (IBR) or only axillary surgery. Final axillary surgery was categorized as sentinel lymph node biopsy (SLNB), axillary lymph node dissection (ALND) or axillary sampling. If BCS was followed by mastectomy with or without IBR, mastectomy +/− IBR was classified as the final type of breast surgery, and if SLNB was followed by ALND, ALND was defined as the final axillary surgery. Number of surgeries was the total number of operations, including the primary operation, in the ipsilateral breast and/or axilla due to tumour data.
Tumour stage (T) and nodal stage (N) were defined according to the eighth edition of the AJCC Cancer Staging Manual41. If upfront surgery was performed TN stage was based on pathology after surgery, but if NAT was given it was based on clinical and/or radiological assessment before NAT. Before 2013, T stage was based on clinical examination only and accordingly non-palpable tumours were classified as T0. Tumour biology was based on pretreatment core needle biopsy if the patient had NAT, and on surgical specimen if upfront surgery was performed. Histological tumour type was defined as no special type (NST), lobular, NST + lobular or other invasive if not NST and/or lobular. ER and PR were considered positive if greater than 10% of tumour cells were stained. HER2 was considered positive with an immunohistochemical (IHC) score of 3+ or if 2+ with a verification of amplification by in situ hybridization (ISH). Ki67 was classified as low, intermediate or high according to local pathology cut offs. Subtype was defined as Luminal A, Luminal B, HER2+ hormone receptor (HR)+, HER2+ HR– or Triple-Negative (ER, PR and HER2–). HR+ tumours were ER+ and/or PR+ and HR– tumours were ER– and PR–. To distinguish between the Luminal A and B subtypes we used NHG (1–3) (Luminal A if NHG 1, Luminal B if NHG 3 and if NHG 2, Ki67 index and PR were used (Luminal A if low Ki67 or if intermediate Ki67 together with PR was greater than 20%, Luminal B if high Ki67 or if intermediate Ki67 together with PR less than 20%)). Due to a large proportion of missing data on Ki67 (31.1%), another subtype definition was used in the multivariable regression analysis: Luminal A (NHG 1), Luminal NHG 2, Luminal B (NHG 3), HER2+ HR+, HER2+ HR– or TNBC (Triple-Negative Breast Cancer).
Analysis of complications other than SSI
Bleeding or wound complications were defined by at least one of the following diagnostic or interventional codes (ICD; T810, T811, T817, HWD00, HWE00, HWA00, T813, HWF00) and unspecified local complication (T854, T856, T858, T859, T812, T815, T818, T818W, T819, T889, HWW99) registered within 90 days of surgery. Any local complication was defined as SSI and/or bleeding or wound complication and/or unspecified local complication. The complication was defined as major if the patient needed readmission or surgery because of the complication. All other complications were proposed as minor.
Analysis of risk factors for an SSI
A separate analysis with primary surgery as exposure and SSI as outcome was performed to assess risk factors for SSI.
Statistical analysis
Descriptive data are presented as numbers with percentages and means(s.d.). OS, BCSS, distant recurrence-free survival (DRFS) and cumulative risk of LRR were calculated with the Kaplan–Meier method and univariable analyses of the effect of exposure were analysed with the log rank test. The association between exposure, predictors and outcome was analysed using multivariable Cox regression. To decide which predictors to include in multivariable analysis, a directed acyclic graph (DAG) was constructed. Results are presented as hazard ratios (HR) with 95% confidence intervals (c.i.).
In a secondary analysis to assess risk factors for SSI, univariable and multiple logistic regression were performed to adjust for clinically relevant confounding predictors. Results are presented as odds ratios (OR) with 95% c.i.
All analyses were performed using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria), through specific study files available by ‘remote server access’ on the research Q-portal administrated by the North Regional Cancer Centre (RCC). All tests were two-sided and P < 0.050 was considered statistically significant.
Power calculation
A power calculation was performed based on findings from previous studies8,11,42,43. The following parameters were considered: an SSI rate after breast cancer surgery of 10% and a rate of 20% for developing systemic recurrence. The δ margin for systemic recurrence was set to 1.08. A sample size of 57 920 patients was calculated with a power 0.80 and type I error of 0.05.
Ethical considerations
The construction of BCBaSe 3.0 was approved by the Ethics Committee (DNR 2019–02610, 2020–00886, 2020–06302) with an amendment for the present study (DNR 2022-01020-02).
Results
The inclusion criteria were met by 87 558 patients. Patients with breast cancer or DCIS in the past, distant metastasis from other cancer or distant metastasis within 3 months of breast cancer surgery were excluded (Fig. 1), leaving 82 102 patients in the cohort, of whom 513 (0.6%) were men. Patient characteristics are shown in Table 1, treatment characteristics in Table S1 and disease characteristics in Table 2. Mean(s.d.) age was 63(13) years, ranging from 19 to 104 years. Of the included patients 73 313 had invasive breast cancer and 8570 in situ breast cancer. Among patients not treated with adjuvant chemotherapy experiencing an SSI, 21.3% started radiotherapy within 60 days, compared with 28.2% (P < 0.001) of patients without an SSI. Median (range) follow-up for systemic recurrence was 4.8 (0–11.8) years, for OS/BCSS was 5.0 (0–11.8) years and for LRR was 4.5 (0–11.0) years.
Flow chart
SSI, surgical site infection; LRR, locoregional recurrence; DCIS, ductal cancer in situ.
Patient characteristics in a population-based cohort of 82 102 individuals with breast cancer diagnosed between 2008 and 2019
| . | No SSI n = 69 227 . | SSI n = 12 875 . | Overall n = 82 102 . |
|---|---|---|---|
| Age group (years) | |||
| <40 | 2513 (3.6) | 584 (4.5) | 3097 (3.8) |
| 40–49 | 10 321 (14.9) | 2130 (16.5) | 12 451 (15.2) |
| 50–64 | 24 084 (34.8) | 4733 (36.8) | 28 817 (35.1) |
| 65–74 | 21 761 (31.4) | 3464 (26.9) | 25 225 (30.7) |
| ≥75 | 10 548 (15.2) | 1964 (15.2) | 12 512 (15.2) |
| Country of birth | |||
| Sweden | 59 049 (85.3) | 10 778 (83.7) | 69 827 (85.1) |
| Europe (not Sweden) | 5489 (7.9) | 1134 (8.8) | 6623 (8.1) |
| Outside Europe | 2721 (3.9) | 583 (4.5) | 3304 (4.0) |
| Missing | 1968 (2.8) | 380 (3.0) | 2348 (2.9) |
| Highest level of education | |||
| ≤9 years | 14 942 (21.6) | 2817 (21.9) | 17 759 (21.6) |
| 10–13 years | 28 818 (41.6) | 5334 (41.4) | 34 152 (41.6) |
| >13 years | 24 737 (35.7) | 4554 (35.4) | 29 291 (35.7) |
| Missing | 730 (1.1) | 170 (1.3) | 900 (1.1) |
| Family income | |||
| Low | 16 963 (24.5) | 3435 (26.7) | 20 398 (24.8) |
| Middle | 34 538 (49.9) | 6389 (49.6) | 40 927 (49.9) |
| High | 17 509 (25.3) | 3017 (23.4) | 20 526 (25.0) |
| Missing | 217 (0.3) | 34 (0.3) | 251 (0.3) |
| Menstrual status | |||
| Premenopausal | 14 103 (20.4) | 2963 (23.0) | 17 066 (20.8) |
| Postmenopausal | 49 808 (72.0) | 8843 (68.7) | 58 651 (71.4) |
| Male | 395 (0.6) | 118 (0.9) | 513 (0.6) |
| Missing | 4921 (7.1) | 951 (7.4) | 5872 (7.2) |
| Hypertension | |||
| No | 55 139 (79.7) | 9788 (76.0) | 64 927 (79.1) |
| Yes | 14 088 (20.4) | 3087 (24.0) | 17 175 (20.9) |
| Obesity | |||
| No | 66 176 (95.6) | 11 791 (91.6) | 77 967 (95.0) |
| Yes | 3051 (4.4) | 1084 (8.4) | 4135 (5.0) |
| Diabetes | |||
| No | 64 432 (93.1) | 11 581 (90.0) | 76 013 (92.6) |
| Yes | 4795 (6.9) | 1294 (10.1) | 6089 (7.4) |
| Autoimmune disease | |||
| No | 64 131 (92.6) | 11 704 (90.9) | 75 835 (92.4) |
| Yes | 5096 (7.4) | 1171 (9.1) | 6267 (7.6) |
| Immunodeficiency | |||
| No | 69 087 (99.8) | 12 842 (99.7) | 81 929 (99.8) |
| Yes | 140 (0.2) | 33 (0.3) | 173 (0.2) |
| Charlson Co-morbidity Index | |||
| 0 | 37 856 (54.7) | 6811 (52.9) | 44 667 (54.4) |
| 1 | 6350 (9.2) | 1382 (10.7) | 7732 (9.4) |
| 2 | 9535 (13.8) | 1949 (15.1) | 11 484 (14.0) |
| 3–5 | 3333 (4.8) | 816 (6.3) | 4149 (5.1) |
| 6–7 | 250 (0.4) | 87 (0.7) | 337 (0.4) |
| ≥8 | 323 (0.5) | 103 (0.8) | 426 (0.5) |
| Missing | 11 580 (16.7) | 1727 (13.4) | 13 307 (16.2) |
| . | No SSI n = 69 227 . | SSI n = 12 875 . | Overall n = 82 102 . |
|---|---|---|---|
| Age group (years) | |||
| <40 | 2513 (3.6) | 584 (4.5) | 3097 (3.8) |
| 40–49 | 10 321 (14.9) | 2130 (16.5) | 12 451 (15.2) |
| 50–64 | 24 084 (34.8) | 4733 (36.8) | 28 817 (35.1) |
| 65–74 | 21 761 (31.4) | 3464 (26.9) | 25 225 (30.7) |
| ≥75 | 10 548 (15.2) | 1964 (15.2) | 12 512 (15.2) |
| Country of birth | |||
| Sweden | 59 049 (85.3) | 10 778 (83.7) | 69 827 (85.1) |
| Europe (not Sweden) | 5489 (7.9) | 1134 (8.8) | 6623 (8.1) |
| Outside Europe | 2721 (3.9) | 583 (4.5) | 3304 (4.0) |
| Missing | 1968 (2.8) | 380 (3.0) | 2348 (2.9) |
| Highest level of education | |||
| ≤9 years | 14 942 (21.6) | 2817 (21.9) | 17 759 (21.6) |
| 10–13 years | 28 818 (41.6) | 5334 (41.4) | 34 152 (41.6) |
| >13 years | 24 737 (35.7) | 4554 (35.4) | 29 291 (35.7) |
| Missing | 730 (1.1) | 170 (1.3) | 900 (1.1) |
| Family income | |||
| Low | 16 963 (24.5) | 3435 (26.7) | 20 398 (24.8) |
| Middle | 34 538 (49.9) | 6389 (49.6) | 40 927 (49.9) |
| High | 17 509 (25.3) | 3017 (23.4) | 20 526 (25.0) |
| Missing | 217 (0.3) | 34 (0.3) | 251 (0.3) |
| Menstrual status | |||
| Premenopausal | 14 103 (20.4) | 2963 (23.0) | 17 066 (20.8) |
| Postmenopausal | 49 808 (72.0) | 8843 (68.7) | 58 651 (71.4) |
| Male | 395 (0.6) | 118 (0.9) | 513 (0.6) |
| Missing | 4921 (7.1) | 951 (7.4) | 5872 (7.2) |
| Hypertension | |||
| No | 55 139 (79.7) | 9788 (76.0) | 64 927 (79.1) |
| Yes | 14 088 (20.4) | 3087 (24.0) | 17 175 (20.9) |
| Obesity | |||
| No | 66 176 (95.6) | 11 791 (91.6) | 77 967 (95.0) |
| Yes | 3051 (4.4) | 1084 (8.4) | 4135 (5.0) |
| Diabetes | |||
| No | 64 432 (93.1) | 11 581 (90.0) | 76 013 (92.6) |
| Yes | 4795 (6.9) | 1294 (10.1) | 6089 (7.4) |
| Autoimmune disease | |||
| No | 64 131 (92.6) | 11 704 (90.9) | 75 835 (92.4) |
| Yes | 5096 (7.4) | 1171 (9.1) | 6267 (7.6) |
| Immunodeficiency | |||
| No | 69 087 (99.8) | 12 842 (99.7) | 81 929 (99.8) |
| Yes | 140 (0.2) | 33 (0.3) | 173 (0.2) |
| Charlson Co-morbidity Index | |||
| 0 | 37 856 (54.7) | 6811 (52.9) | 44 667 (54.4) |
| 1 | 6350 (9.2) | 1382 (10.7) | 7732 (9.4) |
| 2 | 9535 (13.8) | 1949 (15.1) | 11 484 (14.0) |
| 3–5 | 3333 (4.8) | 816 (6.3) | 4149 (5.1) |
| 6–7 | 250 (0.4) | 87 (0.7) | 337 (0.4) |
| ≥8 | 323 (0.5) | 103 (0.8) | 426 (0.5) |
| Missing | 11 580 (16.7) | 1727 (13.4) | 13 307 (16.2) |
Values are n (%). SSI, surgical site infection.
Patient characteristics in a population-based cohort of 82 102 individuals with breast cancer diagnosed between 2008 and 2019
| . | No SSI n = 69 227 . | SSI n = 12 875 . | Overall n = 82 102 . |
|---|---|---|---|
| Age group (years) | |||
| <40 | 2513 (3.6) | 584 (4.5) | 3097 (3.8) |
| 40–49 | 10 321 (14.9) | 2130 (16.5) | 12 451 (15.2) |
| 50–64 | 24 084 (34.8) | 4733 (36.8) | 28 817 (35.1) |
| 65–74 | 21 761 (31.4) | 3464 (26.9) | 25 225 (30.7) |
| ≥75 | 10 548 (15.2) | 1964 (15.2) | 12 512 (15.2) |
| Country of birth | |||
| Sweden | 59 049 (85.3) | 10 778 (83.7) | 69 827 (85.1) |
| Europe (not Sweden) | 5489 (7.9) | 1134 (8.8) | 6623 (8.1) |
| Outside Europe | 2721 (3.9) | 583 (4.5) | 3304 (4.0) |
| Missing | 1968 (2.8) | 380 (3.0) | 2348 (2.9) |
| Highest level of education | |||
| ≤9 years | 14 942 (21.6) | 2817 (21.9) | 17 759 (21.6) |
| 10–13 years | 28 818 (41.6) | 5334 (41.4) | 34 152 (41.6) |
| >13 years | 24 737 (35.7) | 4554 (35.4) | 29 291 (35.7) |
| Missing | 730 (1.1) | 170 (1.3) | 900 (1.1) |
| Family income | |||
| Low | 16 963 (24.5) | 3435 (26.7) | 20 398 (24.8) |
| Middle | 34 538 (49.9) | 6389 (49.6) | 40 927 (49.9) |
| High | 17 509 (25.3) | 3017 (23.4) | 20 526 (25.0) |
| Missing | 217 (0.3) | 34 (0.3) | 251 (0.3) |
| Menstrual status | |||
| Premenopausal | 14 103 (20.4) | 2963 (23.0) | 17 066 (20.8) |
| Postmenopausal | 49 808 (72.0) | 8843 (68.7) | 58 651 (71.4) |
| Male | 395 (0.6) | 118 (0.9) | 513 (0.6) |
| Missing | 4921 (7.1) | 951 (7.4) | 5872 (7.2) |
| Hypertension | |||
| No | 55 139 (79.7) | 9788 (76.0) | 64 927 (79.1) |
| Yes | 14 088 (20.4) | 3087 (24.0) | 17 175 (20.9) |
| Obesity | |||
| No | 66 176 (95.6) | 11 791 (91.6) | 77 967 (95.0) |
| Yes | 3051 (4.4) | 1084 (8.4) | 4135 (5.0) |
| Diabetes | |||
| No | 64 432 (93.1) | 11 581 (90.0) | 76 013 (92.6) |
| Yes | 4795 (6.9) | 1294 (10.1) | 6089 (7.4) |
| Autoimmune disease | |||
| No | 64 131 (92.6) | 11 704 (90.9) | 75 835 (92.4) |
| Yes | 5096 (7.4) | 1171 (9.1) | 6267 (7.6) |
| Immunodeficiency | |||
| No | 69 087 (99.8) | 12 842 (99.7) | 81 929 (99.8) |
| Yes | 140 (0.2) | 33 (0.3) | 173 (0.2) |
| Charlson Co-morbidity Index | |||
| 0 | 37 856 (54.7) | 6811 (52.9) | 44 667 (54.4) |
| 1 | 6350 (9.2) | 1382 (10.7) | 7732 (9.4) |
| 2 | 9535 (13.8) | 1949 (15.1) | 11 484 (14.0) |
| 3–5 | 3333 (4.8) | 816 (6.3) | 4149 (5.1) |
| 6–7 | 250 (0.4) | 87 (0.7) | 337 (0.4) |
| ≥8 | 323 (0.5) | 103 (0.8) | 426 (0.5) |
| Missing | 11 580 (16.7) | 1727 (13.4) | 13 307 (16.2) |
| . | No SSI n = 69 227 . | SSI n = 12 875 . | Overall n = 82 102 . |
|---|---|---|---|
| Age group (years) | |||
| <40 | 2513 (3.6) | 584 (4.5) | 3097 (3.8) |
| 40–49 | 10 321 (14.9) | 2130 (16.5) | 12 451 (15.2) |
| 50–64 | 24 084 (34.8) | 4733 (36.8) | 28 817 (35.1) |
| 65–74 | 21 761 (31.4) | 3464 (26.9) | 25 225 (30.7) |
| ≥75 | 10 548 (15.2) | 1964 (15.2) | 12 512 (15.2) |
| Country of birth | |||
| Sweden | 59 049 (85.3) | 10 778 (83.7) | 69 827 (85.1) |
| Europe (not Sweden) | 5489 (7.9) | 1134 (8.8) | 6623 (8.1) |
| Outside Europe | 2721 (3.9) | 583 (4.5) | 3304 (4.0) |
| Missing | 1968 (2.8) | 380 (3.0) | 2348 (2.9) |
| Highest level of education | |||
| ≤9 years | 14 942 (21.6) | 2817 (21.9) | 17 759 (21.6) |
| 10–13 years | 28 818 (41.6) | 5334 (41.4) | 34 152 (41.6) |
| >13 years | 24 737 (35.7) | 4554 (35.4) | 29 291 (35.7) |
| Missing | 730 (1.1) | 170 (1.3) | 900 (1.1) |
| Family income | |||
| Low | 16 963 (24.5) | 3435 (26.7) | 20 398 (24.8) |
| Middle | 34 538 (49.9) | 6389 (49.6) | 40 927 (49.9) |
| High | 17 509 (25.3) | 3017 (23.4) | 20 526 (25.0) |
| Missing | 217 (0.3) | 34 (0.3) | 251 (0.3) |
| Menstrual status | |||
| Premenopausal | 14 103 (20.4) | 2963 (23.0) | 17 066 (20.8) |
| Postmenopausal | 49 808 (72.0) | 8843 (68.7) | 58 651 (71.4) |
| Male | 395 (0.6) | 118 (0.9) | 513 (0.6) |
| Missing | 4921 (7.1) | 951 (7.4) | 5872 (7.2) |
| Hypertension | |||
| No | 55 139 (79.7) | 9788 (76.0) | 64 927 (79.1) |
| Yes | 14 088 (20.4) | 3087 (24.0) | 17 175 (20.9) |
| Obesity | |||
| No | 66 176 (95.6) | 11 791 (91.6) | 77 967 (95.0) |
| Yes | 3051 (4.4) | 1084 (8.4) | 4135 (5.0) |
| Diabetes | |||
| No | 64 432 (93.1) | 11 581 (90.0) | 76 013 (92.6) |
| Yes | 4795 (6.9) | 1294 (10.1) | 6089 (7.4) |
| Autoimmune disease | |||
| No | 64 131 (92.6) | 11 704 (90.9) | 75 835 (92.4) |
| Yes | 5096 (7.4) | 1171 (9.1) | 6267 (7.6) |
| Immunodeficiency | |||
| No | 69 087 (99.8) | 12 842 (99.7) | 81 929 (99.8) |
| Yes | 140 (0.2) | 33 (0.3) | 173 (0.2) |
| Charlson Co-morbidity Index | |||
| 0 | 37 856 (54.7) | 6811 (52.9) | 44 667 (54.4) |
| 1 | 6350 (9.2) | 1382 (10.7) | 7732 (9.4) |
| 2 | 9535 (13.8) | 1949 (15.1) | 11 484 (14.0) |
| 3–5 | 3333 (4.8) | 816 (6.3) | 4149 (5.1) |
| 6–7 | 250 (0.4) | 87 (0.7) | 337 (0.4) |
| ≥8 | 323 (0.5) | 103 (0.8) | 426 (0.5) |
| Missing | 11 580 (16.7) | 1727 (13.4) | 13 307 (16.2) |
Values are n (%). SSI, surgical site infection.
Disease characteristics in a population-based cohort of 82 102 individuals with breast cancer diagnosed between 2008 and 2019
| > . | No SSI n = 69 227 . | SSI n = 12 875 . | Overall n = 82 102 . |
|---|---|---|---|
| Invasivity | |||
| Invasive | 61 589 (89.0) | 11 724 (91.1) | 73 313 (89.3) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 180 (0.3) | 39 (0.3) | 219 (0.3) |
| Tumour stage | |||
| T0 | 423 (0.6) | 72 (0.6) | 495 (0.6) |
| Tis | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| T1 | 39 075 (56.5) | 6313 (49.0) | 45 388 (55.3) |
| T2 | 18 137 (26.2) | 4266 (33.1) | 22 403 (27.3) |
| T3 | 2809 (4.1) | 782 (6.1) | 3591 (4.4) |
| T4 | 473 (0.7) | 151 (1.2) | 624 (0.8) |
| Unknown | 849 (1.2) | 179 (1.4) | 1028 (1.3) |
| Histological tumour type | |||
| NST | 47 159 (68.1) | 9012 (70.0) | 56 171 (68.4) |
| NST + lobular | 1250 (1.8) | 292 (2.3) | 1542 (1.9) |
| Lobular | 7803 (11.3) | 1575 (12.2) | 9378 (11.4) |
| Other invasive | 4500 (6.5) | 676 (5.3) | 5176 (6.3) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1057 (1.5) | 208 (1.6) | 1265 (1.5) |
| NHG | |||
| 1 | 12 478 (18.0) | 1704 (13.2) | 14 182 (17.3) |
| 2 | 29 363 (42.4) | 5450 (42.3) | 34 813 (42.4) |
| 3 | 15 889 (23.0) | 3590 (27.9) | 19 479 (23.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 4039 (5.8) | 1019 (7.9) | 5058 (6.2) |
| ER | |||
| Positive | 52 522 (75.9) | 9743 (75.7) | 62 265 (75.8) |
| Negative | 8190 (11.8) | 1813 (14.1) | 10 003 (12.2) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1057 (1.5) | 207 (1.6) | 1264 (1.5) |
| PR | |||
| Positive | 44 385 (64.1) | 8136 (63.2) | 52 521 (64.0) |
| Negative | 16 256 (23.5) | 3407 (26.5) | 19 663 (24.0) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1128 (1.6) | 220 (1.7) | 1348 (1.6) |
| HER2 | |||
| Positive | 7701 (11.1) | 1832 (14.2) | 9533 (11.6) |
| Negative | 50 934 (73.6) | 9358 (72.7) | 60 292 (73.4) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 3134 (4.5) | 573 (4.5) | 3707 (4.5) |
| Ki67 | |||
| Low | 16 797 (24.3) | 2681 (20.8) | 19 478 (23.7) |
| Intermediate | 5842 (8.4) | 951 (7.4) | 6793 (8.3) |
| High | 17 981 (26.0) | 3786 (29.4) | 21 767 (26.5) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 21 149 (30.6) | 4345 (33.8) | 25 494 (31.1) |
| Subtype | |||
| Luminal A | 23 861 (34.5) | 3670 (28.5) | 27 531 (33.5) |
| Luminal B | 12 525 (18.1) | 2654 (20.6) | 15 179 (18.5) |
| HER2+ HR+ | 5274 (7.6) | 1242 (9.7) | 6516 (7.9) |
| HER2+ HR– | 2384 (3.4) | 572 (4.4) | 2956 (3.6) |
| Triple-negative | 5175 (7.5) | 1117 (8.7) | 6292 (7.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 12 550 (18.1) | 2508 (19.5) | 15 058 (18.3) |
| Subtype (other definition) | |||
| Luminal A (NHG 1) | 11 555 (16.7) | 1551 (12.1) | 13 106 (16.0) |
| Luminal NHG 2 | 24 945 (36.0) | 4580 (35.6) | 29 525 (36.0) |
| Luminal B (NHG 3) | 7514 (10.9) | 1695 (13.2) | 9209 (11.2) |
| HER2+HR+ | 5274 (7.6) | 1242 (9.7) | 6516 (7.9) |
| HER2+ HR– | 2384 (3.4) | 572 (4.5) | 2956 (3.6) |
| Triple-negative | 5175 (7.5) | 1117 (8.7) | 6292 (7.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 4922 (7.1) | 1006 (7.8) | 5928 (7.2) |
| Nodal stage | |||
| N0 | 50 086 (72.4) | 7225 (56.1) | 57 311 (69.8) |
| N1 | 14 256 (20.6) | 4046 (31.4) | 18 302 (22.3) |
| N2 | 3180 (4.6) | 1039 (8.1) | 4219 (5.1) |
| N3 | 1322 (1.9) | 499 (3.9) | 1821 (2.2) |
| Missing | 383 (0.6) | 66 (0.5) | 449 (0.6) |
| > . | No SSI n = 69 227 . | SSI n = 12 875 . | Overall n = 82 102 . |
|---|---|---|---|
| Invasivity | |||
| Invasive | 61 589 (89.0) | 11 724 (91.1) | 73 313 (89.3) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 180 (0.3) | 39 (0.3) | 219 (0.3) |
| Tumour stage | |||
| T0 | 423 (0.6) | 72 (0.6) | 495 (0.6) |
| Tis | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| T1 | 39 075 (56.5) | 6313 (49.0) | 45 388 (55.3) |
| T2 | 18 137 (26.2) | 4266 (33.1) | 22 403 (27.3) |
| T3 | 2809 (4.1) | 782 (6.1) | 3591 (4.4) |
| T4 | 473 (0.7) | 151 (1.2) | 624 (0.8) |
| Unknown | 849 (1.2) | 179 (1.4) | 1028 (1.3) |
| Histological tumour type | |||
| NST | 47 159 (68.1) | 9012 (70.0) | 56 171 (68.4) |
| NST + lobular | 1250 (1.8) | 292 (2.3) | 1542 (1.9) |
| Lobular | 7803 (11.3) | 1575 (12.2) | 9378 (11.4) |
| Other invasive | 4500 (6.5) | 676 (5.3) | 5176 (6.3) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1057 (1.5) | 208 (1.6) | 1265 (1.5) |
| NHG | |||
| 1 | 12 478 (18.0) | 1704 (13.2) | 14 182 (17.3) |
| 2 | 29 363 (42.4) | 5450 (42.3) | 34 813 (42.4) |
| 3 | 15 889 (23.0) | 3590 (27.9) | 19 479 (23.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 4039 (5.8) | 1019 (7.9) | 5058 (6.2) |
| ER | |||
| Positive | 52 522 (75.9) | 9743 (75.7) | 62 265 (75.8) |
| Negative | 8190 (11.8) | 1813 (14.1) | 10 003 (12.2) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1057 (1.5) | 207 (1.6) | 1264 (1.5) |
| PR | |||
| Positive | 44 385 (64.1) | 8136 (63.2) | 52 521 (64.0) |
| Negative | 16 256 (23.5) | 3407 (26.5) | 19 663 (24.0) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1128 (1.6) | 220 (1.7) | 1348 (1.6) |
| HER2 | |||
| Positive | 7701 (11.1) | 1832 (14.2) | 9533 (11.6) |
| Negative | 50 934 (73.6) | 9358 (72.7) | 60 292 (73.4) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 3134 (4.5) | 573 (4.5) | 3707 (4.5) |
| Ki67 | |||
| Low | 16 797 (24.3) | 2681 (20.8) | 19 478 (23.7) |
| Intermediate | 5842 (8.4) | 951 (7.4) | 6793 (8.3) |
| High | 17 981 (26.0) | 3786 (29.4) | 21 767 (26.5) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 21 149 (30.6) | 4345 (33.8) | 25 494 (31.1) |
| Subtype | |||
| Luminal A | 23 861 (34.5) | 3670 (28.5) | 27 531 (33.5) |
| Luminal B | 12 525 (18.1) | 2654 (20.6) | 15 179 (18.5) |
| HER2+ HR+ | 5274 (7.6) | 1242 (9.7) | 6516 (7.9) |
| HER2+ HR– | 2384 (3.4) | 572 (4.4) | 2956 (3.6) |
| Triple-negative | 5175 (7.5) | 1117 (8.7) | 6292 (7.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 12 550 (18.1) | 2508 (19.5) | 15 058 (18.3) |
| Subtype (other definition) | |||
| Luminal A (NHG 1) | 11 555 (16.7) | 1551 (12.1) | 13 106 (16.0) |
| Luminal NHG 2 | 24 945 (36.0) | 4580 (35.6) | 29 525 (36.0) |
| Luminal B (NHG 3) | 7514 (10.9) | 1695 (13.2) | 9209 (11.2) |
| HER2+HR+ | 5274 (7.6) | 1242 (9.7) | 6516 (7.9) |
| HER2+ HR– | 2384 (3.4) | 572 (4.5) | 2956 (3.6) |
| Triple-negative | 5175 (7.5) | 1117 (8.7) | 6292 (7.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 4922 (7.1) | 1006 (7.8) | 5928 (7.2) |
| Nodal stage | |||
| N0 | 50 086 (72.4) | 7225 (56.1) | 57 311 (69.8) |
| N1 | 14 256 (20.6) | 4046 (31.4) | 18 302 (22.3) |
| N2 | 3180 (4.6) | 1039 (8.1) | 4219 (5.1) |
| N3 | 1322 (1.9) | 499 (3.9) | 1821 (2.2) |
| Missing | 383 (0.6) | 66 (0.5) | 449 (0.6) |
Values are n (%). SSI, surgical site infection; Tis, carcinoma in situ; NST, no special type (former ductal); ER, oestrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NHG, Nottingham histological grade; Ki67, proliferation index.
Disease characteristics in a population-based cohort of 82 102 individuals with breast cancer diagnosed between 2008 and 2019
| > . | No SSI n = 69 227 . | SSI n = 12 875 . | Overall n = 82 102 . |
|---|---|---|---|
| Invasivity | |||
| Invasive | 61 589 (89.0) | 11 724 (91.1) | 73 313 (89.3) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 180 (0.3) | 39 (0.3) | 219 (0.3) |
| Tumour stage | |||
| T0 | 423 (0.6) | 72 (0.6) | 495 (0.6) |
| Tis | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| T1 | 39 075 (56.5) | 6313 (49.0) | 45 388 (55.3) |
| T2 | 18 137 (26.2) | 4266 (33.1) | 22 403 (27.3) |
| T3 | 2809 (4.1) | 782 (6.1) | 3591 (4.4) |
| T4 | 473 (0.7) | 151 (1.2) | 624 (0.8) |
| Unknown | 849 (1.2) | 179 (1.4) | 1028 (1.3) |
| Histological tumour type | |||
| NST | 47 159 (68.1) | 9012 (70.0) | 56 171 (68.4) |
| NST + lobular | 1250 (1.8) | 292 (2.3) | 1542 (1.9) |
| Lobular | 7803 (11.3) | 1575 (12.2) | 9378 (11.4) |
| Other invasive | 4500 (6.5) | 676 (5.3) | 5176 (6.3) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1057 (1.5) | 208 (1.6) | 1265 (1.5) |
| NHG | |||
| 1 | 12 478 (18.0) | 1704 (13.2) | 14 182 (17.3) |
| 2 | 29 363 (42.4) | 5450 (42.3) | 34 813 (42.4) |
| 3 | 15 889 (23.0) | 3590 (27.9) | 19 479 (23.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 4039 (5.8) | 1019 (7.9) | 5058 (6.2) |
| ER | |||
| Positive | 52 522 (75.9) | 9743 (75.7) | 62 265 (75.8) |
| Negative | 8190 (11.8) | 1813 (14.1) | 10 003 (12.2) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1057 (1.5) | 207 (1.6) | 1264 (1.5) |
| PR | |||
| Positive | 44 385 (64.1) | 8136 (63.2) | 52 521 (64.0) |
| Negative | 16 256 (23.5) | 3407 (26.5) | 19 663 (24.0) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1128 (1.6) | 220 (1.7) | 1348 (1.6) |
| HER2 | |||
| Positive | 7701 (11.1) | 1832 (14.2) | 9533 (11.6) |
| Negative | 50 934 (73.6) | 9358 (72.7) | 60 292 (73.4) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 3134 (4.5) | 573 (4.5) | 3707 (4.5) |
| Ki67 | |||
| Low | 16 797 (24.3) | 2681 (20.8) | 19 478 (23.7) |
| Intermediate | 5842 (8.4) | 951 (7.4) | 6793 (8.3) |
| High | 17 981 (26.0) | 3786 (29.4) | 21 767 (26.5) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 21 149 (30.6) | 4345 (33.8) | 25 494 (31.1) |
| Subtype | |||
| Luminal A | 23 861 (34.5) | 3670 (28.5) | 27 531 (33.5) |
| Luminal B | 12 525 (18.1) | 2654 (20.6) | 15 179 (18.5) |
| HER2+ HR+ | 5274 (7.6) | 1242 (9.7) | 6516 (7.9) |
| HER2+ HR– | 2384 (3.4) | 572 (4.4) | 2956 (3.6) |
| Triple-negative | 5175 (7.5) | 1117 (8.7) | 6292 (7.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 12 550 (18.1) | 2508 (19.5) | 15 058 (18.3) |
| Subtype (other definition) | |||
| Luminal A (NHG 1) | 11 555 (16.7) | 1551 (12.1) | 13 106 (16.0) |
| Luminal NHG 2 | 24 945 (36.0) | 4580 (35.6) | 29 525 (36.0) |
| Luminal B (NHG 3) | 7514 (10.9) | 1695 (13.2) | 9209 (11.2) |
| HER2+HR+ | 5274 (7.6) | 1242 (9.7) | 6516 (7.9) |
| HER2+ HR– | 2384 (3.4) | 572 (4.5) | 2956 (3.6) |
| Triple-negative | 5175 (7.5) | 1117 (8.7) | 6292 (7.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 4922 (7.1) | 1006 (7.8) | 5928 (7.2) |
| Nodal stage | |||
| N0 | 50 086 (72.4) | 7225 (56.1) | 57 311 (69.8) |
| N1 | 14 256 (20.6) | 4046 (31.4) | 18 302 (22.3) |
| N2 | 3180 (4.6) | 1039 (8.1) | 4219 (5.1) |
| N3 | 1322 (1.9) | 499 (3.9) | 1821 (2.2) |
| Missing | 383 (0.6) | 66 (0.5) | 449 (0.6) |
| > . | No SSI n = 69 227 . | SSI n = 12 875 . | Overall n = 82 102 . |
|---|---|---|---|
| Invasivity | |||
| Invasive | 61 589 (89.0) | 11 724 (91.1) | 73 313 (89.3) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 180 (0.3) | 39 (0.3) | 219 (0.3) |
| Tumour stage | |||
| T0 | 423 (0.6) | 72 (0.6) | 495 (0.6) |
| Tis | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| T1 | 39 075 (56.5) | 6313 (49.0) | 45 388 (55.3) |
| T2 | 18 137 (26.2) | 4266 (33.1) | 22 403 (27.3) |
| T3 | 2809 (4.1) | 782 (6.1) | 3591 (4.4) |
| T4 | 473 (0.7) | 151 (1.2) | 624 (0.8) |
| Unknown | 849 (1.2) | 179 (1.4) | 1028 (1.3) |
| Histological tumour type | |||
| NST | 47 159 (68.1) | 9012 (70.0) | 56 171 (68.4) |
| NST + lobular | 1250 (1.8) | 292 (2.3) | 1542 (1.9) |
| Lobular | 7803 (11.3) | 1575 (12.2) | 9378 (11.4) |
| Other invasive | 4500 (6.5) | 676 (5.3) | 5176 (6.3) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1057 (1.5) | 208 (1.6) | 1265 (1.5) |
| NHG | |||
| 1 | 12 478 (18.0) | 1704 (13.2) | 14 182 (17.3) |
| 2 | 29 363 (42.4) | 5450 (42.3) | 34 813 (42.4) |
| 3 | 15 889 (23.0) | 3590 (27.9) | 19 479 (23.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 4039 (5.8) | 1019 (7.9) | 5058 (6.2) |
| ER | |||
| Positive | 52 522 (75.9) | 9743 (75.7) | 62 265 (75.8) |
| Negative | 8190 (11.8) | 1813 (14.1) | 10 003 (12.2) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1057 (1.5) | 207 (1.6) | 1264 (1.5) |
| PR | |||
| Positive | 44 385 (64.1) | 8136 (63.2) | 52 521 (64.0) |
| Negative | 16 256 (23.5) | 3407 (26.5) | 19 663 (24.0) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 1128 (1.6) | 220 (1.7) | 1348 (1.6) |
| HER2 | |||
| Positive | 7701 (11.1) | 1832 (14.2) | 9533 (11.6) |
| Negative | 50 934 (73.6) | 9358 (72.7) | 60 292 (73.4) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 3134 (4.5) | 573 (4.5) | 3707 (4.5) |
| Ki67 | |||
| Low | 16 797 (24.3) | 2681 (20.8) | 19 478 (23.7) |
| Intermediate | 5842 (8.4) | 951 (7.4) | 6793 (8.3) |
| High | 17 981 (26.0) | 3786 (29.4) | 21 767 (26.5) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 21 149 (30.6) | 4345 (33.8) | 25 494 (31.1) |
| Subtype | |||
| Luminal A | 23 861 (34.5) | 3670 (28.5) | 27 531 (33.5) |
| Luminal B | 12 525 (18.1) | 2654 (20.6) | 15 179 (18.5) |
| HER2+ HR+ | 5274 (7.6) | 1242 (9.7) | 6516 (7.9) |
| HER2+ HR– | 2384 (3.4) | 572 (4.4) | 2956 (3.6) |
| Triple-negative | 5175 (7.5) | 1117 (8.7) | 6292 (7.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 12 550 (18.1) | 2508 (19.5) | 15 058 (18.3) |
| Subtype (other definition) | |||
| Luminal A (NHG 1) | 11 555 (16.7) | 1551 (12.1) | 13 106 (16.0) |
| Luminal NHG 2 | 24 945 (36.0) | 4580 (35.6) | 29 525 (36.0) |
| Luminal B (NHG 3) | 7514 (10.9) | 1695 (13.2) | 9209 (11.2) |
| HER2+HR+ | 5274 (7.6) | 1242 (9.7) | 6516 (7.9) |
| HER2+ HR– | 2384 (3.4) | 572 (4.5) | 2956 (3.6) |
| Triple-negative | 5175 (7.5) | 1117 (8.7) | 6292 (7.7) |
| In situ | 7458 (10.8) | 1112 (8.6) | 8570 (10.4) |
| Missing | 4922 (7.1) | 1006 (7.8) | 5928 (7.2) |
| Nodal stage | |||
| N0 | 50 086 (72.4) | 7225 (56.1) | 57 311 (69.8) |
| N1 | 14 256 (20.6) | 4046 (31.4) | 18 302 (22.3) |
| N2 | 3180 (4.6) | 1039 (8.1) | 4219 (5.1) |
| N3 | 1322 (1.9) | 499 (3.9) | 1821 (2.2) |
| Missing | 383 (0.6) | 66 (0.5) | 449 (0.6) |
Values are n (%). SSI, surgical site infection; Tis, carcinoma in situ; NST, no special type (former ductal); ER, oestrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NHG, Nottingham histological grade; Ki67, proliferation index.
Overall, 12 875 patients (15.7%) experienced an SSI within 90 days of surgery, of whom 1.3% had a major SSI; 9.5% had an SSI within 30 days of surgery (Table S2).
A total of 2770 patients (3.7%) had an LRR and 7033 (9.6%) systemic recurrence. Five- and 10-year DRFS rates were 91.2% (95% c.i. 90.9 to 91.4) and 84.6% (95% c.i. 84.1 to 85.0) for patients without an SSI, compared with 87.6% (95% c.i. 86.9 to 88.2) and 80.7% (95% c.i. 79.5 to 81.7) for patients with an SSI. Five- and ten-year OS rates were 89.9% (95% c.i. 89.6 to 90.1) and 78.2% (95% c.i. 77.7 to 78.7) for patients without an SSI, compared with 87.1% (95% c.i. 86.4 to 87.8) and 74.1% (95% c.i. 72.8 to 75.3) for patients with an SSI. Five- and 10-year BCSS rates were 95.7% (95% c.i. 95.5 to 95.8) and 92.0% (95% c.i. 91.7 to 92.3) for patients without an SSI compared with 93.5% (95% c.i. 93.0 to 94.0) and 88.6% (95% c.i. 87.7 to 89.5) for patients with an SSI.
On unadjusted analysis, the risk of systemic recurrence (HR 1.36, P < 0.001), overall death (HR 1.26, P < 0.001) and breast cancer death (HR 1.49, P < 0.001) were all significantly increased after SSI, but not the risk of LRR (HR 0.92, P = 0.132) (Fig. 2). After adjustment for age, country of birth, highest level of education, family income, CCI, region of residence, primary treatment (primary surgery or NAT), final breast and axillary surgery, number of surgeries, tumour stage, subtype and nodal stage, the occurrence of SSI was still significantly associated with higher overall death (HR 1.06, P = 0.030), but not with systemic recurrence (HR 1.05, P = 0.089) or breast cancer death (HR 1.07, P = 0.102) (Table 3). In the sensitivity analysis excluding all patients with any kind of malignancy before breast cancer diagnosis (n = 7418), the risk of systemic recurrence was not significant (HR 1.04, P = 0.171) and neither was the association with all-cause death (HR 1.05, P = 0.098).
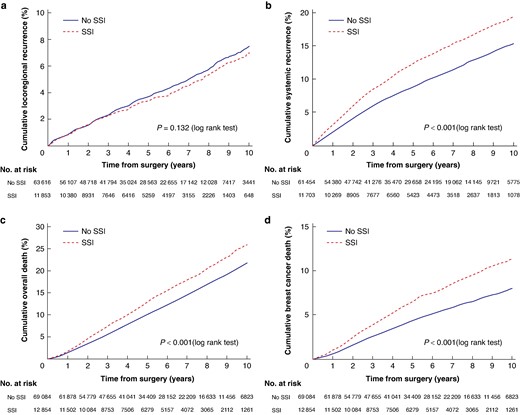
Kaplan–Meier analysis of patients with and without SSI
a Locoregional recurrence. b Systemic recurrence. c Overall death. d Breast cancer death. SSI, surgical site infection.
Adjusted Cox regression analysis of risk of SSI on time to locoregional recurrence, systemic recurrence, overall death and breast cancer death
| Endpoint . | No. of patients . | HR (95% c.i.) . | P . |
|---|---|---|---|
| Locoregional recurrence | 75 469 | 0.98 (0.88,1.09) | 0.657 |
| Systemic recurrence | 73 157 | 1.05 (0.99,1.12) | 0.089 |
| Overall death | 81 938 | 1.06 (1.01,1.11) | 0.030 |
| Breast cancer death | 81 938 | 1.07 (0.99,1.15) | 0.102 |
| Endpoint . | No. of patients . | HR (95% c.i.) . | P . |
|---|---|---|---|
| Locoregional recurrence | 75 469 | 0.98 (0.88,1.09) | 0.657 |
| Systemic recurrence | 73 157 | 1.05 (0.99,1.12) | 0.089 |
| Overall death | 81 938 | 1.06 (1.01,1.11) | 0.030 |
| Breast cancer death | 81 938 | 1.07 (0.99,1.15) | 0.102 |
Adjusted for age, country of birth, highest level of education, family income, CCI, region of residence, primary treatment, final breast and axillary surgery, number of surgeries, tumour stage, subtype and nodal stage. SSI, surgical site infection; CCI, Charlson Co-morbidity Index.
Adjusted Cox regression analysis of risk of SSI on time to locoregional recurrence, systemic recurrence, overall death and breast cancer death
| Endpoint . | No. of patients . | HR (95% c.i.) . | P . |
|---|---|---|---|
| Locoregional recurrence | 75 469 | 0.98 (0.88,1.09) | 0.657 |
| Systemic recurrence | 73 157 | 1.05 (0.99,1.12) | 0.089 |
| Overall death | 81 938 | 1.06 (1.01,1.11) | 0.030 |
| Breast cancer death | 81 938 | 1.07 (0.99,1.15) | 0.102 |
| Endpoint . | No. of patients . | HR (95% c.i.) . | P . |
|---|---|---|---|
| Locoregional recurrence | 75 469 | 0.98 (0.88,1.09) | 0.657 |
| Systemic recurrence | 73 157 | 1.05 (0.99,1.12) | 0.089 |
| Overall death | 81 938 | 1.06 (1.01,1.11) | 0.030 |
| Breast cancer death | 81 938 | 1.07 (0.99,1.15) | 0.102 |
Adjusted for age, country of birth, highest level of education, family income, CCI, region of residence, primary treatment, final breast and axillary surgery, number of surgeries, tumour stage, subtype and nodal stage. SSI, surgical site infection; CCI, Charlson Co-morbidity Index.
Other complications
There were 5710 patients (7.0%) who suffered bleeding or wound complications and 1663 patients (2.0%) experienced an unspecified complication (Table S2). After adjustment for age, country of birth, highest level of education, family income, CCI, region of residence, primary treatment, type of final breast and axillary surgery, number of surgeries, tumour stage, subtype and nodal stage, the occurrence of unspecified complication was significantly associated with systemic recurrence (HR 1.22, P = 0.005) but not with all-cause death (HR 1.07, P = 0.298), breast cancer death (HR 1.14, P = 0.183) or LRR (HR 1.19, P = 0.171) (Table S3). The significant association remained in the sensitivity analysis (HR 1.22, P = 0.006). Bleeding or wound complication were not significantly associated with any of the outcomes. A total of 17 294 patients (21.1%) experienced any local complication within 90 days, of which 3.9% were major (Table S2). After adjustment for the same predictors as above, the occurrence of any major local complication was significantly associated with all-cause death (HR 1.11, P = 0.027), but not with systemic recurrence (HR 1.08, P = 0.184), LRR (HR 0.95, P = 0.628) or breast cancer death (HR 1.05, P = 0.526) (Table S4). The significant association with all-cause death did not remain in the sensitivity analysis (HR 1.09, P = 0.106).
For secondary analysis concerning risk factors for developing SSI, see Table S5.
Discussion
In this nationwide cohort study, SSI was not significantly associated with systemic recurrence, LRR or BCSS, but was associated with worse OS. An elevated relative risk of systemic recurrence less than 8%, corresponding to an absolute risk difference of 0.8–1.6% if risk of recurrence is estimated to be 10–20%, cannot be refuted with the present study. However, such a risk difference is small from a clinical perspective. Other studies have shown a considerably higher relative risk of systemic recurrence in patients experiencing an SSI after breast cancer surgery. For example, Murthy et al.6 showed a more than two-fold increased risk of systemic recurrence in patients with wound complications than in those without (HR 2.52 (1.69–3.77)) and Beecher et al.7 demonstrated a six-fold higher risk of breast cancer recurrence (HR 6.15 (3.33, 11.33)) in patients with SSI following immediate breast reconstruction. Based on the current results, an elevated risk of systemic recurrence of that magnitude after SSI following breast cancer surgery in a general population of breast cancer patients is not likely to be true.
Although the adjusted analysis was non-significant, the unadjusted analysis showed a higher risk of systemic recurrence in patients suffering an SSI, suggesting that confounding influences the risk of recurrence. ALND is a well known risk factor for SSI11,44, and axillary lymph node metastasis also increases the risk of systemic recurrence43. Hence, this is likely an important confounding factor. In the study by Murthy et al., all patients went through ALND, while in the present study most patients underwent sentinel lymph node biopsy only, which may influence the results. Murthy et al. and Beecher et al. also used the Nottingham Prognostic Index (good, intermediate and poor), calculated from histological grade 1, 2 or 3+ nodal status (no positive nodes = 1, 1–3 nodes = 2 and more than 3 nodes positive = 3) + 0.2 × size of tumour in cm, while the present study calculated those predictors one by one. It is important to adjust for potential confounders affecting both risk of SSI and oncological outcome to reduce the risk of overestimation of complication influence. Many of the published studies were comparably small and limited by missing data when performing multivariable adjustments, increasing the risk of uncontrolled confounding6,7,9,13. The definition of complication and length of follow-up also differ among the studies contributing to the diverse results. Moreover, there are several published studies that have not shown an association between complications and oncological outcome11,12,14–16.
Patients suffering an SSI had a significantly longer time to initiation of adjuvant radiotherapy regardless of whether previous adjuvant chemotherapy had been given or not. There are data supporting that cancer treatment delay may have an impact on the oncological outcome in a negative way, with a longer delay further worsening the outcome45–47.
In the present study, patients suffering an SSI had an increased unadjusted absolute 5-year risk of all-cause death of 2.8% (95% c.i. 2.3 to 3.2). This is in line with another large Swedish population-based register study where the 5-year all-cause death rate was 6.2% (95% c.i. 4.6 to 7.8) higher in patients suffering a major local complication after breast cancer surgery10. That study showed, like the present one, that more extensive surgery was significantly associated with a higher rate of local complications. In both studies, patients suffering from postoperative complications had a higher co-morbidity burden and it is likely that this also had an impact on OS. Any major complication was associated with all-cause death in both studies. Thus, in the present study, the association between SSI/any major complication and OS did not remain significant in the sensitivity analysis, possibly due to patients dying from other types of cancer (competing cause of death). One can speculate as to whether a postoperative complication after breast cancer surgery can affect dormant micrometastases from other types of cancer and promote a worse oncological outcome. The HR for all-cause death was almost the same in the sensitivity analysis (SSI 1.05/any major complication 1.09) as in the whole cohort (SSI 1.06/any major complication 1.11), which indicates a power issue. However, there was no big difference in OS between patients suffering an SSI/any major complication and those who did not.
There are many reasons to strive to reduce complications after breast cancer surgery. An SSI can delay the start of adjuvant treatment, cause morbidity, increase costs and lead to failed reconstructions. Crucially, the principle of surgical de-escalation should be prioritized: opting for BCS over mastectomy when both options are viable, utilizing NAT to facilitate BCS and targeted axillary dissection instead of ALND. Additionally, meticulous patient selection for immediate reconstruction and oncoplastic BCS is paramount.
The main strength of this study includes the cohort size, the national population-based setting with registries of high validity, essentially complete coverage and complete follow-up. Another strength is that comprehensive data on many potential confounders were available, allowing for adjustments in the multivariable analysis. Limitations include the absence of information of smoking habits, alcohol consumption, BMI, time to adjuvant chemotherapy and usage of prophylactic antibiotics. Another limitation might be a potential under-reporting of systemic and locoregional recurrences to the registers, which would lead to an underestimation of the true recurrence rate. However, in the present study (with a median follow-up of 4.8 years) 9.6% of the patients developed a systemic recurrence, which is in line with a previous Swedish study, where 7.5% of breast cancer patients diagnosed between 2009 and 2016 developed systemic recurrence48, which supports that the reporting of recurrence within the present study is adequate. The median follow-up is, however, fairly short given the continued risk of late recurrences in ER positive breast cancer.
Also, since the incidence of SSI is in line with previously published studies8,21, it is believed that the current SSI definition is adequate. Regarding patients undergoing reconstruction, the SSI definition is probably not that reliable, since patients with primary implant-based reconstruction are likely to receive prolonged antibiotic prophylaxis or antibiotic treatment even with indefinite SSI symptoms.
The shorter than presumed median follow-up and the lower rates of recurrence than anticipated in the power calculation should be taken into consideration. However, in a post hoc examination of the sample size analysis a higher rate of SSI (15.7%) than anticipated (10%) should also be considered. If looking at the actual adjusted HR of 1.05 of systemic recurrence in patients with SSI and the corresponding 95% c.i. (0.99 to 1.12), it is evident that a relative recurrence rate difference greater than 12% would be statistically unlikely. In the current cohort, with an absolute risk of recurrence of 9.6%, a 12% relative increase would translate to an increase to roughly a 10.8% absolute risk of systemic recurrence, thus an increase in absolute risk of approximately 1.2%.
SSI following breast cancer surgery does not significantly impact the risk of systemic recurrence in this study. These findings hold important clinical implications, providing reassurance to both patients and physicians that the risk of systemic recurrence after SSI is not increased to clinically relevant levels and does not necessitate more aggressive adjuvant treatment or follow-up. However, it remains essential to continue efforts to minimize complication rates.
Funding
This work was funded by the Percy Falk Foundation, Sweden.
Acknowledgements
The authors thank Hans Pettersson, statistician South general hospital Stockholm and Marcus Thuresson, statistician Statisticon Uppsala for help with the statistics.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data are not publicly available due to restrictions by Swedish and European law, in order to protect patient privacy. Data are available from the register holder of BCBaSe 3.0 for researchers with relevant ethical approvals and who meet the criteria for access to confidential data.
Author contributions
Linda Adwall (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing), Irma Fredriksson (Conceptualization, Methodology, Resources, Supervision, Validation, Writing—review & editing), Hella Hultin (Conceptualization, Funding acquisition, Methodology, Writing—review & editing), Maria Mani (Conceptualization, Writing—review & editing) and Olov Norlén (Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing—review & editing).
References
Author notes
A conference abstract of this work was submitted to International Surgical Week, Karlstad, Sweden, August 2024, which was published in BJS.



