-
PDF
- Split View
-
Views
-
Cite
Cite
Stasia Winther, Espen Jimenez-Solem, Martin Sillesen, Association of postoperative opioid type with mortality and readmission rates: multicentre retrospective cohort study, BJS Open, Volume 8, Issue 6, December 2024, zrae113, https://doi.org/10.1093/bjsopen/zrae113
Close - Share Icon Share
Abstract
Opioid treatment in postoperative pain management is crucial, but the impact of administration practices on outcomes is unclear. The hypothesis was that prescription trends remained stable over recent years, and that no difference in mortality and readmission risks is associated with prescription strategies.
Electronic health records of surgical episodes in the Capital and Zealand Regions of Denmark from 2017 to 2021 were analysed. All opioids administered during postoperative admission were converted to oral morphine equivalents (OMEQs) and an average daily dose per patient was calculated. The opioid administered in the highest OMEQ dosages is considered the primary opioid strategy for the surgical case. Administration trends were analysed through linear regression, and Cox regression was used to calculate hazard ratios to assess dominant opioid strategies’ association with 90-day mortality and readmission rates while controlling for confounders.
A total of 183 317 patients met the inclusion criteria. Prescription trends remained steady during the study period. Multivariable analysis revealed increased readmission risk (HR 1.18, P < 0.001) of tramadol and tapentadol compared to morphine. They exhibited decreased 90-day mortality risk (HR 0.63, P < 0.001). Oxycodone had similar readmission risk (HR 1.009, P = 0.24) but lower 90-day mortality risk (HR 0.68, P < 0.001).
Postoperative in-hospital opioid administration remained stable from 2017 to 2021. Tramadol/tapentadol had a higher risk of readmission but lower mortality risk. Oxycodone had comparable readmission but reduced mortality risk. This study provides a framework for future clinical trials assessing this potential impact of opioids in a targeted manner.
Introduction
Worldwide, more than 230 million surgical procedures are performed each year1, addressing an estimated 28% of the global burden of disease2. A key component of ensuring optimal outcomes following surgery is effective pain management strategies capable of alleviating postoperative pain while minimizing unwanted short- and long-term side effects.
Although strategies involving neuraxial and peripheral nervous blockades have proven effective, systemic treatment with analgesics such as opioids are still the basis of perioperative pain management and recommended in consensus guidelines3,4.
Reports have indicated more than a doubling of the use of opioids in the general population from 2001 to 20135, which may in part be related to wide variations in prescription practices across healthcare systems for postoperative pain management6 and an associated risk of moving into chronic usage following surgery7 with an increased mortality risk for long-term users8.
Although studies have indicated differences in efficacy and risk profiles of different subtypes of opioids (for example morphine, oxycodone, tramadol, tapentadol and fentanyl)9, guidelines recommending a multimodal analgesic approach offer no recommendations on specific opioid choice3,4. However, the importance of such data-driven guidelines is underlined by reports indicating a potentially elevated risk of dependency and death associated with oxycodone treatment in surgical and general cohorts10,11, whereas others have reported superior analgesic effects of this opioid subtype9. Elevated mortality risk has also been reported for tramadol in a cohort of osteoarthritis patients12. Whether comparable effects may affect surgical patients remains less well studied.
Collectively, there are conflicting data from general and surgical cohorts. There are no official guidelines to support the use of a specific opioid subtype in the postoperative setting.
The objective of this study was to use a large electronic health record (EHR) data set covering half of the population of Denmark over a 4-year period, to assess the association between the dominant opioid pain management strategy of choice.
The hypothesis was that 90-day mortality and readmission rates would not be associated with opioid prescription practices, and that prescription practices did not change between 2017 and 2021.
Methods
The study adhered to relevant legislature for healthcare studies in Denmark. Institutional Review Board oversight on access to EHR data was approved by the Danish Board of Patient Safety (Styrelsen for patientsikkerhed, approval #31-1521-182) and reported to the Danish Capital Region data security centre (Videncenter for dataanmeldelser, approval #P-2020-180). In accordance with Danish law, research on de-identified retrospective healthcare data is exempt from the requirement of patient consent, but subject to the above-mentioned approvals from the relevant governing bodies. As such, patient consent was not obtained. The study adhered to STROBE guidelines13.
Data source and cohort
The Danish Capital and Zealand regions collectively offer a full range of healthcare services to approximately 2.6 million citizens through public hospitals systems, spanning 18 different hospitals performing surgical procedures. These include both rural and tertiary referral centres caring for a full range of surgical diseases as well as handling readmissions and postoperative follow-up.
These two regions share the same EHR system based on the EPIC platform. Of note, the EHR system only covers in-hospital treatment and medications, with out-of-hospital prescriptions covered by a separate, national system unavailable for the purpose of this study.
Information on both in-hospital and out-of-hospital mortality rates is, however, logged in the EHR system.
From the EHR system, data were extracted from all in-patient surgical procedures performed between 2017 and 2021 (both included) across all surgical subspecialties and included hospitals. Patients with hospital admission < 24 h were excluded from the data set. Patients having surgical procedures performed at private clinics outside of the public healthcare system did not have data in the public EHR system and thus were not included in this study.
Cohort groups
The administration of all systemic opioids during admission was extracted as defined by relevant Anatomical Therapeutical Chemical (ATC) groups N02A* (please see Table S1 for definitions) and converted into oral morphine equivalent dosages (OMEQs) using the conversion factors shown in Table S2. Then, the mean daily OMEQs per patient during their hospital admission was calculated. Administration of the opioid was defined as the sequence of prescription with subsequent administration and verified intake as recorded in the EHR system.
As patients may receive treatment with multiple opioid subtypes during admission, the cohort groups were categorized based on the most prevalent opioid treatment subtype during the primary surgical admission. Therefore, the dominant opioid treatment strategy was defined as the type of opioid administered in the highest OMEQ dosages per patient during hospital admission.
Groups were defined as patients treated predominantly with morphine, oxycodone, tramadol/tapentadol and other (fentanyl, ketobemidone, hydromorphone and buprenorphine) as well as no opioid treatment. All groups could also receive non-opioid analgesics not registered in this study.
Study endpoints
The primary endpoint was all-cause 90-day mortality rate. Time of the surgical procedure was defined as the follow-up start time. Both in- and out-of-hospital mortality rates were registered.
The secondary endpoint was defined as all-cause 90-day readmission rate, defined as any admission registered in the EHR system to any public hospital within 90 days of the surgical procedure. Patients admitted for more than 90 days following surgery were excluded from this endpoint.
Statistical models
Data are presented as medians and interquartile range or percentages where appropriate. For assessing the association of dominant opioid treatment strategy see the cohort groups above. Univariable and multivariable Cox regression models were constructed to assess the opioid group with primary and secondary outcomes. HRs were used as the effect measure, using dominant morphine treatment as the reference group. For the multivariable approach, models were controlled for the potential effects of confounders, including age, BMI, sex, most recent estimated glomerular filtration rate (eGFR) before surgery and the Elixhauser co-morbidity score14. eGFR was treated as a continuous variable and values >90 were included as 90. Covariates were chosen from domain knowledge of relevant confounding factors from the scientific literature. The ASA score was not included due to shared collinearity with the Elixhauser score.
Associations were graphically visualized using Kaplan–Meier plots.
To assess whether prescription practices for the individual opioid treatments had changed over the course of the study time frame, regression modelling was used to associate median daily opioid dose with the study year. To provide more granular insight into differences in trends for individual surgical subtypes, the 25 most frequently performed surgeries were also assessed individually.
Data processing and statistical analysis was performed using Python 3.8. The Elixhauser co-morbidity score was calculated using the co-morbidity library15 with ICD-10 codes and Van Walraven weights. Kaplan–Meier analysis and Cox proportional hazards regression were conducted using the lifelines16 library. P < 0.05 was considered statistically significant.
Results
In total, data from 1 396 069 surgical episodes from 2017 to 2021 were extracted from the EHR system, with 183 317 patients meeting inclusion criteria. Figure 1 presents a graphical overview of the patient selection process.
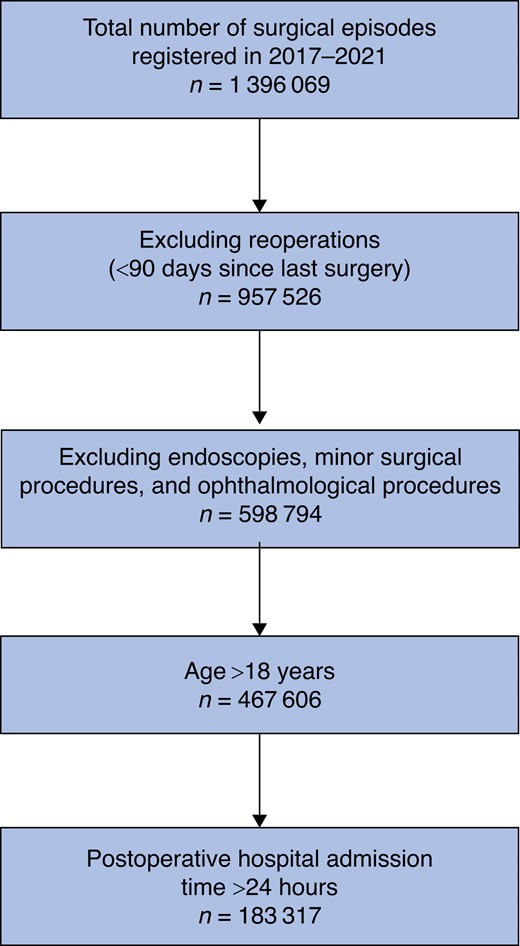
Table 1 presents a demographic overview of the cohort stratified on predominant opioid treatment strategy. Table S3 demonstrates the results of significance testing between groups. Oxycodone was the most prominent dominant opioid treatment strategy (67 059 episodes) followed by morphine (60 065 episodes), tramadol/tapentadol (10 364) episodes and other strategies (2378 episodes). In 43 451 surgical episodes, opioid treatment was not used.
| . | Morphine . | Oxycodone . | Tramadol + tapentadol . | Other . | No in-hospital opioids . |
|---|---|---|---|---|---|
| Surgical episodes, n | 60 065 | 67 059 | 10 364 | 2378 | 43 451 |
| Sex (male), % | 33.5 | 39.7 | 43.4 | 45.4 | 40.8 |
| ASA score | 2.0 (2.0–2.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 2.0 (2.0–3.0) |
| BMI (kg/m2) | 26.2 (23.1–30.2) | 26.1 (23.1–30.1) | 25.6 (22.6–29.4) | 24.8 (21.8–28.1) | 26.0 (23.1–29.6) |
| Creatinine (µmol/l) | 69.0 (59.0–82.0) | 73.0 (62.0–87.0) | 74.0 (63.0–88.0) | 74.0 (63.0–91.0) | 73.0 (62.0–87.0) |
| Age (years) | 53.6 (34.5–70.8) | 69.6 (56.6–77.9) | 66.4 (51.3–76.3) | 72.0 (62.2–79.8) | 62.7 (38.0–74.8) |
| eGFR | 90.0 (77.0–90.0) | 84.0 (68.0–90.0) | 85.0 (69.0–90.0) | 81.0 (64.0–90.0) | 87.0 (71.0–90.0) |
| Elixhauser score | 0.0 (0.0–3.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) | 4.0 (0.0–8.0) | 0.0 (0.0–4.0) |
| Hospital admission (h) | 68 (46–122) | 73 (43–149) | 76 (46–167) | 217 (139–387) | 48 (28–77) |
| . | Morphine . | Oxycodone . | Tramadol + tapentadol . | Other . | No in-hospital opioids . |
|---|---|---|---|---|---|
| Surgical episodes, n | 60 065 | 67 059 | 10 364 | 2378 | 43 451 |
| Sex (male), % | 33.5 | 39.7 | 43.4 | 45.4 | 40.8 |
| ASA score | 2.0 (2.0–2.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 2.0 (2.0–3.0) |
| BMI (kg/m2) | 26.2 (23.1–30.2) | 26.1 (23.1–30.1) | 25.6 (22.6–29.4) | 24.8 (21.8–28.1) | 26.0 (23.1–29.6) |
| Creatinine (µmol/l) | 69.0 (59.0–82.0) | 73.0 (62.0–87.0) | 74.0 (63.0–88.0) | 74.0 (63.0–91.0) | 73.0 (62.0–87.0) |
| Age (years) | 53.6 (34.5–70.8) | 69.6 (56.6–77.9) | 66.4 (51.3–76.3) | 72.0 (62.2–79.8) | 62.7 (38.0–74.8) |
| eGFR | 90.0 (77.0–90.0) | 84.0 (68.0–90.0) | 85.0 (69.0–90.0) | 81.0 (64.0–90.0) | 87.0 (71.0–90.0) |
| Elixhauser score | 0.0 (0.0–3.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) | 4.0 (0.0–8.0) | 0.0 (0.0–4.0) |
| Hospital admission (h) | 68 (46–122) | 73 (43–149) | 76 (46–167) | 217 (139–387) | 48 (28–77) |
Values are median (i.q.r.) unless otherwise stated. ASA, American Society of Anesthesiologists; BMI, body mass index; eGFR, estimated glomerular filtration rate; i.q.r., interquartile range.
| . | Morphine . | Oxycodone . | Tramadol + tapentadol . | Other . | No in-hospital opioids . |
|---|---|---|---|---|---|
| Surgical episodes, n | 60 065 | 67 059 | 10 364 | 2378 | 43 451 |
| Sex (male), % | 33.5 | 39.7 | 43.4 | 45.4 | 40.8 |
| ASA score | 2.0 (2.0–2.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 2.0 (2.0–3.0) |
| BMI (kg/m2) | 26.2 (23.1–30.2) | 26.1 (23.1–30.1) | 25.6 (22.6–29.4) | 24.8 (21.8–28.1) | 26.0 (23.1–29.6) |
| Creatinine (µmol/l) | 69.0 (59.0–82.0) | 73.0 (62.0–87.0) | 74.0 (63.0–88.0) | 74.0 (63.0–91.0) | 73.0 (62.0–87.0) |
| Age (years) | 53.6 (34.5–70.8) | 69.6 (56.6–77.9) | 66.4 (51.3–76.3) | 72.0 (62.2–79.8) | 62.7 (38.0–74.8) |
| eGFR | 90.0 (77.0–90.0) | 84.0 (68.0–90.0) | 85.0 (69.0–90.0) | 81.0 (64.0–90.0) | 87.0 (71.0–90.0) |
| Elixhauser score | 0.0 (0.0–3.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) | 4.0 (0.0–8.0) | 0.0 (0.0–4.0) |
| Hospital admission (h) | 68 (46–122) | 73 (43–149) | 76 (46–167) | 217 (139–387) | 48 (28–77) |
| . | Morphine . | Oxycodone . | Tramadol + tapentadol . | Other . | No in-hospital opioids . |
|---|---|---|---|---|---|
| Surgical episodes, n | 60 065 | 67 059 | 10 364 | 2378 | 43 451 |
| Sex (male), % | 33.5 | 39.7 | 43.4 | 45.4 | 40.8 |
| ASA score | 2.0 (2.0–2.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 2.0 (2.0–3.0) |
| BMI (kg/m2) | 26.2 (23.1–30.2) | 26.1 (23.1–30.1) | 25.6 (22.6–29.4) | 24.8 (21.8–28.1) | 26.0 (23.1–29.6) |
| Creatinine (µmol/l) | 69.0 (59.0–82.0) | 73.0 (62.0–87.0) | 74.0 (63.0–88.0) | 74.0 (63.0–91.0) | 73.0 (62.0–87.0) |
| Age (years) | 53.6 (34.5–70.8) | 69.6 (56.6–77.9) | 66.4 (51.3–76.3) | 72.0 (62.2–79.8) | 62.7 (38.0–74.8) |
| eGFR | 90.0 (77.0–90.0) | 84.0 (68.0–90.0) | 85.0 (69.0–90.0) | 81.0 (64.0–90.0) | 87.0 (71.0–90.0) |
| Elixhauser score | 0.0 (0.0–3.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) | 4.0 (0.0–8.0) | 0.0 (0.0–4.0) |
| Hospital admission (h) | 68 (46–122) | 73 (43–149) | 76 (46–167) | 217 (139–387) | 48 (28–77) |
Values are median (i.q.r.) unless otherwise stated. ASA, American Society of Anesthesiologists; BMI, body mass index; eGFR, estimated glomerular filtration rate; i.q.r., interquartile range.
Table S4 shows the number of included episodes by surgical subspecialties. Orthopaedic surgery accounted for most episodes (69 059) followed by obstetrics and gynaecology (33 450 episodes) and general surgery (28 210 episodes).
Associations with predominant opioid strategy and outcomes
Results of the Cox regression models associating opioid groups with primary endpoint (90-day mortality rate) are shown in Figs. 2, 3a,b.
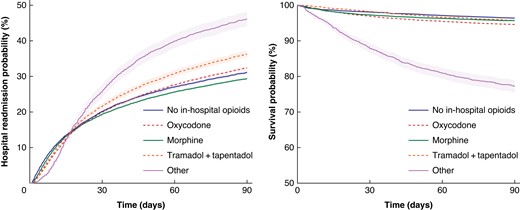
Hospital readmission risk (left) and survival probability (right) within 90 days postoperatively by opioid strategy (unadjusted)
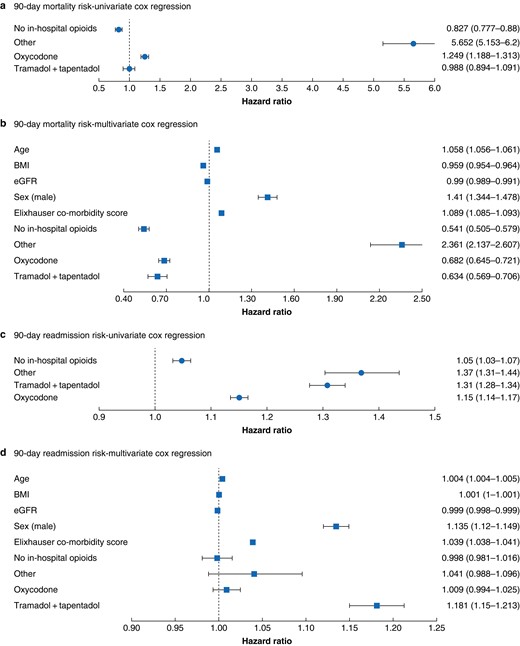
Ninety-day mortality
a,b Readmission risk. c,d Univariable and multivariable Cox regressions. Numbers represent hazard ratios (95% confidence intervals). BMI, body mass index; eGFR, estimated glomerular filtration rate.
In the univariable approach when compared with morphine as the dominant treatment strategy, oxycodone treatment was associated with higher mortality risk (HR 1.25, P < 0.001). Other opioid strategies were associated with increased mortality risks (HR 5.6, P < 0.001), whereas no opioid treatment was associated with lower mortality risk (HR 0.83, P < 0.001).
No association was found between tramadol/tapentadol treatment and mortality risk in the univariate approach (HR 0.98, P = 0.8).
When corrected for confounders in the multivariate model, no opioid treatment was associated with lower mortality rate (HR 0.54, P < 0.001) compared with morphine treatment, as was the case for oxycodone treatment (HR 0.68, P < 0.001) and tramadol/tapentadol (HR 0.63, P < 0.001). Patients treated with other opioid strategies had higher mortality risks (HR 2.36, P < 0.001).
Secondary outcome (90-day readmission risk) Cox regression results are shown in Figs. 2, 3c,d. A total of 417 patients had hospital length of stay over 90 days and were thus excluded from this analysis. When compared with morphine as the dominant strategy, univariate modelling indicated an increased risk associated with all opioid strategies including oxycodone (HR 1.15, P < 0.001), tramadol/tapentadol (HR 1.31, P < 0.001) and other strategies (HR 1.37, P < 0.001). An increased risk was also observed for no opioid treatment (HR 1.05, P < 0.001).
When assessed through a multivariable approach, association remained similar for tramadol/tapentadol (HR 1.18, P < 0.001). However, when adjusting for confounders, oxycodone treatment could no longer be associated with increased readmission risk (HR 1.009, P = 0.24) compared to morphine. Likewise, no association was found for other strategies (HR 1.04, P = 0.13) and no opioid treatment (HR 0.99, P = 0.85).
Trends in prescription practices
Figure 4 and Table S5 provide an overview of the development of in-hospital prescription trends over the course of the study for both overall morphine equivalents as well as individual opioid groups. Table S6 provides an overview of how prescription practices have developed for the 25 most frequently performed surgical procedures. Overall, no significant trend in prescription practices could be identified when opioid strategies were converted to morphine equivalents (P = 0.88).
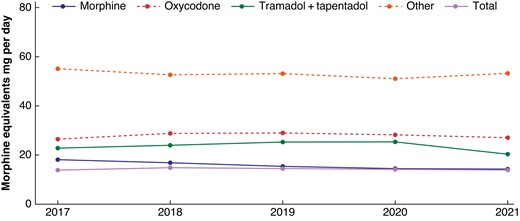
Median opioid usage (in oral morphine equivalents (OMEQs)) within 30 days of surgical procedure, per day of hospitalization over time course of the study period (2017–2021)
When analysing the differential use of opioid prescription practices, a significant reduction in morphine use was observed. Specifically, the median dosage of morphine (in OMEQs) dropped from 18.0 mg in 2017 to 14.3 mg in 2021, indicating a yearly reduction of 0.96 mg (P = 0.007). Prescription practices within the remaining pain medication strategy groups remained stable, with no significant changes observed.
Discussion
In this study, association between dominant opioid treatment strategies and outcomes (90-day mortality and readmission rates) was assessed in a large cohort of surgical patients.
The study demonstrated that both oxycodone- and tramadol/tapentadol-dominant treatment strategies were associated with lower mortality risks when compared with morphine treatment as a dominant opioid strategy, albeit other strategies (for example fentanyl-based) were associated with higher mortality risk. Not surprisingly, no use of in-hospital opioids was associated with the lowest mortality risk, presumably owing to the use in minor surgical procedures rather than intrinsic risks associated with morphine treatment.
Conversely, tramadol/tapentadol-dominant strategies were associated with a slightly higher 90-day risk of readmission compared with morphine-dominant strategies.
Over the course of the study period, administration practices did not appear to change significantly as assessed by comparing the dominant strategy for each year of the study period.
The underlying reasons for the observed results are likely multifaceted and can be affected by both in-hospital– and out-of-hospital–related factors. Improved analgesic efficacy of oxycodone over morphine has been suggested specifically for visceral pain17, a modality often occurring in non-orthopaedic operations collectively accounting for the majority of surgical subspecialties in this study. This could be due to a subtle difference in the interaction with k- and m-opioid receptors between morphine and oxycodone18, with specifically the k-receptor being important for transmitting visceral pain19. A recent meta-analysis of randomized trials comparing morphine and oxycodone treatment for acute postoperative pain did, however, find comparable analgesic as well as side effects between these two modalities9.
Although the multivariable analysis was controlled for renal function (eGFR), there could be confounding by indication, as oxycodone might have been chosen over morphine for patients with renal impairment. However, this would be expected to be associated with higher mortality and readmission rates in oxycodone-treated patients due to the underlying renal impairment, which was not observed in this study.
Finally, choice of opioid strategy could be affected by both in- and out-of-hospital differences in pricing (although these are currently minor) as well as differences in post-discharge pain strategies between regions. In line with this, it should be noted that there are currently no national guidelines dictating the use of a specific opioid strategy for postoperative patients in Denmark.
Interestingly, choice of opioids may also affect surgically relevant factors such as postoperative immune system competency. Reports have indicated that whereas morphine and tramadol treatment may be equipotent in terms of providing postoperative analgesia, morphine may be associated with a more pronounced suppression of the immune system perpetuated by the surgical stress response, specifically through suppression of natural killer cell function when compared with tramadol20. Comparable data have also been reported when morphine is compared to oxycodone21 as well as a dose-dependent immune suppression effect of fentanyl22. This collectively indicates that modulation of the surgical stress response may provide part of the explanation of the observed results. Although this should theoretically translate into higher levels of immune-mediated postoperative infectious complications, studies associating opioid prescription strategies with specific complications are lacking.
Whereas differential modulation of the surgical stress response between opioid strategy may thus be of importance for mortality rates, readmission rates should theoretically be less affected by this, although it is important to underline that there is a well-established relationship between postoperative complications and hospital readmission rates23. This study observed an association between tramadol/tapentadol strategies and readmission risk when compared to morphine. No other significant associations between dominant opioid treatment strategy and 90-day readmission risk could be demonstrated. Theoretically, the increased risk observed for tramadol-dominant strategies could be due to either insufficient pain management or underlying co-morbidities not captured in these analyses, resulting in the deviation from a morphine-dominant strategy. Conflicting reports on the association between tramadol and readmission risks following surgery exist24,25.
Although the reason for readmission cannot be assessed in this study, a potential explanation could include late complications due to inadequate pain control through increased opioid dependency and tolerance. An overview of the prescription trends over the course of the study period did not suggest that practices had changed significantly. As such, temporal variations in prescription practices are unlikely to affect the presented results.
This study investigates outcomes from a Danish surgical cohort. Whether results will be comparable on external validation in other healthcare systems including potential differences in private versus public hospital settings cannot be concluded here but could be the focus of future studies.
The study has several limitations that should be addressed. First, this is a retrospective study and only allows us to observe associations and not conclude on causality. It is important to underline that the study cannot conclude on the superiority of one opioid prescription strategy over another, but merely observe associations. In line with this, although the Cox regression models have been adjusted for relevant confounders, other clinically relevant data points (for example surgical procedure, previous opioid usage and out of hospital drug exposure) would likely have been important and could influence the presented results.
Figures S1, S2 display Cox regressions on specific subsets of the population: age < 65, males, females, and Elixhauser score < 4 (75th percentile). Overall, our findings indicate consistent associations between the dominant opioid treatment strategy and the risks of readmission and mortality in the stratified analyses across various patient characteristics. These associations align with those presented for the entire population.
A significant difference is likely to exist between opioid-naïve and opioid-tolerant patients preoperatively, but data on preoperative opioid use were not available. Differences in post-discharge prescription patterns could have affected the results. Data on these aspects were, unfortunately, not available for this study. A follow-up study assessing this is currently being planned.
In line with this, it could be speculated that rational post-discharge uses as opposed to opioid use driven by opioid dependency could impact on results. As the follow-up period is only 90 days and without data on the rationale for any post-discharge use, it is difficult to assess the potential impact this may have had.
In addition, mortality and readmission rates are high-level postoperative outcomes and are affected by the surgical procedure in question. Although this cannot be accounted for directly, the broad nature of the cohort as mentioned above ensures general applicability of the presented findings across surgical subspecialties and procedures. It would, however, be of value to assess the effects of individual complications such as postoperative infection rates in future studies.
Differences in hospital prescription patterns could affect results. Although this issue is interesting, the included hospitals form an integrated care network, with many patients being transferred between geographical locations as part of their routine care. As such, the data do not directly allow us to investigate whether any potential differences could be hospital- or patient-centric.
Finally, this study does not assess post-discharge opioid use or dominant strategy. Because the outcomes being assessed can occur after discharge, these factors could obviously affect the presented findings. Although the duration of opioid treatment would be interesting to assess, it is less likely that there will be a change in dominant strategy (for example morphine to oxycodone) following discharge.
Funding
Funded by a grant (#NNF19OC0055183) from the Novo Nordisk Foundation to Martin Sillesen.
Disclosure
The authors report no conflicts of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
Data are considered sensitive, and the authors are not at liberty to share them without permission from the governing bodies (Capital and Zealand Regions of Denmark as well as the National Board of Patient Safety of Denmark).
Author contributions
Stasia Winther (Conceptualization, Data curation, Formal analysis, Writing—original draft, Writing—review & editing), Espen Jimenez-Solem (Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing—review & editing), and Martin Sillesen (Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing—review & editing)



