-
PDF
- Split View
-
Views
-
Cite
Cite
Hongyan Zhu, Xiaowen Li, Mengxue Qiao, Xiaowen Sun, Guorong Li, Resveratrol Alleviates Inflammation and ER Stress Through SIRT1/NRF2 to Delay Ovarian Aging in a Short-Lived Fish, The Journals of Gerontology: Series A, Volume 78, Issue 4, April 2023, Pages 596–602, https://doi.org/10.1093/gerona/glad009
Close - Share Icon Share
Abstract
Aging is a complex process in which the structure and function of various tissues and organs gradually decline with age, and ovarian aging affects the reproductive capacity of females and induces age-related diseases. Resveratrol, a natural polyphenol compound, extends the life span and has a protective effect on the ovaries of vertebrates. However, the effects and underlying mechanisms of resveratrol delaying ovarian aging are unclear. In this study, using an annual fish Nothobranchius guentheri, we demonstrated that senescence-associated-beta-galactosidase (SA-β-gal) activity and lipofuscin accumulation increased with age in the ovaries, and resveratrol reversed this phenomenon. Resveratrol increased proliferating cell nuclear antigen (PCNA) expression and the oocyte proportions of the primary growth stage, cortical alveolus stage and vitellogenesis stage, and decreased the number of atretic follicles in the ovaries of 6-, 9-, and 12-month-old fish. Moreover, the expression of SIRT1 and NRF2 decreased and the levels of NF-κB, pro-inflammatory cytokines IL-1β, TNF-α, and IL-8 and endoplasmic reticulum (ER) stress markers GRP78 and CHOP increased with aging, while resveratrol up-regulated SIRT1 and NRF2 expression and down-regulated NF-κB, IL-1β, TNF-α, IL-8, GRP78, and CHOP levels in the ovaries of 6- and 9-month-old fish. In HEK293T cells, knockdown SIRT1 decreased NRF2 and increased NF-κB p65, pro-inflammatory cytokines (IL-1β and TNF-α), and ER stress marker GRP78 expression markedly. Silencing SIRT1 and then treating the cells with resveratrol significantly reversed the phenomenon. Collectively, resveratrol might activate SIRT1/NRF2 to reduce inflammation and ER stress, and finally delay ovarian aging in a short-lived fish. This study highlights the protective effect and mechanism of resveratrol on ovarian aging.
Ovary is an important reproductive organ of female organisms, and ovarian aging refers to the decline of ovarian function with age, accompanied by the decrease in the quantity and quality of ovarian follicles (1). With the development of the economy and the change in lifestyles over the last decade, more women tend to delay their child-bearing age. Thus, ovarian aging-induced reproductive problems have become a big issue (2). Therefore, it is urgent to explore the methods and mechanisms of delaying ovarian aging.
Inflammation is one of the main causes that promote aging, and nuclear factor-kappa B (NF‐κB) is a crucial mediator of inflammatory responses (3). Overexpression of NF-κB stimulates an increased inflammatory response and knockout of the nfkb1 subunit of the NF-κB activates the NF-κB-COX-2-ROS axis to induce chronic inflammation and accelerate aging in multiple tissues of mice (3,4). UVB-induced IKKα/NF-κB activation leads to the inflammatory state in the skin of mice and accelerates skin aging (5). Endoplasmic reticulum (ER) stress drives the oocyte aging process and involves in several age-related diseases such as diabetes, arteriosclerosis, cancer, and so on (6). The expression of ER stress markers, GRP78 and CHOP, is significantly increased in the heart and cerebral cortex of aging mice (7,8). ER stress-induced PI3K activation leads to follicular cell apoptosis and premature ovarian failure in rats (9). From these facts, the management of inflammation and ER stress may be effective in protecting ovaries from aging.
Sirtuin1(SIRT1), a NAD+-sensitive deacetylase, modulates the organismal life span and has beneficial effects on delaying aging (2,10,11). Nuclear factor-erythroid 2-related factor 2 (NRF2) is an important transcription factor that is involved in the anti-inflammatory and anti-oxidative responses in chronic diseases (12). Activation of the SIRT1/NRF2 signaling pathway alleviates lung injury symptoms in poisoned mice (13). Depletion of NRF2 induces the activation of NF-κB-mediated inflammation after scratch injury in mouse primary cultured astrocytes (14), and activation of NRF2 reduces ER stress and prevents senescence of canine adipose-derived mesenchymal stem cells (15).
Resveratrol, a phytoalexin polyphenolic compound mainly found in grape, peanut, soybeans, berries, and polygonum cuspidatum, has many biological effects such as anti-oxidation, anti-inflammatory, anti-aging, and anti-tumor (16). Resveratrol and organic selenium-rich fermented milk reduce cognitive dysfunction induced by D-galactose in mice (17). Resveratrol alleviates rheumatoid arthritis by reducing ROS, inflammation, and angiogenesis, and inhibiting MAPK signaling pathways in rats (18). Resveratrol treatment restores the morphology and weight of the ovaries and alleviates oogonial stem cell apoptosis and ovarian aging in chemotherapy mice (19,20). However, little is known about the mechanisms of resveratrol delaying ovarian aging. Therefore, this study aimed to investigate the effect and mechanisms of resveratrol preventing ovarian aging in a short-lived fish Nothobranchius guentheri.
Materials and Methods
Fish Culture and Treatment
This study was carried out according to the guidelines for Ethics Committee on Animal Experiments of Shandong Normal University (Permit Number: AEECSDNU2018006). The annual fish N. guentheri, with a 12-month-old mean life span, were reared in our laboratory according to the previous description (21), and the procedure for food preparation was also according to Yu et al. (21). When the fish grew to sexual maturity at 3 months old, a total of 126 female fish were randomly and equally separated into a control group feeding with standard food and resveratrol group with resveratrol-supplemented food (200 μg/g food, resveratrol, V900386-5G, Sigma, MO) until they were sacrificed in 3 stages, 6-, 9-, and 12-month old, respectively. The ovaries were freshly obtained from the fish as rapidly as possible to minimize suffering and fixed in 4% formaldehyde solution for 24 hours.
Cell Culture and siRNA Knockdown
The human embryonic kidney 293T (HEK293T) cells were obtained from American Type Culture Collection (Manassas, VA). HEK293T cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin sulfate (Solarbio, Beijing, China) at 37°C in a humidified incubator with 5% CO2.
Control and specific siRNA directed against of SIRT1 were from Guangzhou RiboBio. The cells were transfected with (50 nM) control siRNA or anti-SIRT1 siRNA by using lipofectamine 2000 (Invitrogen, Waltham, MA) according to the manufacturer’s instruction. The transfection medium was replaced with fresh low-serum medium 6 hours post-transfection. The cells were incubated for 36 hours, and then collected for subsequent experiments.
SA-β-gal and Lipofuscin
The fixed ovaries were dehydrated with 30% sucrose solution and embedded in optical cutting temperature compound (Sakura Finetek, Tokyo, Japan) at −20°C and sectioned at thickness of 10 μm on a microtome (MEV, Slee Medical, Mainz, Germany). The frozen sections were stained with X-gal staining solution overnight at 37°C according to the manufacturer’s instructions (Cell Signaling Technology, MA) to detect SA-β-gal activity. The frozen sections were sealed with mounting medium mixed with glycerin and gelatin to observe lipofuscin accumulation using a fluorescent microscope after drying.
Histological Evaluation of Ovarian Development
The frozen sections were stained with hematoxylin and eosin, and the histological structure of the ovaries was observed using a light microscope and computer (3DHISTECH, Budapest, Hungary). Five fish were used for oocyte counts in the ovaries of control and resveratrol-treated fish, respectively. Each section was scanned for morphological analysis, and every fifth section was used to quantify the total number of oocytes at different developmental stages and the oocyte development process was classified as previous description (22).
Immunohistochemical Analysis
The frozen sections were dried at room temperature for 10 minutes and incubated with primary antibodies proliferating cell nuclear antigen (PCNA) (1:200, Santa Cruz Biotechnology, TX), SIRT1 (1:200, Cell Signaling Technology) and GRP78 (1:600, ABclonal Technology, Wuhan, China) overnight at 4°C according to previous description of immunohistochemical staining with some modification (23).
RNA Extraction and Quantitative Real-time PCR Assay
The expression of NRF2, NF-κB, IL-1β, TNF-α, IL-8, GRP78, and CHOP at mRNA level was detected using quantitative real-time PCR (qPCR). Total RNA was extracted from the ovaries using the RNA simple Total RNA Kit (TIAN GEN, Beijing, China) and rapidly reverse-transcribed to cDNA with Fast-Quant cDNA (TIAN GEN, Beijing, China) according to the manufacturer’s instructions, and qPCR was performed as described previously using SYBR green real-time PCR master mix (TIAN GEN, Beijing, China) (22). The primer sequences are listed in Table 1.
| Gene . | Primer Sequence (5′-3′) . |
|---|---|
| NRF2 | F : GCACAGCTATTGCCTTCTCC |
| R : CAGCGTGGCAATGATCTCTA | |
| NF-κB | F : CTTCAGCCACTCCACCTAGC |
| R : GTCCACTCTTCAGCTCCTGG | |
| IL-1β | F : GGTGCTGGAGAGCATCGTAG |
| R : GGAAAACTTTGCGGGAGTCG | |
| TNF-α | F : GCGGCCTGTACTTCGTCTAC |
| R : AGGTAAATGGCGCTGTAGCA | |
| IL-8 | F : GCACAGCTATTGCCTTCTCC |
| R : CAGCGTGGCAATGATCTCTA | |
| GRP78 | F : GCCGTGTTGAGATCATTGCC |
| R : GAACAGAAGAGTCGCCCCAA | |
| CHOP | F : TCCACGGGAACCTGACTCT |
| R : GACTTCACCCTCATCCGGTTC | |
| β-actin | F : CACCTTCTACAATGAGCTCCGT |
| R : GCAGGAGTGTTGAAGGTCTCAA |
| Gene . | Primer Sequence (5′-3′) . |
|---|---|
| NRF2 | F : GCACAGCTATTGCCTTCTCC |
| R : CAGCGTGGCAATGATCTCTA | |
| NF-κB | F : CTTCAGCCACTCCACCTAGC |
| R : GTCCACTCTTCAGCTCCTGG | |
| IL-1β | F : GGTGCTGGAGAGCATCGTAG |
| R : GGAAAACTTTGCGGGAGTCG | |
| TNF-α | F : GCGGCCTGTACTTCGTCTAC |
| R : AGGTAAATGGCGCTGTAGCA | |
| IL-8 | F : GCACAGCTATTGCCTTCTCC |
| R : CAGCGTGGCAATGATCTCTA | |
| GRP78 | F : GCCGTGTTGAGATCATTGCC |
| R : GAACAGAAGAGTCGCCCCAA | |
| CHOP | F : TCCACGGGAACCTGACTCT |
| R : GACTTCACCCTCATCCGGTTC | |
| β-actin | F : CACCTTCTACAATGAGCTCCGT |
| R : GCAGGAGTGTTGAAGGTCTCAA |
| Gene . | Primer Sequence (5′-3′) . |
|---|---|
| NRF2 | F : GCACAGCTATTGCCTTCTCC |
| R : CAGCGTGGCAATGATCTCTA | |
| NF-κB | F : CTTCAGCCACTCCACCTAGC |
| R : GTCCACTCTTCAGCTCCTGG | |
| IL-1β | F : GGTGCTGGAGAGCATCGTAG |
| R : GGAAAACTTTGCGGGAGTCG | |
| TNF-α | F : GCGGCCTGTACTTCGTCTAC |
| R : AGGTAAATGGCGCTGTAGCA | |
| IL-8 | F : GCACAGCTATTGCCTTCTCC |
| R : CAGCGTGGCAATGATCTCTA | |
| GRP78 | F : GCCGTGTTGAGATCATTGCC |
| R : GAACAGAAGAGTCGCCCCAA | |
| CHOP | F : TCCACGGGAACCTGACTCT |
| R : GACTTCACCCTCATCCGGTTC | |
| β-actin | F : CACCTTCTACAATGAGCTCCGT |
| R : GCAGGAGTGTTGAAGGTCTCAA |
| Gene . | Primer Sequence (5′-3′) . |
|---|---|
| NRF2 | F : GCACAGCTATTGCCTTCTCC |
| R : CAGCGTGGCAATGATCTCTA | |
| NF-κB | F : CTTCAGCCACTCCACCTAGC |
| R : GTCCACTCTTCAGCTCCTGG | |
| IL-1β | F : GGTGCTGGAGAGCATCGTAG |
| R : GGAAAACTTTGCGGGAGTCG | |
| TNF-α | F : GCGGCCTGTACTTCGTCTAC |
| R : AGGTAAATGGCGCTGTAGCA | |
| IL-8 | F : GCACAGCTATTGCCTTCTCC |
| R : CAGCGTGGCAATGATCTCTA | |
| GRP78 | F : GCCGTGTTGAGATCATTGCC |
| R : GAACAGAAGAGTCGCCCCAA | |
| CHOP | F : TCCACGGGAACCTGACTCT |
| R : GACTTCACCCTCATCCGGTTC | |
| β-actin | F : CACCTTCTACAATGAGCTCCGT |
| R : GCAGGAGTGTTGAAGGTCTCAA |
Western Blotting
Harvested cells and obtained ovaries were lysed in RIPA lysis buffer (Beyotime, Shanghai, China) with protease inhibitors (PMSF, Solarbio, Beijing, China) for 30 minutes at 4°C. Cell and ovary lysates were centrifuged for 20 minutes at 12,000 rpm at 4°C, then supernatant was extracted and the protein concentrations were determined using a BCA protein assay kit (Tiangen, Beijing, China). The proteins were separated by SDS/PAGE (10%–12% gels), and then transferred to PVDF membranes. Blots were blocked with 5% non-fat milk for 1 hour and then probed with primary antibodies against SIRT1 and NF-κB p65 (1:1 000, Cell Signaling Technology), NRF2, IL-1β, and TNF-α (1:1 000, Santa Cruz Biotechnology), GRP78 (1:1 000, ABclonal Technology, Wuhan, China), and β-actin (1:1 000, Affinity, Cincinnati, OH) overnight at 4°C. After extensive washing with TBS-T, blots were incubated with appropriate HRP-conjugated secondary antibody for 2 hours. The protein bands were revealed by chemiluminescence using an ECL detection kit (Millipore, Billerica, MA).
Statistical Analysis
All of the assays were repeated in 3 fish independently at least. For image analysis, the right ovary of each fish was obtained and 5 transverse sections were taken at different positions from anterior to posterior, and 3 fields were selected randomly from each section. Overall, 45 images were analyzed per group to draw the results for SA-β-gal, lipofuscin and immunohistochemical staining. Image J, Image Pro Plus 6.0, GraphPad Prism 9.0 and Adobe Illustrator CC 2019 software were used to process figures. Statistical analyses were performed using Student’s t-test and 1-way analysis of variance for experiments. Results were presented as mean ± SD and values of p < .05 were considered statistically significant.
Results
Resveratrol Retarded SA-β-gal Activity and Lipofuscin Accumulation in Ovaries of the Fish
SA-β-gal accumulates with age in different animal models, and lipofuscin, an endogenous fluorescence complex, can reflect the aging of the organism intuitively. Our results showed that positive staining of SA-β-gal appeared mainly in the interfollicular stroma of the ovaries and increased significantly with the ovarian aging process by Image J analysis, and feeding resveratrol decreased the positive staining of SA-β-gal significantly at different ages (Figure 1A). There were only a small number of pale yellow pigment particles in the ovaries at the age of 6 months, and lipofuscin accumulation increased at the age of 9 months and the particles were fused together occasionally. At the age of 12 months, the pale yellow transformed into bright yellow fluorescent pigment bodies, forming clumps. Feeding resveratrol reduced the accumulation of lipofuscin significantly in the ovaries of 6-, 9-, and 12-month-old fish by Image J analysis (Figure 1B). The above results indicated that resveratrol delayed the ovarian aging of the fish.
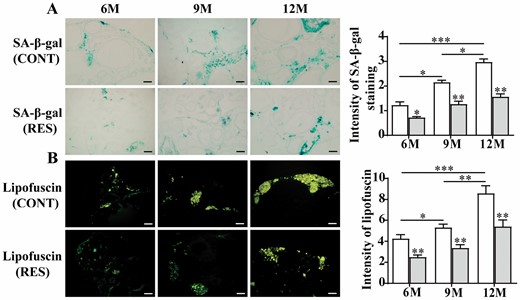
Effects of resveratrol on SA-β-gal activity and lipofuscin accumulation in the ovaries of 6-, 9-, and 12-month-old fish. (A) SA-β-gal staining and its quantification by Image J (n = 3 per group). (B) Lipofuscin accumulation and its quantification by Image J (n = 3 per group). 6M = 6-month-old; 9M = 9-month-old, 12M = 12-month-old. White: control (CONT); Gray: resveratrol (RES). Scale bar = 50 μm. The values are shown as the mean ± SD. *p < .05, **p < .01, ***p < .001.
Resveratrol Improved the Ability of Cell Proliferation and Ovarian Reserve in Ovaries of the Fish
The expression of PCNA in the nucleus of oocytes and granulosa cells was detected by immunohistochemistry. The positive staining intensity of PCNA in the ovaries of 9- and 12-month-old fish was lower than that in 6-month-old fish, and so did the staining intensity of 12-month-old than 9-month-old fish, although there is no significant difference. After feeding resveratrol, PCNA-positive staining was increased significantly in the ovaries of 6-, 9-, and 12-month-old fish (Figure 2A). Histological results showed that the ovarian tissue structure of young fish was compact, while the ovaries of old fish became loose. From the perspective of histology and nutrient accumulation, the oocyte development process was divided into oogonium (Stage 1), primary growth stage (Stage 2), cortical alveolus stage (Stage 3), vitellogenesis stage (Stage 4), and atretic stage (Atretic). With ovarian aging, the oocyte proportions of the primary growth stage, cortical alveolus stage and vitellogenesis stage decreased, and the atretic stage increased. Resveratrol treatment significantly increased the oocyte proportions of the primary growth stage, cortical alveolus stage and vitellogenesis stage, and decreased the number of atretic follicles (Figure 2B). It reflected that resveratrol improved the ability of cell proliferation and ovarian reserve in ovaries of the fish.
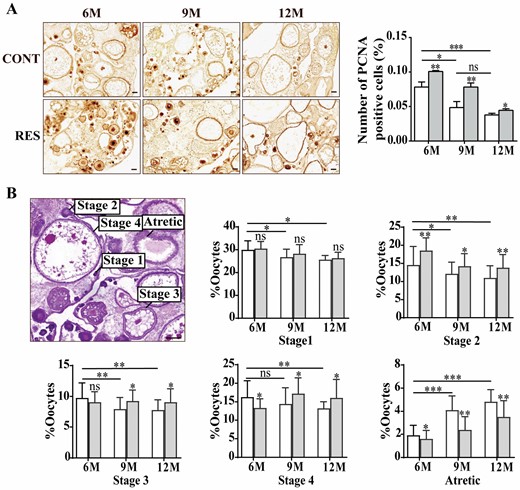
Effects of resveratrol on cell proliferation and ovarian reserve at different ages. (A) Staining of PCNA detected by immunohistochemistry and its quantitation in ovaries of the fish at 6-, 9-, and 12-month old (n = 3 per group). Scale bar = 50 μm. (B) Developmental stages and quantification of the oocytes in the ovaries of 6-, 9-, and 12-month-old fish (n = 5 per group). Stage 1, oogonium; Stage 2, primary growth stage; Stage 3, cortical alveolus stage; Stage 4, vitellogenesis stage; Atretic, atretic stage. Scale bar = 100 μm. 6M = 6-month-old; 9M = 9-month-old; 12M = 12-month-old. White: control (CONT); Gray: resveratrol (RES). The values are shown as the mean ± SD. *p < .05, **p < .01, ***p < .001, ns: no significance.
Mechanisms of Resveratrol to Retard Ovarian Aging of the Fish
Resveratrol activated SIRT1/NRF2 pathway and alleviated inflammation in the ovaries
SIRT1 plays an important role in the process of cell apoptosis, cell senescence, and tumor development. Resveratrol is an activator of SIRT1, so we detected the expression of SIRT1 in the ovaries of 6- and 9-month-old fish by immunohistochemistry. The results showed that SIRT1 protein expression in the ovaries of 9-month-old fish was significantly lower than that in 6-month-old fish, while resveratrol increased the expression of SIRT1 in the ovaries of 9-month-old fish, but had no significant effect on that in 6-month-old fish (Figure 3A). NRF2 has a variety of biological effects and promotes the maturation of mouse oocytes through the SIRT1-NRF2-Cyclin B1/CDK1 signaling pathway, which is a key factor affecting the senescence of oocytes (24). The NRF2 expression was detected using qPCR assay in ovaries of the fish at 6- and 9-month old. The results showed that the level of NRF2 decreased with age, and feeding resveratrol significantly reversed its decrease in the ovaries of 6- and 9-month-old fish (Figure 3B). The expression of NF-κB, IL-1β, TNF-α, and IL-8 was also measured by qPCR assay. The results showed that levels of NF-κB and proinflammatory cytokines IL-1β, TNF-α, and IL-8 were significantly higher in the ovaries of 9-month-old fish than that in 6-month-old fish, and resveratrol markedly down-regulated NF-κB, IL-1β, TNF-α, and IL-8 mRNA levels in the ovaries of 6- and 9-month-old fish (Figure 3C and D). Western blotting was used to confirm the expression of NRF2, NF-κB p65, and TNF-α in the ovaries of 9-month-old fish, and the results showed that feeding resveratrol significantly increased NRF2 and decreased NF-κB p65 and TNF-α levels in the ovaries of 9-month-old fish (Figure 3E). The results indicated that resveratrol increased SIRT1 and NRF2 levels and decreased inflammation response.
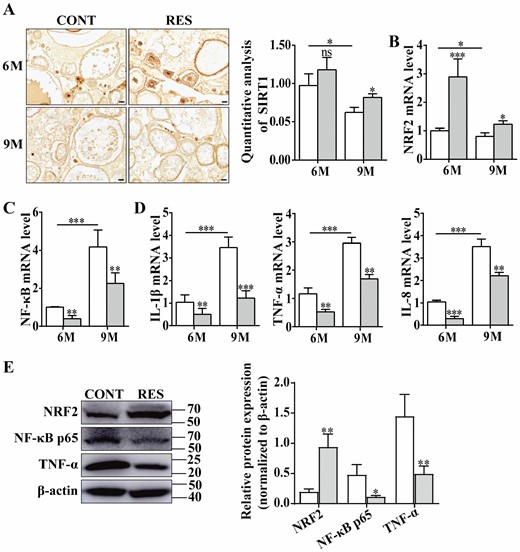
Resveratrol increased levels of SIRT1 and NRF2 and decreased the expression of NF-κB and proinflammatory cytokines in the ovaries of 6- and 9-month-old fish. (A) Immunohistochemical staining and quantitative analysis of SIRT1 in ovaries of the fish at 6- and 9-month old (n = 3 per group). Scale bar = 50 μm. (B, C, and D) Relative levels of NRF2, NF-κB, IL-1β, TNF-α, and IL-8 in the ovaries of 6- and 9-month-old fish measured by qPCR assay (n = 3 per group). (E) Western blotting was performed to detect the expression of NRF2, NF-κB p65, and TNF-α in the ovaries of 9-month-old fish compared to control group (n = 3 per group). 6M = 6-month-old; 9M = 9-month-old. White: control (CONT); Gray: resveratrol (RES). The values are shown as the mean ± SD. *p < .05, **p < .01, ***p < .001, ns: no significance.
Resveratrol inhibited ER stress in the ovaries
GRP78 normally binds to PERK, IRE1 and ATF-6, UPR transmembrane stress sensors, in a stable state of inactivation, and GRP78 dissociates from the UPR sensors when ER stress occurs (25). CHOP is the downstream signal molecule of GRP78, and the increased expression of GRP78 and CHOP reflects the activation of ER stress. ER stress is involved in mechanisms of mouse oocyte aging, and GRP78 expression is higher in aged oocytes than in fresh oocytes significantly (6). We detected the expression of GRP78 and CHOP in the ovaries of 6- and 9-month-old fish by qPCR assay. The results showed that levels of GRP78 and CHOP increased significantly with age, and feeding resveratrol markedly down-regulated the expression of GRP78 and CHOP in the ovaries of 6- and 9-month-old fish (Figure 4A and B). GRP78 protein level was further verified by immunohistochemical staining, and it mainly located in the cytoplasm (the nuclei were counterstained with hematoxylin), and its protein level elevated with age significantly and resveratrol reduced its positive staining remarkably in the ovaries of 6- and 9-month-old fish by statistical analysis, which was consistent with its mRNA level (Figure 4C), implying that resveratrol reversed the increase of ER stress with age.
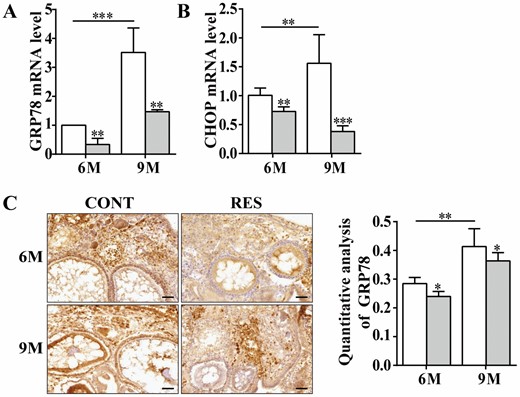
Resveratrol decreased the expression of GRP78 and CHOP in ovaries of the 6- and 9-month-old fish. (A and B) Relative levels of GRP78 and CHOP measured by qPCR assay (n = 3 per group). (C) Immunohistochemical staining and quantitative analysis of GRP78 in ovaries of the fish at 6- and 9-month old. Hematoxylin staining showed the location of the nucleus (n = 3 per group). Scale bar = 50 μm. 6M = 6-month-old; 9M = 9-month-old. White: control (CONT); Gray: resveratrol (RES). The values are shown as the mean ± SD. *p < .05, **p < .01, ***p < .001.
Resveratrol activated SIRT1/NRF2 signaling pathway to alleviate inflammation and ER stress in HEK293T cells
In order to verify the effects of SIRT1/NRF2 signaling pathway in inflammation and ER stress, we used siRNA knockdown in HEK293T cells. When SIRT1 siRNA was transfected in HEK293T cells to silence endogenous SIRT1 expression, western blotting results showed that NRF2 level decreased significantly, and the expression of NF-κB p65, pro-inflammatory cytokines (IL-1β and TNF-α) and ER stress marker GRP78 increased markedly. Silencing SIRT1 and then treating the cells with resveratrol significantly reversed the decrease of NRF2 and increase of NF-κB p65, IL-1β, TNF-α, and GRP78 induced by SIRT1 knockdown (Figure 5). The data suggested that resveratrol activated SIRT1/NRF2 signaling pathway to reduce inflammation and ER stress in HEK293T cells. Together with the above results in the fish, we speculated that resveratrol delayed ovarian aging with age by activating SIRT1/NRF2 signaling pathway to reduce inflammation and ER stress.
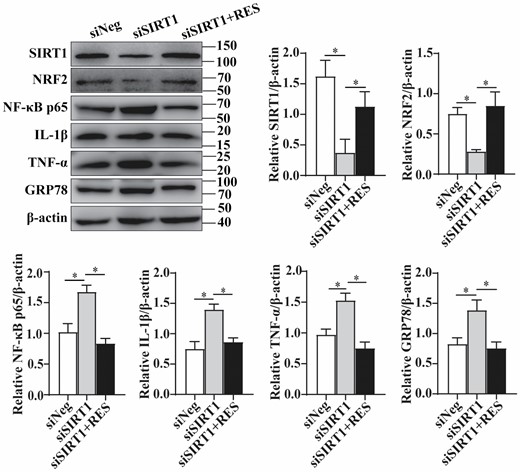
Activation of SIRT1/NRF2 signaling pathway reduced inflammation and ER stress in HEK293T cells upon resveratrol treatment. Protein levels and the quantification of SIRT1, NRF2, NF-κB p65, IL-1β, TNF-α, and GRP78 after SIRT1 siRNA transiently transfected into HEK293T cells without or with 20 mM resveratrol treatment (n = 3 per group). The values are shown as the mean ± SD. *p < .05.
Discussion
The genus Nothobranchius is a novel emerging vertebrate model for studying aging due to their short life cycle, easy rearing under laboratory conditions, and similar aging mechanism with higher vertebrates. Using N. guentheri, studies have shown that natural products and drugs such as salidroside, β-1,3-Glucans delay the onset of age-related biomarkers and extend the life span (26,27). This fish also recapitulates the age-associated changes of ovarian aging that adversely affect female fertility (28). Therefore, N. guenther is an ideal model to explore the effects and mechanisms of natural products and drugs on delaying ovarian aging. By using this excellent fish model, our previous results show that the proportion of developing oocytes decreases and the number of atretic follicles increases in the ovaries with age, and resveratrol extends the life span, improves the cognitive ability via behavioral assays and delays gut aging by SIRT1/NF-κB pathway (11,21,22). In this study, our results showed that resveratrol alleviated inflammation and ER stress through SIRT1/NRF2 pathway to delay ovarian aging in the annual fish.
Resveratrol increases the number of eggs and mid-life fecundity of female N. guentheri and protects against age-associated infertility in mice (28,29). Both resveratrol and genistein increase the ovarian follicular reserve and prolong the ovarian life span in rats (30). Resveratrol reduces arterial and kidney aging in mice and delays retinal aging in zebrafish (31–33). SA-β-gal activity and lipofuscin accumulation increase with age in the gill epithelium of the annual fish N. rachovii (34). In this study, our results showed that SA-β-gal activity and lipofuscin accumulation increased significantly in the ovaries with age, and feeding resveratrol decreased SA-β-gal activity and lipofuscin accumulation in the ovaries of 6-, 9-, and 12-month-old fish. Therefore, the results suggested that resveratrol delayed ovarian aging process.
PCNA, a marker of cell proliferation, is detected at all stages of the process of folliculogenesis in rats (35), and the expression of PCNA decreases in ovaries of cyclophosphamide-induced premature ovarian failure mice (36). Our experimental result here showed that resveratrol reversed the decline of PCNA expression with the aging process in the ovaries of 6-, 9-, and 12-month-old fish, indicating that resveratrol promoted the cell proliferation in the ovaries. Ovarian reserve is used to indicate the quantity and quality of follicles in the ovary, and depletion of the follicle pool represents a decline in the function of ovarian reserve (30). Feeding resveratrol increases the proportion of the healthy follicles and decreases the number of atretic follicles in the ovaries of aged mice and rats (29,30). This study showed that resveratrol treatment reduced the proportion of atretic follicles increased with age and increased the oocyte numbers of primary growth stage, cortical alveolus stage, and vitellogenesis stage decreased with age, indicating that resveratrol increased the ovarian reserve of the fish to a certain extent.
The activation of SIRT1 by caloric restriction extends ovarian life span, and suppressing SIRT1 signaling by high-fat diet accelerates ovarian follicle loss in rats (37). The expression of NRF2 decreases with age in mouse and human ovarian granulosa cells and NRF2-/- mice have fewer follicles and accelerated ovarian aging (24,38). In mouse type II alveolar epithelial cells, SIRT1 deacetylates NRF2 and protects against paraquat-induced injury (39). NRF2 negatively regulates NF-κB signaling activation in intracerebral hemorrhage rats (40). NF-κB activation promotes the secretion of pro-inflammatory cytokines and accelerates inflammatory responses and DNA damage-induced aging, and NF-κB inhibition slows the rate of primordial follicle growth activation and extends ovarian function in mice (41,42). NRF2 pharmacologic activation inhibits ER stress, inflammatory and oxidative stress to alleviate nonalcoholic steatohepatitis in mice (43). ER stress caused by excessive ROS induces senescence of cumulus granulosa cells from endometriosis patients (44), and alleviation of ER stress delays senescence of mouse ovarian surface epithelium cells (45). Reducing ER stress, inflammation, and apoptosis enhances oocyte quality and embryo development and attenuates ovarian aging and dysfunction in aged mice (46). GRP78 and CHOP are at the core of ER stress signaling pathway and the expression of GRP78 and CHOP increases in palmitic acid-induced apoptotic mouse granulosa cells (47). Our previous studies have shown that resveratrol activates SIRT1 to delay gut aging process and reduce the generation of age-dependent spontaneous tumors of the liver in N. guentheri (11,48). In this study, we found that resveratrol reversed the decrease of SIRT1 and NRF2 expression and the increase of NF-κB, pro-inflammatory cytokine, and ER stress marker levels with age in ovaries of the fish. We speculated that resveratrol activates SIRT1 to deacetylate NRF2 and plays a protective role in inhibition of inflammation and ER stress, finally delays ovarian aging of the fish. In HEK293T cells, we verified the effects of SIRT1/NRF2 signaling pathway in inflammation and ER stress by knockdown SIRT1 upon resveratrol treatment.
In summary, this study showed that resveratrol delayed ovarian aging, which was manifested in reducing aging-related markers SA-β-gal activity and lipofuscin accumulation and enhancing PCNA-positive staining intensity and ovarian reserve. Resveratrol might activate SIRT1/NRF2 signaling to alleviate inflammatory response and ER stress, and finally delay ovarian aging in a short-lived fish. Our findings identify a novel mechanism for resveratrol to delay ovarian aging in vertebrates and natural product resveratrol might be a prospective dietary supplement to delay ovarian aging and enhance female fertility in the near future.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31672377).
Conflict of Interest
The authors have declared no conflicts of interest.
References
Author notes
These authors contributed equally to this work.



