-
Views
-
Cite
Cite
Anchun Hu, Yanli Mu, Guanyou Huang, Zhongan Wang, Shuyun Zhao, Wenchi Xu, Panpan Chen, Xin Guo, IDO can improve ovarian function in premature ovarian insufficiency via AhR and regulatory T cells, Biology of Reproduction, 2025;, ioaf102, https://doi.org/10.1093/biolre/ioaf102
Close - Share Icon Share
Abstract
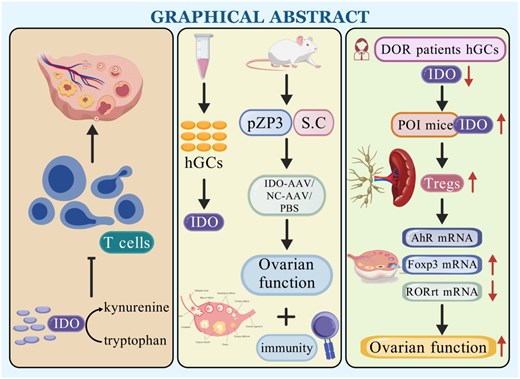
Background: Premature ovarian insufficiency (POI) is the loss of ovarian function among women <40 years of age, and immune disorders play a critical role in POI development. Indoleamine 2,3-dioxygenase (IDO) catalyses tryptophan metabolism via the kynurenine pathway and plays a key role in preventing and treating immune-related diseases. Methods: Human ovarian granulosa cells (hGCs) were collected via the density gradient method, pZP3 was used to establish an immune POI mouse model, and an AAV vector carrying IDO1 (IDO-AAV) was injected into mouse ovaries to induce IDO overexpression. Ovarian function was measured by the oestrous cycle, serum AMH concentration, degree of ovarian fibrosis and number of follicles. Findings: IDO protein levels and mRNA expression in hGCs were lower in the POI group than in the control group (P < 0.05). Both the ovarian function and IDO levels in the POI + Water group and the POI + Glu group were significantly lower than those in the control group. In POI model mice injected with IDO-AAV, ovarian function and the CD4 + CD25 + Foxp3+ regulatory T (Treg) cell proportion were increased compared with those in mice injected with the natural control AAV. The FoxP3 mRNA expression level in Treg cells was positively correlated with the IDO mRNA expression level, whereas the RORγt mRNA expression level in Th17 cells was negatively correlated with the IDO mRNA expression level, further suggesting that IDO may be related to Treg and Th17 cells through AhR and subsequently regulate immunity and ovarian function. Conclusion: Increasing ovarian IDO levels in POI mice improved ovarian function.