-
PDF
- Split View
-
Views
-
Cite
Cite
Marzia Salgarello, Mariachiara Fabbri, Giuseppe Visconti, Liliana Barone Adesi, Implant-Based Breast Reconstruction After Nipple-Sparing and Skin-Sparing Mastectomy in Breast-Augmented Patients: Prepectoral or Submuscular Direct-to-Implant Reconstruction?, Aesthetic Surgery Journal, Volume 44, Issue 5, May 2024, Pages 503–515, https://doi.org/10.1093/asj/sjad383
Close - Share Icon Share
Abstract
Patients with breast augmentation facing a breast cancer diagnosis pose unique challenges for both breast and plastic surgeons in terms of treatment and reconstruction. Traditional submuscular direct-to-implant (DTI) breast reconstruction is often considered the standard approach, regardless of the previous implant pocket. However, recent trends in prepectoral reconstruction provide an innovative solution for patients with previous subglandular and submuscular implants.
In this study we aimed to share our experiences with DTI breast reconstruction in patients with a history of breast augmentation, with a specific focus on the viability of prepectoral reconstruction.
A retrospective review was conducted on 38 patients with previous breast augmentation who underwent either skin-sparing mastectomy or nipple-sparing mastectomy for breast cancer followed by DTI reconstruction between January 2015 and July 2023. Our analysis considered various factors, including previous implant positioning, capsular and implant status, and mastectomy flap thickness (MFT), offering insights into the rationale behind choosing the new implant positioning.
Patients with a history of subglandular breast augmentation and an MFT greater than 1 cm were candidates for prepectoral reconstruction. When the MFT was less than 1 cm but flap vascularity was sufficient, a prepectoral reconstruction was performed; otherwise, retropectoral reconstruction was preferred. Patients with submuscular breast augmentation were evaluated similarly, with submuscular reconstruction chosen when the MFT was less than 1 cm and prepectoral reconstruction preferred when the MFT exceeded 1 cm.
Immediate prepectoral DTI reconstruction represents a feasible option for specific patients with a history of breast augmentation. Decisions regarding the reconstructive approach are influenced by variables such as mastectomy flap thickness, implant status, and capsular conditions.

Breast cancer is the most prevalent malignancy among females, with nearly 55,700 new cases diagnosed in Italy in 2022, and an overall 5-year survival rate of 88%.1 On the other hand, breast augmentation surgery is the most common cosmetic procedure in Italy, with over 41,412 surgeries performed in 2021.2 Consequently, we are witnessing a growing number of patients who receive a breast cancer diagnosis after having undergone breast augmentation surgery.
The choice of treatment, whether conservative surgery (quadrantectomy or lumpectomy) with subsequent radiation therapy or nonconservative procedures (mastectomy), depends on both the tumor characteristics and patient considerations.3 Patients with a history of breast augmentation surgery often present a technical challenge for immediate breast reconstruction, also due to their high aesthetic expectations. This includes young patients with a low body mass index and middle-aged females who have specific aesthetic demands related to their appearance.
Performing conservative surgeries on this patient population while retaining implants can increase the risk of implant-related complications after radiotherapy, such as infection, capsular contracture, implant exposure, and unsatisfactory cosmetic outcomes.4,5 The aesthetic results are often suboptimal due to breast deformities resulting from conservative therapy. Most surgeons agree that mastectomy with immediate reconstruction is the preferred option for patients who have previously undergone breast augmentation, because it maximizes aesthetic outcomes and reduces complication rates.6-8
However, questions remain about whether to retain the previous implant or replace it during reconstruction and how to manage both subglandular and subpectoral implants. Many authors have shared their experiences and approaches in the literature. Some choose to maintain submuscular implants in situ, incorporating them into the reconstruction, while others prefer replacing the implants, either subglandular or submuscular, with a new subpectoral implant.9-17 Given the increasing use of prepectoral reconstruction, which represents a recent paradigm shift in immediate breast reconstruction, it is essential to consider the option of placing subglandular implants in the prepectoral plane whenever possible. Additionally, for some subpectoral reconstructions, switching to prepectoral reconstruction should also be considered when feasible. In this study our aim was to report our experiences in breast reconstruction after nipple-sparing and skin-sparing mastectomies in patients with breast augmentation and provide a rationale for the new implant positioning based on patient and previous implant characteristics.
METHODS
In this retrospective study we examined 38 patients who had undergone previous breast augmentation, either subglandular or submuscular, and later received unilateral or bilateral nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM) with direct-to-implant (DTI) reconstruction.
The authors obtained informed consent from all patients and adhered to the ethical principles outlined in the Declaration of Helsinki.
The patients included in the study were consecutively operated on between January 2015 and July 2023 at our hospital. The average age of the patients at the time of surgery was 54 years, of whom the youngest was 34 years old and the oldest was 77 years old. Full demographic information can be found in Table 1.
| . | Subglandular breast augmentation . | Submuscular breast augmentation . |
|---|---|---|
| Tot. patients | 17 | 21 |
| Mean BMI | 21.26 | 21.92 |
| Smoking habits | 1 | 7 |
| PMRT | 6 | 4 |
| Arterial hypertension | 0 | 1 |
| Autoimmune disease | 1 | 0 |
| Implant rupture | 3 | 1 |
| Capsular contracture III/IV | 4 | 3 |
| Seroma | 0 | 1 |
| Calcified capsule | 1 | 0 |
| Waterfall deformity | 0 | 3 |
| Double bubble deformity | 0 | 1 |
| Original incision: periareolar | 9 | 8 |
| Original incision: inframammary | 5 | 11 |
| Original incision: mastopexy | 3 | 2 |
| Unilateral mastectomy | 11 | 15 |
| Bilateral mastectomy | 6 | 6 |
| . | Subglandular breast augmentation . | Submuscular breast augmentation . |
|---|---|---|
| Tot. patients | 17 | 21 |
| Mean BMI | 21.26 | 21.92 |
| Smoking habits | 1 | 7 |
| PMRT | 6 | 4 |
| Arterial hypertension | 0 | 1 |
| Autoimmune disease | 1 | 0 |
| Implant rupture | 3 | 1 |
| Capsular contracture III/IV | 4 | 3 |
| Seroma | 0 | 1 |
| Calcified capsule | 1 | 0 |
| Waterfall deformity | 0 | 3 |
| Double bubble deformity | 0 | 1 |
| Original incision: periareolar | 9 | 8 |
| Original incision: inframammary | 5 | 11 |
| Original incision: mastopexy | 3 | 2 |
| Unilateral mastectomy | 11 | 15 |
| Bilateral mastectomy | 6 | 6 |
BMI, body mass index; PMRT, postmastectomy radiation therapy .
| . | Subglandular breast augmentation . | Submuscular breast augmentation . |
|---|---|---|
| Tot. patients | 17 | 21 |
| Mean BMI | 21.26 | 21.92 |
| Smoking habits | 1 | 7 |
| PMRT | 6 | 4 |
| Arterial hypertension | 0 | 1 |
| Autoimmune disease | 1 | 0 |
| Implant rupture | 3 | 1 |
| Capsular contracture III/IV | 4 | 3 |
| Seroma | 0 | 1 |
| Calcified capsule | 1 | 0 |
| Waterfall deformity | 0 | 3 |
| Double bubble deformity | 0 | 1 |
| Original incision: periareolar | 9 | 8 |
| Original incision: inframammary | 5 | 11 |
| Original incision: mastopexy | 3 | 2 |
| Unilateral mastectomy | 11 | 15 |
| Bilateral mastectomy | 6 | 6 |
| . | Subglandular breast augmentation . | Submuscular breast augmentation . |
|---|---|---|
| Tot. patients | 17 | 21 |
| Mean BMI | 21.26 | 21.92 |
| Smoking habits | 1 | 7 |
| PMRT | 6 | 4 |
| Arterial hypertension | 0 | 1 |
| Autoimmune disease | 1 | 0 |
| Implant rupture | 3 | 1 |
| Capsular contracture III/IV | 4 | 3 |
| Seroma | 0 | 1 |
| Calcified capsule | 1 | 0 |
| Waterfall deformity | 0 | 3 |
| Double bubble deformity | 0 | 1 |
| Original incision: periareolar | 9 | 8 |
| Original incision: inframammary | 5 | 11 |
| Original incision: mastopexy | 3 | 2 |
| Unilateral mastectomy | 11 | 15 |
| Bilateral mastectomy | 6 | 6 |
BMI, body mass index; PMRT, postmastectomy radiation therapy .
The patients were categorized into 2 groups: those with previous subglandular implants (Group A) and those with previous submuscular implants (Group B). For each group, we analyzed patient BMI, the condition of the original capsule and implant, the previous incisions, and whether the mastectomy was unilateral or bilateral.
In cases of unilateral mastectomy, when the condition of the contralateral periprosthetic capsule and implant allowed it (no need for implant replacement due to rupture, high-grade capsular contracture, or poor aesthetic appearance of the breast), our goal was to achieve a reconstruction that closely resembled the healthy side. Patients were informed about the possibility of future breast fat grafting to address contour deformities and better match the contralateral healthy breast. However, if the contralateral breast had issues like implant rupture, capsular contracture (Baker grade III/IV), or poor aesthetics, we planned to replace the contralateral implant and treat the breast parenchyma to achieve symmetry. Capsules removed by total or partial capsulectomy were sent for histological examination.
During SSM, the mastectomy involved the excision of the nipple-areola complex (NAC), regardless of the previous incision for breast augmentation. In NSM cases, when the previous incision for breast augmentation was at the level of the inframammary fold or vertically in the lower quadrants, consistent with previous mastopexy, we made the mastectomy incision along the previous one. However, if the previous access for breast augmentation was periareolar, we opted for a radial incision to better preserve the NAC’s vascularization.18
The operative details are summarized in Table 2. We retrospectively analyzed our reconstruction decision-making based on mastectomy flap thickness (MFT) and the overall perfusion status of the mastectomy flaps, the need to intervene with the previous capsule, and the use of acellular dermal matrix (ADM). We also observed early and late complications that occurred.
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Breast cancer stage | ||
| G1 | 4 | 7 |
| G2 | 10 | 11 |
| G3 | 4 | 2 |
| Mastectomy incision | ||
| Radial | 9 | 9 |
| IMF | 9 | 11 |
| Axillary surgery | ||
| SLB | 13 | 15 |
| Radical lymphadenectomy | 5 | 5 |
| Preoperative breast augmentation implant volume | ||
| 100-200 cc | 1 | 2 |
| 201-300 cc | 13 | 7 |
| 301-400 cc | 4 | 11 |
| Reconstructive implant volume | ||
| 200-300 cc | 6 | 2 |
| 301-400 cc | 12 | 10 |
| 401-500 cc | 5 | 10 |
| 501-600 cc | 1 | 3 |
| >601 cc | 0 | 1 |
| Mastectomy specimen weight | ||
| 0-50 ml | 3 | 1 |
| 51-100 ml | 10 | 7 |
| 101-200 ml | 9 | 11 |
| 201-300 ml | 1 | 3 |
| 301-400 ml | 1 | 3 |
| >401 ml | 0 | 1 |
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Breast cancer stage | ||
| G1 | 4 | 7 |
| G2 | 10 | 11 |
| G3 | 4 | 2 |
| Mastectomy incision | ||
| Radial | 9 | 9 |
| IMF | 9 | 11 |
| Axillary surgery | ||
| SLB | 13 | 15 |
| Radical lymphadenectomy | 5 | 5 |
| Preoperative breast augmentation implant volume | ||
| 100-200 cc | 1 | 2 |
| 201-300 cc | 13 | 7 |
| 301-400 cc | 4 | 11 |
| Reconstructive implant volume | ||
| 200-300 cc | 6 | 2 |
| 301-400 cc | 12 | 10 |
| 401-500 cc | 5 | 10 |
| 501-600 cc | 1 | 3 |
| >601 cc | 0 | 1 |
| Mastectomy specimen weight | ||
| 0-50 ml | 3 | 1 |
| 51-100 ml | 10 | 7 |
| 101-200 ml | 9 | 11 |
| 201-300 ml | 1 | 3 |
| 301-400 ml | 1 | 3 |
| >401 ml | 0 | 1 |
G, grade; IMF, inframammary fold; SLNB, sentinel lymph node biopsy
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Breast cancer stage | ||
| G1 | 4 | 7 |
| G2 | 10 | 11 |
| G3 | 4 | 2 |
| Mastectomy incision | ||
| Radial | 9 | 9 |
| IMF | 9 | 11 |
| Axillary surgery | ||
| SLB | 13 | 15 |
| Radical lymphadenectomy | 5 | 5 |
| Preoperative breast augmentation implant volume | ||
| 100-200 cc | 1 | 2 |
| 201-300 cc | 13 | 7 |
| 301-400 cc | 4 | 11 |
| Reconstructive implant volume | ||
| 200-300 cc | 6 | 2 |
| 301-400 cc | 12 | 10 |
| 401-500 cc | 5 | 10 |
| 501-600 cc | 1 | 3 |
| >601 cc | 0 | 1 |
| Mastectomy specimen weight | ||
| 0-50 ml | 3 | 1 |
| 51-100 ml | 10 | 7 |
| 101-200 ml | 9 | 11 |
| 201-300 ml | 1 | 3 |
| 301-400 ml | 1 | 3 |
| >401 ml | 0 | 1 |
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Breast cancer stage | ||
| G1 | 4 | 7 |
| G2 | 10 | 11 |
| G3 | 4 | 2 |
| Mastectomy incision | ||
| Radial | 9 | 9 |
| IMF | 9 | 11 |
| Axillary surgery | ||
| SLB | 13 | 15 |
| Radical lymphadenectomy | 5 | 5 |
| Preoperative breast augmentation implant volume | ||
| 100-200 cc | 1 | 2 |
| 201-300 cc | 13 | 7 |
| 301-400 cc | 4 | 11 |
| Reconstructive implant volume | ||
| 200-300 cc | 6 | 2 |
| 301-400 cc | 12 | 10 |
| 401-500 cc | 5 | 10 |
| 501-600 cc | 1 | 3 |
| >601 cc | 0 | 1 |
| Mastectomy specimen weight | ||
| 0-50 ml | 3 | 1 |
| 51-100 ml | 10 | 7 |
| 101-200 ml | 9 | 11 |
| 201-300 ml | 1 | 3 |
| 301-400 ml | 1 | 3 |
| >401 ml | 0 | 1 |
G, grade; IMF, inframammary fold; SLNB, sentinel lymph node biopsy
RESULTS
Group A
Seventeen out of 38 patients had undergone subglandular breast augmentation. The mean BMI was 21.26. We categorized patients in 2 groups based on mastectomy flap thickness greater (thick) or less (thin) than 1 cm.
Thick Patients
Four out of 17 patients had an MFT > 1 cm. In these cases, we opted for prepectoral reconstruction. If we encountered an intact implant and capsule, we proceeded with anterior capsulectomy, either after the mastectomy or by excising the anterior capsule along with the mastectomy specimen. We inserted textured anatomical implants with full ADM coverage (Braxon; Decomed, Lisbon, Portugal) in the first part of our experience (2015-2018) and polyurethane (PU) covered anatomical implants in the second part (2018-2023) (Figure 1). Our move to PU-covered implants from 2018 onward was motivated by a reduced rate of complications, due to infections and seromas associated with ADM. In case of implant rupture, calcified capsule, or double capsule phenomenon, we opted for total capsulectomy and inserted textured anatomical implants with full ADM coverage or PU-covered anatomical implants. One patient with full ADM coverage underwent bilateral upper quadrant fat grafting for aesthetic purposes (Table 3).
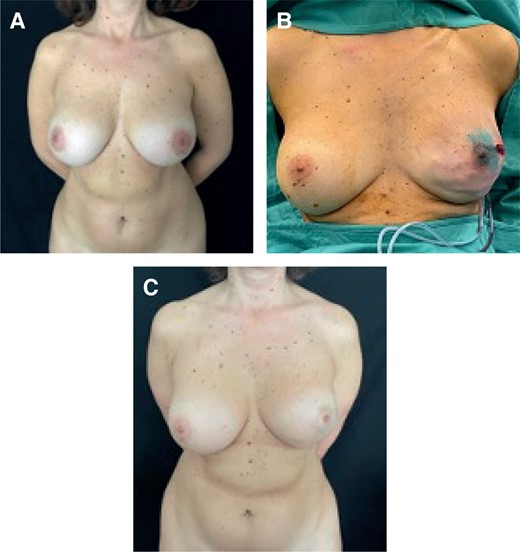
(A) Preoperative image of a 45-year-old female patient who underwent a subglandular breast augmentation, classified as a thick patient. (B) Left NSM and immediate prepectoral reconstruction with textured anatomical implants with full ADM coverage. (C) Postoperative result at 2 years. ADM, acellular dermal matrix; NSM, nipple-sparing mastectomy.
| . | Prepectoral reconstruction . | Submuscular reconstruction . | Percentage prepectoral reconstruction . | Percentage subpectoral reconstruction . |
|---|---|---|---|---|
| Early infections | 2 | 0 | 11.1 | 0 |
| Early wound dehiscence | 0 | 1 | 0 | 5 |
| Rippling | 0 | 0 | 0 | 0 |
| Capsular contracture III/IV | 0 | 3 | 0 | 15 |
| Implant dislocation | 0 | 1 | 0 | 5 |
| Delayed lipofilling | 3 | 2 | 16.7 | 10 |
| Delayed autologous conversion | 0 | 1 | 0 | 5 |
| Delayed implant exchange | 0 | 0 | 0 | 0 |
| Delayed implant conversion | 0 | 0 | 0 | 0 |
| Contour deformity | 3 | 0 | 16.7 | 0 |
| Animation deformity | 0 | 2 | 0 | 10 |
| . | Prepectoral reconstruction . | Submuscular reconstruction . | Percentage prepectoral reconstruction . | Percentage subpectoral reconstruction . |
|---|---|---|---|---|
| Early infections | 2 | 0 | 11.1 | 0 |
| Early wound dehiscence | 0 | 1 | 0 | 5 |
| Rippling | 0 | 0 | 0 | 0 |
| Capsular contracture III/IV | 0 | 3 | 0 | 15 |
| Implant dislocation | 0 | 1 | 0 | 5 |
| Delayed lipofilling | 3 | 2 | 16.7 | 10 |
| Delayed autologous conversion | 0 | 1 | 0 | 5 |
| Delayed implant exchange | 0 | 0 | 0 | 0 |
| Delayed implant conversion | 0 | 0 | 0 | 0 |
| Contour deformity | 3 | 0 | 16.7 | 0 |
| Animation deformity | 0 | 2 | 0 | 10 |
| . | Prepectoral reconstruction . | Submuscular reconstruction . | Percentage prepectoral reconstruction . | Percentage subpectoral reconstruction . |
|---|---|---|---|---|
| Early infections | 2 | 0 | 11.1 | 0 |
| Early wound dehiscence | 0 | 1 | 0 | 5 |
| Rippling | 0 | 0 | 0 | 0 |
| Capsular contracture III/IV | 0 | 3 | 0 | 15 |
| Implant dislocation | 0 | 1 | 0 | 5 |
| Delayed lipofilling | 3 | 2 | 16.7 | 10 |
| Delayed autologous conversion | 0 | 1 | 0 | 5 |
| Delayed implant exchange | 0 | 0 | 0 | 0 |
| Delayed implant conversion | 0 | 0 | 0 | 0 |
| Contour deformity | 3 | 0 | 16.7 | 0 |
| Animation deformity | 0 | 2 | 0 | 10 |
| . | Prepectoral reconstruction . | Submuscular reconstruction . | Percentage prepectoral reconstruction . | Percentage subpectoral reconstruction . |
|---|---|---|---|---|
| Early infections | 2 | 0 | 11.1 | 0 |
| Early wound dehiscence | 0 | 1 | 0 | 5 |
| Rippling | 0 | 0 | 0 | 0 |
| Capsular contracture III/IV | 0 | 3 | 0 | 15 |
| Implant dislocation | 0 | 1 | 0 | 5 |
| Delayed lipofilling | 3 | 2 | 16.7 | 10 |
| Delayed autologous conversion | 0 | 1 | 0 | 5 |
| Delayed implant exchange | 0 | 0 | 0 | 0 |
| Delayed implant conversion | 0 | 0 | 0 | 0 |
| Contour deformity | 3 | 0 | 16.7 | 0 |
| Animation deformity | 0 | 2 | 0 | 10 |
Thin Patients
Thirteen out of 17 patients had an MFT < 1 cm. We performed intraoperative ICG (indocyanine green) angiography to assess mastectomy flap perfusion. If the flaps demonstrated uniform viability, we proceeded with prepectoral reconstruction. In cases of intact implants and capsules, we performed anterior capsulectomy, if not already removed with the mastectomy specimen (Figure 2) and inserted textured anatomical implants with full ADM coverage (Braxon) or PU-covered anatomical implants. When faced with implant rupture, calcified capsule, or a double capsule phenomenon, we performed total capsulectomy (Figure 3). Two patients developed early infections, which we treated with antibiotic therapy. Another patient developed concavities in the upper quadrants and underwent 2 fat grafting sessions (Table 3). If the mastectomy flaps did not exhibit uniformly good perfusion, we chose submuscular reconstruction. We performed anterior capsulectomy, created the submuscular pocket by elevating the pectoralis major muscle with an ADM sling for the lower pole (Surgimend; Integra LifeSciences, Princeton, NJ), and then inserted textured anatomical implants. If the mastectomy was unilateral, the patient also required contralateral surgery to move the contralateral implant under the muscle. In case of implant rupture with silicone leakage, calcified capsule, or double capsule phenomenon, we followed the same strategy: total capsulectomy, creation of the submuscular pocket with an ADM sling for the lower pole, and insertion of textured anatomical implants. One patient experienced implant dislocation, while another developed grade III implant contracture and subsequently underwent autologous conversion (Table 3).
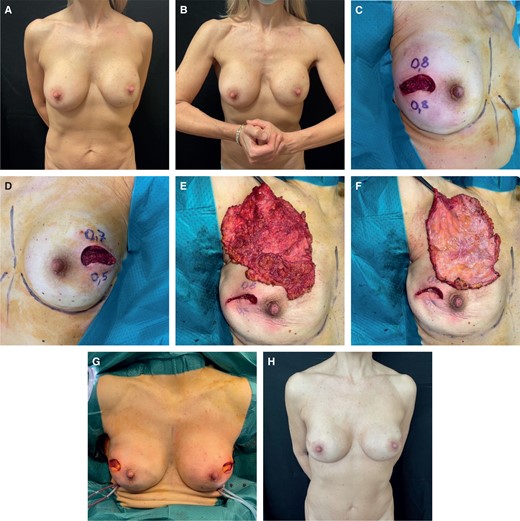
(A) Preoperative image of a 60-year-old female patient in static position who underwent a subglandular breast augmentation. (B) Patient in dynamic position. (C) Intraoperative MFT < 1 cm right side, ICG angiography showed good mastectomy flap perfusion. (D) Intraoperative MFT < 1 cm left side, ICG angiography showed good mastectomy flap perfusion. (E) Right mastectomy specimen. (F) Right anterior capsule removed together with the gland. (G) Intraoperative view of prepectoral reconstruction with PU implants. (H) Postoperative result at 1 year. ICG, indocyanine green; MFT, mastectomy flap thickness; PU, polyurethane.
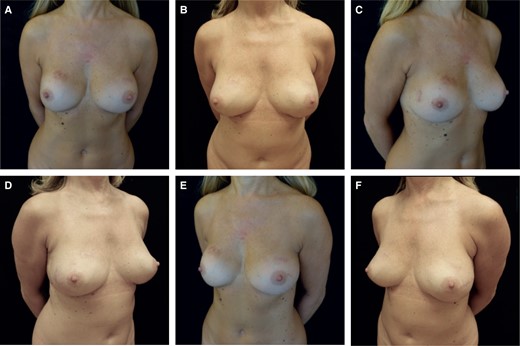
(A) Preoperative frontal view image of a 58-year-old female patient who underwent subglandular breast augmentation with a calcified capsule, MFT < 1 cm. ICG angiography showed good mastectomy flap perfusion. (B) 14 months postoperative frontal view, after bilateral NSM with total capsulectomy and prepectoral reconstruction with textured anatomical implants with full ADM coverage. (C) Preoperative left oblique view. (D) 14 months postoperative left oblique view. (E) Preoperative right oblique view. (F) 14 months postoperative right oblique view. ADM, acellular dermal matrix; ICG, indocyanine green; MFT, mastectomy flap thickness; NSM, nipple-sparing mastectomy.
Group B
Twenty-one out of 38 patients had undergone submuscular breast augmentation. The mean BMI was 21.92. We categorized patients based on mastectomy flap thickness (greater or less than 1 cm).
Thick Patients
Three out of 21 patients had an MFT > 1 cm. In these cases, we chose prepectoral reconstruction with anatomical PU implants. After removing the previous submuscular implant, we performed anteroinferior capsulectomy and anchored the muscle to the chest wall, slightly stretched. Then, we inserted PU implants (Figure 4). We performed total capsulectomy only in cases of implant rupture with silicone leakage, calcified capsule, or a double capsule phenomenon. One patient underwent autologous fat grafting of the upper quadrant to correct concavities (Table 3).
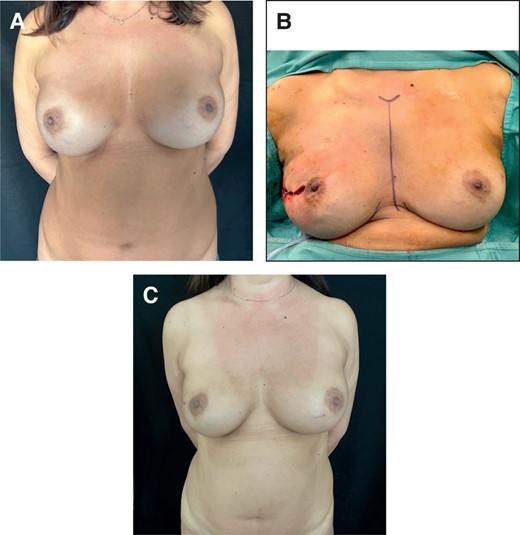
(A) Preoperative image of a 47-year-old female patient who underwent submuscular breast augmentation, MFT > 1 cm. (B) Intraoperative view of prepectoral immediate implant conversion with anatomical PU-covered implant after right NSM. (C) Postoperative result at 12 months. MFT, mastectomy flap thickness; NSM, nipple-sparing mastectomy; PU, polyurethane.
Thin Patients
Eighteen out of 21 patients had an MFT < 1 cm. In these cases, we opted for the submuscular plane. If the previous capsule and implant were intact, we inserted the new device into the same capsule after selective capsulotomies and pocket enlargement to accommodate the new, larger textured anatomical implants with higher projection (Figure 5). In cases of previous capsular contracture (grade III or IV), we inserted textured anatomical implants and placed ADM patches (Surgimend) at the capsulotomy sites to reduce the risk of capsular contracture recurrence. Total capsulectomy was performed in cases of silicone-embedded capsules, calcified capsules, or a double capsule phenomenon. In these patients, the submuscular pocket was completed with an ADM sling for the lower pole (Surgimend), and textured anatomical implants were inserted. Among patients with no previous capsular contracture, 2 developed high-grade capsular contracture. They were treated with fat grafting sessions, and one of them is scheduled for future prepectoral implant conversion. Another patient experienced early wound dehiscence and underwent revision. Two patients developed animation deformity (Table 3).
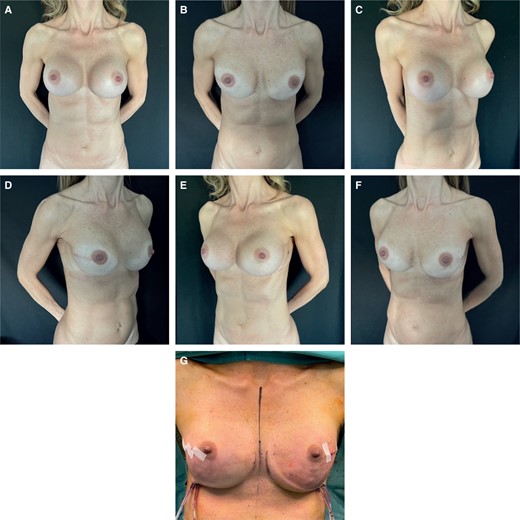
(A) Preoperative frontal view of a 49-year-old female patient who underwent submuscular breast augmentation with bilateral capsular contracture, MFT < 1 cm. (B) 1 year postoperative frontal view of submuscular reconstruction, she shows recurrent capsular contracture. (C) Preoperative left oblique view. (D) 1 year postoperative left oblique view. (E) Preoperative right oblique view. (F) 1 year postoperative right oblique view. (G) Intraoperative view of submuscular breast reconstruction. MFT, mastectomy flap thickness. NSM, nipple-sparing mastectomy.
DISCUSSION
Although 2-stage implant-based reconstruction remains the most common choice among plastic surgeons worldwide, direct-to-implant (DTI) breast reconstruction is gaining increasing popularity due to its potential to minimize the number of surgeries for the patient and deliver better cosmetic outcomes and satisfaction.19-22 DTI reconstruction can involve placing the implant in the subpectoral or prepectoral position.
However, submuscular implant positioning is associated with well-known complications, such as animation deformity, pectoralis muscle spasm, chronic pain, and reduced upper limb mobility. Placing the implant in the prepectoral position, on the other hand, avoids damage to the pectoralis major muscle and offers a more natural appearance, reduced pain, no upward implant dislocation, and better aesthetics, often eliminating the need for contralateral symmetrization procedures.23-26
Given the new opportunities afforded by DTI prepectoral reconstruction, this study is an update of our previous article on immediate breast reconstruction after skin-sparing and nipple-sparing mastectomies for previously augmented patients.9
Females who have undergone breast augmentation often have high expectations regarding the aesthetic results of their reconstructive surgeries. Consequently, it is crucial to maintain clarity and provide a realistic assessment of the potential outcomes of the proposed surgery. Whenever possible, we make every effort to accommodate their wishes by considering slightly larger implants or intervening on the contralateral healthy side.
Since 2015, we have adopted prepectoral reconstruction. Initially, we placed textured anatomical implants with full ADM coverage (Braxon) for prepectoral reconstruction. However, due to an increased early complication rate associated with ADM, in 2018 we shifted to polyurethane-coated implants (Microthane; Polytech Health and Aesthetics, Dieburg, Germany) as our primary choice for prepectoral reconstruction. Research indicates that these implants provide excellent adhesion to surrounding tissues, significantly reducing early complications such as seroma, wound dehiscence, and infections when compared to implants with ADM coverage. They also help prevent implant rotation, dislocation, and reduce long-term capsular contracture rates.27-29 Furthermore, polyurethane-covered implants offer cost-effective benefits.30
It is for this reason that, in this paper, we describe 2 different implant choices for prepectoral reconstruction. We transitioned from textured anatomical implants with full ADM coverage in the initial 3 years of our practice to polyurethane-coated implants from 2018 onward.
We have no experience with smooth round implants; they are not so commonly utilized in Italy. Nevertheless, the prepectoral placement of smooth round implants with full ADM coverage can be a valuable alternative to either textured anatomical implants with full ADM coverage or PU-covered implants. This concept also extends to subpectoral reconstruction, in which textured anatomical implants with a lower ADM sling could be replaced with smooth round implants and ADM. However, despite their potential to imitate anatomical implants under the influence of gravity and the pressure exerted by the pectoralis major muscle on the upper pole, the aesthetic results of round smooth submuscular implants are uncertain due to their lack of stability. This is especially relevant for patients with large breasts and previous anatomical implants who have high aesthetic expectations.
In Table 4, we have summarized the total number of prepectoral and submuscular reconstructions. Patients were categorized as “thick” or “thin” based on their mastectomy flap thickness, which was greater or less than 1 cm.
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Subglandular BA | 15 | 2 |
| Submuscular BA | 3 | 18 |
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Subglandular BA | 15 | 2 |
| Submuscular BA | 3 | 18 |
BA, breast augmentation.
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Subglandular BA | 15 | 2 |
| Submuscular BA | 3 | 18 |
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Subglandular BA | 15 | 2 |
| Submuscular BA | 3 | 18 |
BA, breast augmentation.
Thick patients with previous subglandular breast augmentation are considered ideal candidates for prepectoral reconstruction. We perform anterior capsulectomy, preferably in conjunction with mastectomy, and then place polyurethane-covered implants. Total capsulectomy is performed only in cases of implant rupture, calcified capsules, or the double capsule phenomenon.
Thin patients with previous subglandular breast augmentation undergo intraoperative ICG angiography. If mastectomy flaps demonstrate uniform viability, we perform anterior capsulectomy to ensure direct contact between the polyurethane-covered implant and the surrounding tissues. This is followed by prepectoral reconstruction, with the same technique applied to thick patients.
In cases in which mastectomy flap perfusion is not uniformly adequate, we conduct anterior capsulectomy to prevent the gliding effect. A new submuscular pocket is created, and textured anatomical implants with ADM coverage of the lower pole are inserted. Total capsulectomy is reserved for cases of implant rupture, calcified capsules, or the double capsule phenomenon (Figure 6).
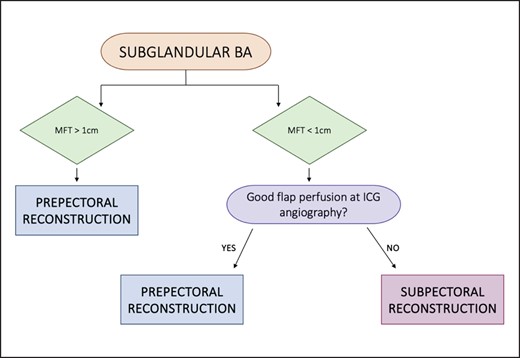
Algorithm for subglandular breast augmented patients. BA, breast augmentation; ICG, indocyanine green; MFT, mastectomy flap thickness.
For thick patients with submuscular breast augmentation, our preferred choice is prepectoral immediate implant conversion to PU implants. This involves explanting the submuscular implant, performing anteroinferior capsulectomy and anchoring the lower border of the pectoralis muscle to the posterior capsule on the chest wall. The polyurethane-covered implant is then placed in the prepectoral position, with total capsulectomy undertaken only in cases of implant rupture with silicone-embedded capsules, calcified capsules, or the double capsule phenomenon.
Thin patients with submuscular breast augmentation are generally considered candidates for submuscular reconstruction, regardless of flap perfusion status. In unilateral mastectomies, prepectoral reconstruction would require surgery on the contralateral healthy side to achieve symmetry, potentially involving the contralateral implant’s relocation to the prepectoral position. However, this might lead to questionable aesthetic outcomes due to the thin subcutaneous tissue.
The new implant can be inserted into the same submuscular pocket if feasible, typically by entering the submuscular plane at the superior portion of the lateral border of the pectoralis major muscle. Selective capsulotomies are performed to enlarge the pocket, with a preference for medial and lateral capsulotomies while avoiding the inferior one, unless lowering the implant is necessary. In such cases, inferior capsulorrhaphies may be added. We then insert textured anatomical implants with a larger volume and higher projection compared to the previous implant.
When dealing with patients who have previously experienced high-grade capsular contracture, we prefer to incorporate ADM patches in the capsulotomy regions to reduce the risk of capsular contracture recurrence. Total capsulectomy is performed in cases of silicone-embedded tissues following implant rupture, calcified capsules, and double capsule phenomenon. The submuscular pocket is then augmented with an ADM sling for the lower pole (Surgimend), and textured anatomical implants are inserted (Figure 7).
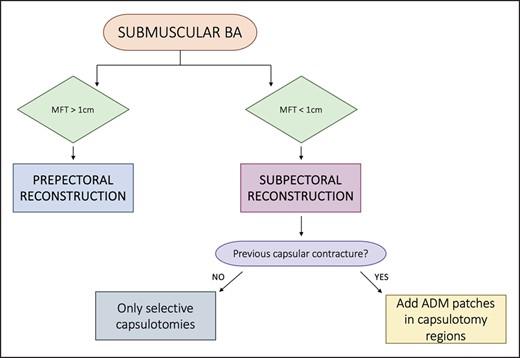
Algorithm for submuscular breast augmented patients. ADM, acellular dermal matrix; BA, breast augmentation; MFT, mastectomy flap thickness.
Our observations of early and late complications (Table 3) indicate the absence of grade III and IV capsular contracture in prepectoral reconstructions. Additionally, we noted a reduced recurrence rate of capsular contracture in patients undergoing submuscular reconstruction when ADM patches were placed in capsulotomy regions compared to those without ADM. For thin patients without previous capsular contracture, developing it after capsulotomies prompted us to plan multiple periprosthetic fat grafting procedures, with the ultimate goal of a delayed prepectoral implant conversion.
It is essential to recognize that in our study we had an average follow-up period of 3 years, with a maximum of 7 years and a minimum of 2 months. Five patients had follow-up times of less than 6 months, insufficient for a thorough evaluation of the development of contracture. Of these, 2 underwent submuscular reconstructions, while the other 3 had prepectoral reconstructions.
In the analysis of patients who underwent adjuvant radiotherapy, we observed that none of the patients with prepectoral reconstruction developed high-grade (grade III-IV) capsular contracture. In contrast, 2 patients with submuscular reconstruction developed grade III and IV capsular contractures, consistent with our experience and the existing literature.
An important consideration is whether the mastectomy is bilateral or unilateral. In bilateral reconstruction, achieving symmetry is more straightforward. In unilateral reconstruction, the goal of symmetrizing with the contralateral breast poses greater challenges. Therefore, our inclination to prefer prepectoral reconstruction whenever possible aims to reduce contralateral surgery. In cases where the contralateral implant is damaged, and aesthetic results are asymmetric, unilateral submuscular reconstruction may require more frequent adjustments to the contralateral breast.
Regarding the overall reconstruction results, we compiled a Likert scale and averaged the results, classifying them according to prepectoral and submuscular. The results are presented in Table 5.
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Volume | ||
| 0 Marked discrepancy | 1.6 | 1.4 |
| 1 Moderate discrepancy | ||
| 2 Symmetric | ||
| Contour | ||
| 0 Marked deformity | 1.9 | 1.2 |
| 1 Moderate deformity | ||
| 2 Little/no deformity | ||
| Inframammary crease | ||
| 0 Poorly defined | 1.8 | 1.7 |
| 1 Demarcated, asymmetric | ||
| 2 Well defined, symmetric | ||
| Breast position | ||
| 0 Marked discrepancy | 1.8 | 1.5 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Breast projection | ||
| 0 Marked discrepancy | 1.7 | 1.4 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Breast shape | ||
| 0 Marked discrepancy | 1.8 | 1 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Scars | ||
| 0 Poor/hypertrophic/contracted | 2 | 2 |
| 1 Wide scars/poor color match | ||
| 2 Eutrophic scars/good color match | ||
| Overall results | ||
| 0 Poor/needing further operations | 1.7 | 1.4 |
| 1 Fair/needing second minor surgery | ||
| 2 Good/definitive results |
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Volume | ||
| 0 Marked discrepancy | 1.6 | 1.4 |
| 1 Moderate discrepancy | ||
| 2 Symmetric | ||
| Contour | ||
| 0 Marked deformity | 1.9 | 1.2 |
| 1 Moderate deformity | ||
| 2 Little/no deformity | ||
| Inframammary crease | ||
| 0 Poorly defined | 1.8 | 1.7 |
| 1 Demarcated, asymmetric | ||
| 2 Well defined, symmetric | ||
| Breast position | ||
| 0 Marked discrepancy | 1.8 | 1.5 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Breast projection | ||
| 0 Marked discrepancy | 1.7 | 1.4 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Breast shape | ||
| 0 Marked discrepancy | 1.8 | 1 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Scars | ||
| 0 Poor/hypertrophic/contracted | 2 | 2 |
| 1 Wide scars/poor color match | ||
| 2 Eutrophic scars/good color match | ||
| Overall results | ||
| 0 Poor/needing further operations | 1.7 | 1.4 |
| 1 Fair/needing second minor surgery | ||
| 2 Good/definitive results |
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Volume | ||
| 0 Marked discrepancy | 1.6 | 1.4 |
| 1 Moderate discrepancy | ||
| 2 Symmetric | ||
| Contour | ||
| 0 Marked deformity | 1.9 | 1.2 |
| 1 Moderate deformity | ||
| 2 Little/no deformity | ||
| Inframammary crease | ||
| 0 Poorly defined | 1.8 | 1.7 |
| 1 Demarcated, asymmetric | ||
| 2 Well defined, symmetric | ||
| Breast position | ||
| 0 Marked discrepancy | 1.8 | 1.5 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Breast projection | ||
| 0 Marked discrepancy | 1.7 | 1.4 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Breast shape | ||
| 0 Marked discrepancy | 1.8 | 1 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Scars | ||
| 0 Poor/hypertrophic/contracted | 2 | 2 |
| 1 Wide scars/poor color match | ||
| 2 Eutrophic scars/good color match | ||
| Overall results | ||
| 0 Poor/needing further operations | 1.7 | 1.4 |
| 1 Fair/needing second minor surgery | ||
| 2 Good/definitive results |
| . | Prepectoral reconstruction . | Submuscular reconstruction . |
|---|---|---|
| Volume | ||
| 0 Marked discrepancy | 1.6 | 1.4 |
| 1 Moderate discrepancy | ||
| 2 Symmetric | ||
| Contour | ||
| 0 Marked deformity | 1.9 | 1.2 |
| 1 Moderate deformity | ||
| 2 Little/no deformity | ||
| Inframammary crease | ||
| 0 Poorly defined | 1.8 | 1.7 |
| 1 Demarcated, asymmetric | ||
| 2 Well defined, symmetric | ||
| Breast position | ||
| 0 Marked discrepancy | 1.8 | 1.5 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Breast projection | ||
| 0 Marked discrepancy | 1.7 | 1.4 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Breast shape | ||
| 0 Marked discrepancy | 1.8 | 1 |
| 1 Moderate discrepancy | ||
| 2 Little/no discrepancy | ||
| Scars | ||
| 0 Poor/hypertrophic/contracted | 2 | 2 |
| 1 Wide scars/poor color match | ||
| 2 Eutrophic scars/good color match | ||
| Overall results | ||
| 0 Poor/needing further operations | 1.7 | 1.4 |
| 1 Fair/needing second minor surgery | ||
| 2 Good/definitive results |
In addition, we distributed the postoperative BREAST-Q questionnaire to every participant in the study. We divided the outcomes based on the reconstruction type, distinguishing between prepectoral and submuscular approaches. A mean value was computed for each assessed parameter, displayed in the accompanying graphs (Figures 8, 9). Last, we designed a third graph for a comparative analysis of the results from both groups (Figure 10).
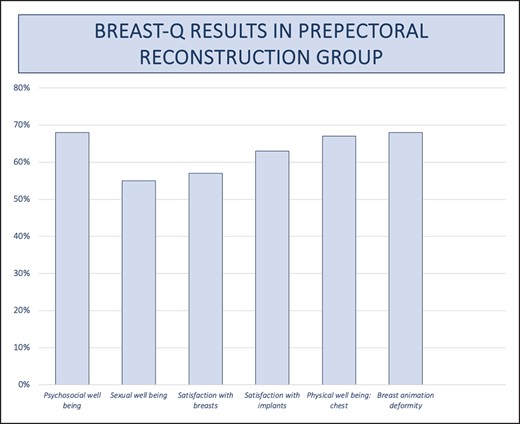
Graph for BREAST-Q results in prepectoral reconstructive group.
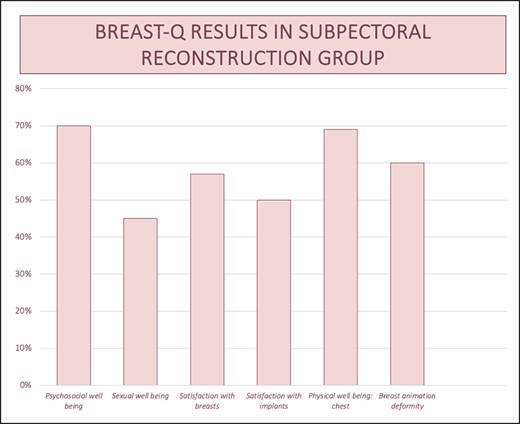
Graph for BREAST-Q results in subpectoral reconstructive group.
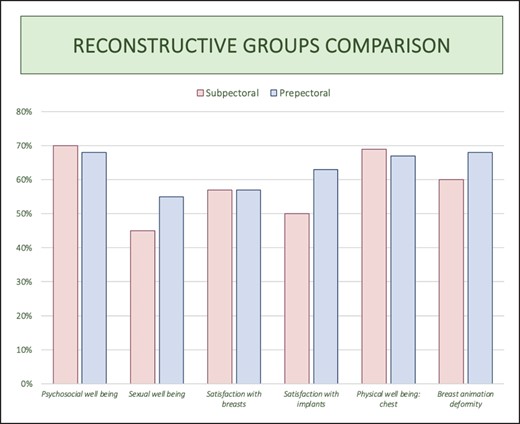
Graph comparing BREAST-Q results of the 2 reconstructive groups.
Based on the obtained results, it is evident that prepectoral reconstructions demonstrate a higher level of sexual well-being, satisfaction regarding breasts and implants, and notably, reduced breast animation deformity. In terms of psychosocial well-being and chest health, the subpectoral group displays only a marginal advantage (70% vs 68% and 69% vs 67%, respectively), which lacks statistical significance.
Females with breast augmentation who are subsequently diagnosed with breast cancer present a multifaceted challenge for both breast and plastic surgeons. These patients are typically lean and have elevated aesthetic expectations. Moreover, they are often less suitable candidates for conservative breast surgery.
While nipple-sparing and skin-sparing mastectomy with immediate DTI reconstruction is considered the gold standard for treating augmented patients, ongoing discussions persist regarding the ideal implant positioning and managing the periprosthetic capsule. Several surgeons advocate for submuscular reconstruction, whether the previous implant’s position was subglandular or subpectoral.
The authors herein propose a systematic approach to choosing the reconstruction plane, taking into account mastectomy flap thickness, perfusion, implant type, and periprosthetic capsular status.
Prepectoral reconstruction has gained recognition as a viable option for immediate breast reconstruction after nipple-sparing mastectomy and skin-sparing mastectomy. This approach helps mitigate muscle animation deformity and chronic pain, and offers advantages such as faster recovery, reduced discomfort, prevention of upward implant dislocation, and superior aesthetic results compared to submuscular reconstruction.24,31-33
Furthermore, for patients experiencing issues such as animation deformity, upper limb movement impairment, chronic pain, and severe capsular contracture following subpectoral reconstruction, a delayed prepectoral implant conversion can be considered, potentially involving multiple fat grafting procedures to prepare for the transition.34-36
The study had its limitations, including being based on single-center experience with a small sample of patients. The average follow-up time was 3 years, with a minimum of 2 months, making it difficult to draw definitive conclusions about the development of capsular contracture in this subgroup of patients. Potential biases may be associated with patient selection and choice of operative techniques.
CONCLUSIONS
This work highlights the potential of prepectoral reconstruction as a promising option for selected patients with previous subglandular and submuscular breast augmentation, offering favorable outcomes with low complication rates and pleasing aesthetic results. When prepectoral reconstruction is not feasible, submuscular reconstruction remains a viable alternative, with careful attention to capsulotomies and capsulectomies as needed to accommodate the new implant.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
Dr Salgarello is a professor of plastic surgery and Dr Visconti and Dr Barone Adesi are plastic surgeons, Plastic Surgery Unit, Department of Women’s Health, Children, and Public Health, Gemelli University Hospital, Rome, Italy.
Dr Fabbri is a plastic surgery resident, Residency Program in Plastic Surgery, Catholic University of Sacred Heart, Rome, Italy.



