-
PDF
- Split View
-
Views
-
Cite
Cite
Annie Chiu, Jose Raul Montes, Girish Munavalli, Ava Shamban, Smita Chawla, Steve Abrams, Improved Patient Satisfaction With Skin After Treatment of Cheek Skin Roughness and Fine Lines With VYC-12L: Participant-Reported Outcomes From a Prospective, Randomized Study, Aesthetic Surgery Journal, Volume 43, Issue 11, November 2023, Pages 1367–1375, https://doi.org/10.1093/asj/sjad111
Close - Share Icon Share
Abstract
VYC-12L is a hyaluronic acid filler to improve skin quality. A prospective study showed safety and effectiveness of VYC-12L for improving cheek skin smoothness and fine lines.
To report participant-reported outcomes, subgroup analyses, and physician experience from the prospective study.
Adults with moderate to severe Allergan Cheek Skin Smoothness (ACSS) scale scores were randomized 2:1 to VYC-12L or control (no treatment with optional treatment). Participant assessments included FACE-Q Satisfaction With Skin, FACE-Q Appraisal of Lines, natural look and feel, the Global Aesthetic Improvement Scale (GAIS), and safety. Subgroup analyses examined ACSS responder rate (≥1-grade improvement from baseline to 1 month).
FACE-Q Satisfaction With Skin overall mean scores improved from baseline to 1 month posttreatment by 32.0 and 1.4 in the VYC-12L and control groups, respectively. FACE-Q Appraisal of Lines overall mean scores improved from baseline to 1 month posttreatment by 23.3 and 0.4 in VYC-12L and control, respectively. Treated participants had high median scores (≥9.0) of natural look and feel of their cheek skin. GAIS responder rate was high at Month 1 (85.5%; 95% CI, 79.3%-91.7%) and through Month 6 (83.1%; 95% CI, 76.5%-89.7%). Mean participant-rated pain scores were low (<3). The most common injection site reactions were redness, swelling, and lumps/bumps; most resolved within 3 days. Subgroup analyses showed significant differences in ACSS responder rate between VYC-12L and control groups 1 month posttreatment. Physician injectors reported that VYC-12L was easily injected at the superficial skin and integrated quickly.
VYC-12L treatment produced significant improvements in satisfaction with skin and cheek skin smoothness, as measured by participant-reported outcome measures.

Skin quality comprises multiple attributes such as texture, hydration, radiance, and distribution of pigmentation.1-3 Poor skin quality can result from both intrinsic (eg, aging) and extrinsic (eg, smoking, environmental pollution) factors altering extracellular matrix dynamics, ultimately leading to dysfunction and reductions in hyaluronic acid (HA), collagen, and elastin.4-9 The quality of one's skin can impact self-perception and quality of life.10,11 Minimally invasive aesthetic treatments, including HA fillers, chemical peels, and skin resurfacing, represent an increasingly popular approach to improve skin quality and patients’ satisfaction with their overall appearance. Indeed, treatment with HA fillers has been shown to improve multiple aspects of skin quality and is associated with high patient satisfaction.12-14
VYC-12L (Allergan Aesthetics, an AbbVie company, Irvine, CA) is a recently developed HA filler that was designed to correct superficial cutaneous depressions, such as fine lines, and for additional improvements in skin quality. VYC-12L is a member of the VYCROSS family of HA fillers containing 12-mg/mL HA and 0.3% w/w lidocaine, with lower G' and cohesivity compared with other VYCROSS fillers.15 The effectiveness and safety of VYC-12L for the treatment of facial skin quality were recently evaluated in a randomized, multicenter, evaluator-blind, delayed treatment control study of 135 treated participants.16 The primary effectiveness endpoint, the percentage of participants with a ≥1-grade improvement from baseline on the validated Allergan Cheek Skin Smoothness (ACSS) scale on both cheeks, was met in 57.9% of participants at Month 1 and remained ≥55% through Month 6.16,17 A ≥1-grade improvement from baseline on the Allergan Fine Lines Scale (AFLS) for both cheeks was also achieved in 58.3% of treated participants, and skin hydration was improved compared to baseline at Month 1 and lasted through Month 6.16,18 This paper presents results of subgroup analyses of the primary endpoint and participant-reported outcome (PRO) measures from the primary study.
METHODS
Study Design
The design of this randomized, multicenter, evaluator-blind, delayed control study conducted at 14 US sites from November 2018 through July 2020 has been published previously.16 Briefly, eligible participants were healthy adults (≥22 years) who had scores of moderate (2) or severe (3) on the validated, 5-point photonumeric ACSS scale, as assessed by a blinded investigator, and a FACE-Q Satisfaction With Skin questionnaire score of ≤69. Participants were excluded if they had received dermal fillers, including cross-linked HA fillers, or other cosmetic procedures (eg, laser treatment) within 12 months of screening; had received botulinum toxin injections in the cheek area within 6 months of screening; or had received semipermanent or permanent facial implants. Participants were randomized 2:1 to the VYC-12L treatment or no-treatment control group with a computer-generated study level randomization mechanism. The VYC-12L treatment group received initial treatment and an optional touch-up treatment at Month 1 if needed to achieve optimal correction. Control group participants were followed for 1 month after randomization and then either completed the study or received optional treatment (initial and touch-up, if needed) in the delayed treatment period.
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki, was conducted in compliance with good clinical practice, and all participants provided informed consent before treatment. IRB approval was obtained from Copernicus IRB (Cary, NC). This study is registered at clinicaltrials.gov (NCT#03728309).
Participant-Reported Outcomes
Participants completed the validated FACE-Q Satisfaction With Skin scale at baseline before treatment and at months 1, 2, 4, and 6 after final injection.19 The FACE-Q Satisfaction With Skin scale comprises a 12-item questionnaire on which responses are rated on a 4-point scale from 1 (very dissatisfied) to 4 (very satisfied). Scores from each question are summed and Rasch-transformed to a scale score that ranges from 0 to 100, with higher scores indicating increased satisfaction. Participants also completed the validated FACE-Q Appraisal of Fine Lines scale at baseline before treatment and at months 1, 2, 4, and 6 after final injection.19 The Appraisal of Fine Lines scale comprises a 10-item questionnaire on which responses are rated on a 4-point scale from 1 (not at all bothered) to 4 (extremely bothered). Scores from each question are summed and Rasch-transformed to a scale score that ranges from 0 to 100, with higher scores indicating being less bothered by lines on the face.
Participants assessed the natural look of their skin on an 11-point scale ranging from 0 (unnatural looking) to 10 (natural looking) and assessed the natural feel of their skin with an 11-point scale ranging from 0 (unnatural feeling) to 10 (natural feeling). Participants also completed the Global Aesthetic Improvement Scale (GAIS) at months 1, 2, 4, and 6 after final injection. The GAIS responder rate was defined as the proportion of participants responding “improved” or “much improved.” Exploratory analyses tested whether GAIS scores correlated with a change in MoistureMeter instrument probe (Delfin Technologies, Kuipio, Finland) measurements of skin hydration (note: hydration data published previously).16
Procedural pain was assessed by the participant after the initial injection on an 11-point scale ranging from 0 (no pain) to 10 (worst pain imaginable). Participants also recorded the incidence, severity, and duration of injection site reactions (ISRs) in an e-diary for each cheek for up to 1 month after any treatment, starting on the day of treatment. The treating investigator reviewed the participants’ e-diaries at 3-day and 1-month follow-ups to determine whether any reported ISRs qualified as adverse events.
Subgroup Analyses
Subgroup analyses of the primary effectiveness endpoint (ACSS responder rate at 1 month following the last injection [initial or touch-up], defined as a ≥1-grade improvement from baseline) were conducted to compare across baseline ACSS grade, age, sex, and Fitzpatrick skin phototype and ethnicity. Additional analyses of AFLS responder rate (≥1-grade improvement from baseline to 1 month following the last injection), GAIS, and FACE-Q Satisfaction With Skin scores across age subgroups were also conducted.
Physician Experience
A subset of treating investigators was asked during the course of manuscript development to describe their experience administering VYC-12L treatment.
Statistical Analyses
All analyses were performed on the modified intent-to-treat (mITT) population, which included all randomized participants. A paired t test was used to test the mean change from baseline to Month 1 in FACE-Q Satisfaction With Skin scores. For subgroup analyses of the ACSS responder rate, a 2-sided Fisher's exact test with α = .05 was used to test whether the ACSS responder rate at Month 1 in the treatment group was significantly greater than that in the control group. A 2-way repeated measures analysis of variance (ANOVA) was used to assess the relationship between the GAIS score and hydration over time. Results on all other measures were summarized descriptively.
RESULTS
Participants and Treatment Administration
The participant demographics and disposition for this study have been reported previously; briefly, 209 participants were randomized 2:1 to receive VYC-12L (n = 136) or the no-treatment control (n = 73). A total of 195 participants (VYC-12L, n = 131; control, n = 74) completed the Month 1 control period, and the majority of participants (81.8%, n = 171) completed the study (ie, completed a follow-up visit 6 months after initial or touch-up or 4 months after repeat treatment). Participants in the mITT population were majority female (86.1%), White (87.6%), not of Hispanic ethnicity (72.3%), and had a mean age of 57.1 years (range: 31-83 years).
Details of treatment administration have been reported separately; briefly, the median total volume injected was 4.0 mL for both cheeks in the VYC-12L (initial and touch-up treatments combined; range, 0.8-6.0 mL) and control (initial and touch-up treatments combined; range, 1.0-6.0 mL) groups. VYC-12L treatment was predominantly via intradermal microdepot injections with 32G 1/2-inch needles.
Participant-Reported Outcomes
In the VYC-12L treatment group (n = 131), the FACE-Q Satisfaction With Skin overall mean score at baseline was 34.3 and improved by a mean of 32.0 to an overall mean score of 66.5 at Month 1 post–final injection (initial or touch-up) (Figure 1). In the untreated control group (n = 71), the baseline overall mean score was 32.5 and improved by a mean of 1.4 to an overall mean score of 35.0 at Month 1. The difference between VYC-12L treatment and control at Month 1 was 28.0 (P < .001). Individual components of the FACE-Q Satisfaction With Skin assessment showed notable treatment-related improvements, including a 60.4% increase from baseline in how smooth participants felt their facial skin looks; a 55% increase from baseline in how hydrated participants felt their facial skin looks, and a 64.6% increase from baseline in how radiant participants felt their skin looks. Improvements in overall mean satisfaction with skin scores remained consistent through the 6-month study period.
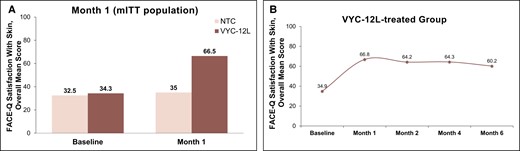
FACE-Q Satisfaction With Skin. (A) Overall mean scores on FACE-Q Satisfaction With Skin for the mITT population at baseline and Month 1 after VYC-12L treatment. (B) Overall mean score on FACE-Q Satisfaction With Skin at months 1, 2, 4, and 6 (study end) posttreatment for the VYC-12-L group. mITT, modified intent-to-treat; NTC, no-treatment control.
In the VYC-12L treatment group, the overall FACE-Q Appraisal of Lines mean score at baseline was 31.0 and improved by a mean of 23.3 to an overall mean score of 54.1 at Month 1 after final injection (initial or touch-up) (Figure 2). In the untreated control group, the baseline overall mean score was 32.5 and improved by a mean of 0.4 to an overall mean score of 34.0 at Month 1. Improvements in FACE-Q Appraisal of Lines scores remained consistent through the 6-month study period.
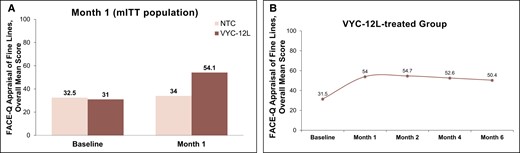
FACE-Q Appraisal of Lines. (A) Overall mean scores on FACE-Q Appraisal of Fine Lines for the mITT population at baseline and Month 1 after VYC-12L treatment. (B) Overall mean score on FACE-Q Appraisal of Lines at months 1, 2, 4, and 6 posttreatment for the VYC-12-L group. mITT, modified intent-to-treat; NTC, no-treatment control.
When asked to rate the look of their cheek skin on an 11-point scale from 0 (unnatural looking) to 10 (natural looking), treated participants had a median score of 9.0 or 10.0 at all time points post–final injection. Median scores were also 9.0 at every visit after repeat treatment. When asked to rate the feel of their cheek skin on an 11-point scale from 0 (unnatural feeling) to 10 (natural feeling), treated participants had a median score of 9.0 or 10.0 at all time points post–final injection. Median scores were also 9.0 at every visit after repeat treatment. In the VYC-12L treatment group, the GAIS responder rate was high at Month 1 (85.5%; 95% CI [79.3%-91.7%]) and remained consistent through Month 6 (83.1%; 95% CI [76.5%-89.7%]).
MoistureMeter measurements of skin hydration, which were significantly improved by VYC-12L treatment, were done to determine the threshold of clinically meaningful difference (CMD) in skin hydration.16 This threshold of CMD was determined by calculating the average of differences in skin hydration between GAIS categories (ie, hydration rate for participants with GAIS responses of “much improved” minus hydration rate for participants with GAIS responses of “improved,” “improved” minus “no change,” etc.). With this value (1.456) as the estimated CMD, there was a significantly higher (P < .05) proportion of participants in the VYC-12L (54.9%) group than in the control (39.1%) group who had clinically meaningful changes in skin hydration (15.9%; 95% CI [1.0%-30.7%]). Time trend analysis also showed that these clinically meaningful changes in skin hydration persisted through the Month 6 follow-up.
Mean participant-rated pain scores immediately after initial treatment were 3.0 for the VYC-12L treatment group and 2.4 for the control group. During the 1-month period after initial treatment, at least 1 ISR was reported in participant e-diaries by 110 participants (81.5%; n = 110/135) of the VYC-12L group and by 48 participants (75.0%; n = 48/64) of the control group. The most frequently reported ISRs after initial treatment were redness, swelling, and lumps/bumps. The majority of lumps/bumps resolved without intervention by the Month 2 follow-up visit. Three participants (1.4%) reported lumps/bumps (2 mild, 1 moderate) that resolved without intervention at approximately 12 to 15 months after initial treatment; these were not considered adverse events by the treating investigators, and review of baseline photography of 1 of these participants showed that mild lumps/bumps were present before study treatment. After repeat treatment, the most frequently reported ISRs were redness, bruising, and swelling. The majority of ISRs were mild or moderate in severity and resolved within 7 days (Figure 3). ISRs were similar in incidence and severity across Fitzpatrick skin types. There were no reports of late-onset nodules in this study.
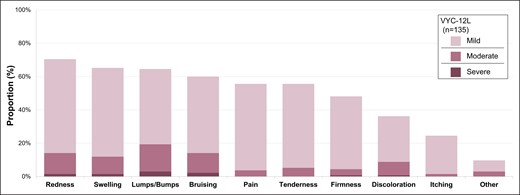
Incidence and severity of injection site responses (ISRs) in VYC-12L treatment group. ISRs were captured by participant e-diaries during the first 30 days posttreatment.
Subgroup Analyses
Subgroup analyses of the primary effectiveness endpoint (ACSS responder rate, defined as a ≥1-grade improvement from baseline) (Figure 4) showed that the ACSS responder rate was significantly greater for the VYC-12L group than the control group in participants with baseline ACSS scores of 2 (difference: 46.2%; 95% CI [31.4%-61.0%]; P < .001) or 3 (63.5%; 95% CI [47.6%-79.5%]; P < .001). The responder rate was also significantly greater for treated participants than control group participants across all Fitzpatrick skin phototypes; I/II (41.0%; 95% CI [16.8%-65.2%]; P < .05), phototypes III/IV (57.7%; 95% CI [44.6%-70.7%]; P < .001), and phototypes V/VI (58.3%; 95% CI [30.4%-86.2%]; P < .05). There was not a significant difference in ACSS responder rate between VYC-12L (n = 12) and control (n = 4) group male participants (P = .1); however, the responder rate was significantly higher in treated female participants than control group female participants (53.9%; 95% CI [42.0%-65.8%]; P < .001). The responder rate was also significantly greater for the VYC-12L group than control in all age subgroups evaluated: participants aged <50 years (82.4%; 95% CI [64.2%-100.0%]; P < .001), 50-60 years (62.5%; 95% CI [49.8%-75.2%]; P < .001), and >60 years (46.9%; 95% CI [33.0%-60.9%]; P < .05).
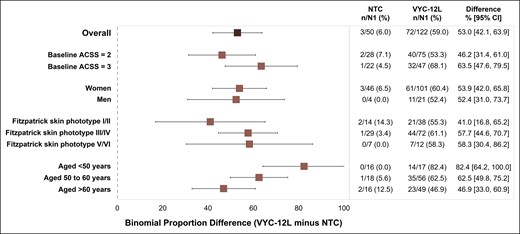
Subgroup analyses of Allergan Cheek Skin Smoothness Scale responder rates at Month 1 in the mITT population. The figure shows the binomial proportion difference between VYC-12L responders and no-treatment control responders at Month 1 for the overall mITT population and for each subgroup. Responder rate was defined as a ≥1-grade improvement in the ACSS score on both cheeks from baseline to Month 1. N1 = number of participants with data at baseline and Month 1 of control period. Horizontal bars in figure indicate 95% CI. ACSS, Allergan Cheek Skin Smoothness Scale; CI, confidence interval; mITT, modified intent-to-treat; NTC, no-treatment control.
Subgroup analyses of the AFLS responder rate, defined as a ≥1-grade improvement from baseline, by age showed that AFLS responder rate was significantly greater for the VYC-12L group than the control group in participants aged <50 years (75.0%; 95% CI [50.5%-99.5%]; P < .001), age 50-60 years (67.5%; 95% CI [53.0%-82.0%]; P < .001), and age >60 years (40.6%; 95% CI [23.6%-57.6%]; P < .05). The GAIS responder rate was also high for all participants treated with VYC-12L across all age subgroups that were evaluated: participants age <50 years (81.3%; 95% CI [62.1%-100.0%]), aged 50-60 years (86.2%; 95% CI [77.3%-95.1%]), and aged >60 years (86.0%; 95% CI [76.4%-95.6%]). For FACE-Q Satisfaction With Skin, the difference between the VYC-12L and control groups for the mean change from baseline to 1 month post–last injection was significantly different for participants aged <50 years (28.1; 95% CI [11.5-44.6]; P < .01), aged 50-60 years (32.8; 95% CI [23.2-42.3]; P < .001), and aged >60 years (27.3; 95% CI [12.7-42.0]; P < .001).
Physician Experience
Physician injectors reported that VYC-12L was easily injected at the superficial skin and gets quickly integrated, leaving no signs of injection in a short time, and that the injections were quickly performed and very well tolerated by patients.
DISCUSSION
This is the first prospective, randomized, multicenter, delayed-treatment control study to include multiple participant-reported outcome (PRO) measures, including the validated FACE-Q, to assess the effectiveness and safety of an HA filler for the treatment of cheek skin roughness and fine lines from the participants’ perspective. In this study of 135 participants who received intradermal injections of VYC-12L in the cheek area, significant improvements were seen in participant ratings of satisfaction with skin, appraisal of lines, and the natural look and feel of their skin. Furthermore, subgroup analyses of the study's primary effectiveness endpoint, the proportion of participants with a ≥1-grade improvement from baseline on the validated Allergan Cheek Skin Smoothness (ACSS) scale, showed that VYC-12L was an effective treatment for moderate and severe baseline ACSS scores, male and female participants, all Fitzpatrick skin phototypes, and all age groups treated.16,17
On the FACE-Q Satisfaction With Skin scale, the majority of participants reported significantly improved satisfaction with their skin after VYC-12L treatment, with improvements persisting through the 6-month study period. Significant improvements were seen from baseline to Month 1 in responses of “somewhat satisfied” or “very satisfied” on each of the individual questions of the FACE-Q Satisfaction With Skin scale for VYC-12L–treated participants. Indeed, the proportion of participants who were somewhat or very satisfied with how smooth their facial skin looks improved from 19.1% at baseline to 79.5% a 1 month posttreatment, with similar improvements in the proportion of participants somewhat or very satisfied with how hydrated their facial skin looks (baseline: 22.9%; Month 1: 77.9%) and how radiant their facial skin looks (baseline: 9.2%; Month 1: 73.8%). Importantly, the proportion of participants who were satisfied with how healthy and hydrated their facial skin looks remained >70% through Month 6 after VYC-12L treatment. Subgroup analyses by age (<50, 50-60, and >60 years) showed that improvements in Satisfaction With Skin overall mean scores from baseline to 1 month post–last injection were significantly greater in participants treated with VYC-12L than control, regardless of age category. These results for treatment of the cheeks are similar to those reported in a recent study of VYC-12L injections in the full face, and provide additional data supporting lasting increases in participant satisfaction with multiple aspects of the skin's appearance after VYC-12L treatment.12,13
On the FACE-Q Appraisal of Lines scale, which measures how bothered participants are by the appearance of lines on their face in various situations (eg, in photographs, under bright lights, when smiling), participants reported being significantly less bothered by facial lines 1 month after VYC-12L treatment. Indeed, the proportion of participants who reported being “not at all” or “a little” bothered by the appearance of lines when their face was relaxed improved from 25.2% at baseline to 67.2% 1 month after VYC-12L treatment. Treated participants also reported being less bothered by how old lines on their face made them look, as measured by responses of “not at all” or “a little” bothered at the 1-month posttreatment visit (50%) compared with baseline (15.3%). Together, these FACE-Q data show that intradermal VYC-12L injections in the cheek produce robust improvements in participant satisfaction with multiple aspects of facial skin appearance (eg, hydration, smoothness), as well as self-perceptions of age. Representative photographs showing treatment-related improvements can be seen in Figures 5, 6.
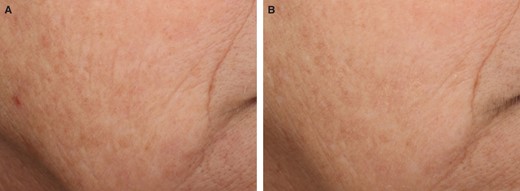
Representative images of VYC-12L–related improvements in cheek skin smoothness and fine lines. (A) Participant is shown at baseline before treatment and (B) 1 month after VYC-12L treatment. The participant was a 51-year-old White female participant with baseline ACSS and AFLS scores of 3 and 4, respectively, on the right cheek. The participant received 1.8 mL of VYC-12L for the initial treatment and 0.9 mL for touch-up treatment in the right cheek. At the 1-month posttreatment follow-up, the participant had ACSS and AFLS scores of 2 and 3, respectively, on the right cheek. ACSS, Allergan Cheek Smoothness Scale; AFLS, Allergan Fine Lines Scale.
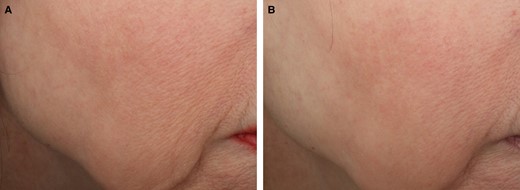
Representative images of VYC-12L-related improvements in cheek skin smoothness and fine lines. (A) Participant is shown at baseline before treatment and (B) 1 month after VYC-12L treatment. The participant was a 63-year-old White female participant with baseline ACSS and AFLS scores of 2 on the right cheek. The participant received 1.8 mL of VYC-12L for the initial treatment and 0.8 mL for touch-up treatment in the right cheek. At the 1-month posttreatment follow-up, the participant had ACSS and AFLS scores of 1 on the right cheek. ACSS, Allergan Cheek Smoothness Scale; AFLS, Allergan Fine Lines Scale.
Participants also reported that VYC-12L treatment of the cheeks produced natural-looking and natural-feeling outcomes, and the participant GAIS responder rate (defined as responses of “improved” or “much improved”) was high (≥80%) at all posttreatment visits, including all age subgroups. Additional analyses correlating GAIS scores with tissue dielectric constant probe-measured skin hydration showed that VYC-12L treatment was associated with lasting and clinically meaningful improvements in hydration. Importantly, PRO measures of skin hydration on the FACE-Q also showed substantial improvements after VYC-12L treatment, suggesting concordance between objective clinical measurements and participant assessments of improved facial skin hydration due to VYC-12L.
The sizable study population also allowed us to examine primary and secondary effectiveness endpoints across multiple subgroups. Analysis of the primary endpoint, ACSS responder rate (defined as a ≥1-grade improvement from baseline to 1 month), showed that VYC-12L improved ACSS scores regardless of baseline cheek skin smoothness/roughness, age, or Fitzpatrick skin type. Female participants also showed significant improvements in ACSS responder rate after VYC-12L treatment. Although there was not a significant treatment effect among male participants, this may have been due to the small number of male participants in the mITT population (n = 22; 16.8%), and future studies focusing on this treatment in men are warranted. An age-focused subgroup analysis of AFLS, GAIS, and FACE-Q Satisfaction With Skin scores also showed significant treatment-related improvements for all analyzed age groups, showing that VYC-12L was an effective means of improving cheek skin smoothness, fine lines, and participant satisfaction with the appearance of the skin across generations.
Of note, participants had low ratings of pain following VYC-12L treatment, the majority of ISRs reported were mild in severity and resolved within a week, the incidence and severity of ISRs were similar across Fitzpatrick skin types, and there were no reports of late-onset nodules, supporting the favorable safety profile of intradermal VYC-12L injections in the cheek. Physician injectors queried regarding their experience administering VYC-12L noted that the product was easily and quickly injected, integrated into the skin quickly, and was well tolerated by participants.
Our study complements the recent European study of VYC-12L for skin quality improvements, and extends those findings through the large sample size, inclusion of an untreated control group with a randomized design, and the multitude of PRO measures that demonstrated significant and sustained improvements in skin quality from the participants’ perspective.12,13,20 Additionally, VYC-12L is distinct from other fillers due to its lower concentration of HA and resulting differences in rheologic properties, which make it ideal for targeting skin quality improvements such as roughness and fine lines, rather than more general plumping of facial areas. Although other dermal fillers may be available outside the US for skin quality improvements, published data are limited by small sample size, requirement for multiple treatments, more time between when treatments are administered and when noticeable and significant results are achieved, or are not treating the face. In contrast, this is the first large, randomized, no-treatment control study of a filler to improve skin quality, and it shows significant, lasting improvements in skin quality after 1 treatment from both the clinician and patient perspectives.
A potential limitation of this study is the small number of participants with Fitzpatrick skin types I, V, and VI, and future studies of these participant groups are warranted to better understand the effectiveness of VYC-12L treatment on clinical and participant-reported outcome measures. Further, 2-dimensional photography is limited in its ability to capture the full degree of treatment-related improvements in skin texture and fine lines. However, clinical effectiveness measures in this study were completed by live investigator assessment, allowing for a more complete evaluation of improvements following VYC-12L treatment.
CONCLUSIONS
Using multiple PRO measures, this study shows that VYC-12L treatment of the cheeks is a safe, effective means to produce natural looking and feeling improvements in cheek skin smoothness and fine lines, and is associated with lasting improvements in participant satisfaction with multiple aspects of facial appearance and skin quality (eg, smoothness, hydration, radiance, and lines).
Disclosures
Financial arrangements of the authors with companies whose products may be related to the present report are as follows, as declared by the authors: Dr Chiu is a consultant and investigator for AbbVie (North Chicago, IL); Dr Montes is a consultant for AbbVie, Galderma (Lausanne, Switzerland), and Merz (Frankfurt, Germany); Dr Munavalli is an investigator and consultant for AbbVie and Merz; Dr Shamban is an investigator for AbbVie, Galderma, and Merz, and is a consultant for AbbVie, Merz, and Revance (Nashville, TN); Drs Chawla and Abrams are employees of AbbVie Inc, and may own stock/stock options in the company. Medical writing and editorial assistance were provided by Sarah J. Cross, PhD, of AbbVie, and funded by AbbVie Inc.
Funding
The design, study conduct, and financial support for the study were provided by Allergan (Irvine, CA) before its acquisition by AbbVie Inc. (North Chicago, IL). AbbVie participated in the interpretation of data, review, and approval of the publication.
REFERENCES
Author notes
Dr Chiu is a dermatologist in private practice in Redondo Beach, CA, USA.
Dr Montes is a oculoplastic surgeon, Department of Ophthalmology, University of Puerto Rico, San Juan, Puerto Rico.
Dr Munavalli is a dermatologist in private practice in Charlotte, NC, USA.
Dr Shamban is a dermatologist in private practice in Santa Monica, CA, USA.
Dr. Chawla is a director of clinical development, AbbVie Inc, Irvine, CA, USA.
Dr. Abrams is a executive director of clinical development, AbbVie Inc, Irvine, CA, USA.



