-
PDF
- Split View
-
Views
-
Cite
Cite
Anand K Deva, Commentary on: BIA-ALCL Epidemiology in an Aesthetic Breast Surgery Cohort of 1501 Patients, Aesthetic Surgery Journal, Volume 43, Issue 11, November 2023, Pages 1269–1272, https://doi.org/10.1093/asj/sjad199
Close - Share Icon Share
See the Original Article here.
“To err is human. To blame someone else is politics.” (Hubert Humphrey)1
This paper examines a cohort of 1501 patients who underwent cosmetic breast augmentation over a 10-year period.2 These patients were, with 2 exceptions, all exposed to textured breast implants with a range of texture grades: Polytech Microthane (9.2%; Dieburg, Germany), Allergan Biocell (38.6%; Irvine, CA), and Mentor Siltex (52.0%; Irvine, CA). The patients were all operated by 2 surgeons, who used well-accepted intraoperative bacterial mitigation strategies and then followed patients prospectively for outcomes postsurgery.2 The authors also performed a retrospective analysis of clinical records, presumably to obtain details of the original surgery. Mean follow-up was just over 3 years, considerably less than the mean time for development of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).3-5 The series showed a relatively high rate of implant revision (16.9%) including implant replacement and revisional mastopexy. Interestingly, 1 patient was diagnosed with breast cancer during the period of surveillance.2
Twenty-one patients (1.4%) presented with a delayed onset seroma, with 5 of these patients diagnosed with BIA-ALCL. Four of these patients had Allergan Biocell implants and 1 had a Mentor Siltex implant. The authors performed validated statistical analysis and concluded that in this cohort, the prevalence for BIA-ALCL was 1 in 300 patients with a higher risk associated with Allergan Biocell compared with Mentor Siltex implants,2 consistent with previously published comparative risks.4-6
Since its emergence in the last decade as a significant risk of textured breast implants, BIA-ALCL has been a focus of much research, regulatory action, and media attention.7,8 The issues of breast implant safety, patient informed (and educated) consent, and the assessment and reporting of medium- to long-term risk of these devices has now come into sharp focus.7 Before we look at the findings of this study and what it adds to the emerging picture of risk of BIA-ALCL, it is important we acknowledge the history and context of breast implants and the pattern of recurrent crises, court, and regulatory action that has surrounded these devices since their introduction into clinical practice in the 1960s.9
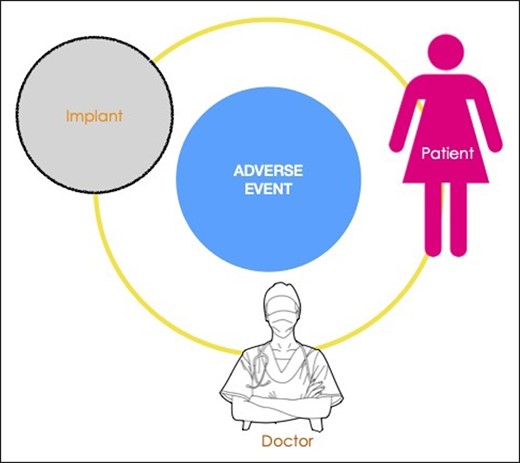
Any medical implantable device is associated with a risk matrix, in which 3 interrelated factors—the patient, the device, and the clinician deploying the device—play variable roles in generating adverse events (Figure 1). The transvaginal mesh crisis, now the subject of settled class actions, found that a lack of proper training of clinicians was a significant factor in generating harm to women.10 For the metal-on-metal hip prosthesis, also now settled in class action, the main issue was the material utilized in the device, leading to significant morbidity and revision surgery.11 For BIA-ALCL, the relative contribution of each of these 3 overarching factors will be difficult to elucidate as the disease is relatively rare with a long indolent period before onset. We have previously described a unifying hypothesis to explain the likely etiologic factors involved in T-cell lymphomagenesis,12 namely, bacteria, texture, genetics, and time. Newly published data suggest an important role in stimulating tumor cell proliferation is played by Gram-negative bacterial antigens, a response that is unique to this tumor cell13 (Figure 2). Although this may not be proof of an etiologic role for bacterial antigens, it is interesting to note that the presence of bacteria and biofilm has been linked to a dose-dependent degree of chronic inflammation, T-cell activation, and capsular contracture.14 Clinical strategies to reduce the risk of bacterial contamination at the time of implant insertion have also been shown to translate to a significant reduction in the incidence of capsular contracture.15 Bacterial inflammation may also play a role in the growing number of patients who are presenting with systemic symptoms associated with breast implants.16,17 It is interesting that the authors note that ascribing clinician factors to etiology, particularly in relation to technical factors and implant contamination, constitute “surgeon-shaming” and expose individuals to liability and more specifically legal action. Any attempt to reduce the significance of case clusters linked to clinicians/clinical units, however, tends to ignore the clinician’s role, clinical assessment, implant choice, and technical skill in performing the procedure. Clusters of adverse events can certainly be ascribed to more thorough follow-up and better surveillance as well as to a higher genetic risk of patients in a specific cohort. There remain, however, some high-risk clusters of BIA-ALCL from cosmetic breast augmentation where both follow-up and patient populations match other practices that report little or no resulting cases. In this setting, it is reasonable to assume that the reason for clustering is related to specific factors associated with the clinician and his/her clinical practice. A more accurate reflection of BIA-ALCL risk should involve the pooling of cohorts from a variety of clinicians, rather than focusing on clinicians who generate a higher number of cases.
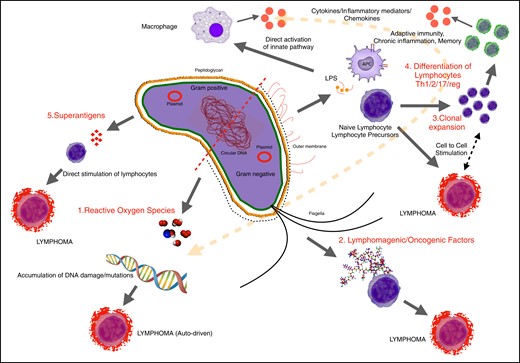
The role of bacteria in T-cell transformation and lymphomagenesis.
As more data emerge with better capture of prospective data from registries, it will be important to continue to study the role of training and certification and its association with adverse events, especially when it comes to cosmetic breast augmentation. We have looked at certification both in BIA-ALCL and other breast implant–related complications and have found a higher risk of both the risk of BIA-ALCL5 and malposition/double bubble18 when the primary cosmetic augmentation is performed by a noncertified clinician. Furthermore, analysis of implant-related complications emerging within 5 years of original surgery shows a much higher incidence of early capsular contracture, malposition, and rupture arising from surgery performed by noncertified practitioners.18 It is important that the role of the practitioner, and his/her clinical skill and training, be studied in all reported adverse events related to breast implants. To ignore this would be to perpetuate the myth that the clinician remains blameless and to falsely ascribe risk solely to the device or the patient. Recent guidelines released in this author’s state (New South Wales, Australia) have called for annual surveillance of all women with breast implants, including the incorporation of imaging of both breast and implant. This, coupled with mandatory reporting of adverse events related to the device, and the maturing of breast device registries, will provide us with a clearer picture of the relative contributions of doctor, patient, and device to adverse events.
For this study, in spite of a relatively short median follow-up, there appears to be a far greater incidence of BIA-ALCL in this cosmetic cohort,2 which will no doubt continue to rise with time. The authors do not report whether these cases of BIA-ALCL arose exclusively or predominantly from 1 of the 2 clinicians who contributed to this cohort, which would be of some interest. The risk reported here stands in stark contrast to the risk rates already published for BIA-ALCL, particularly in Australia, where implant usage is captured and adverse event reporting is now mandatory.4-6 This reported cohort may represent a high-risk cluster and thus analysis of other cosmetic cohorts arising from single/dual practitioners over time will be key in providing context to these findings. The gathering of any clusters of adverse events around a single practitioner should prompt a thorough and independent evaluation of that clinician, his/her training and credentialing, and operative techniques as well as the choice of device and the patient cohort. The identification of clusters of adverse events related to medical devices in postmarket surveillance remains the most common pathway to elucidation of risk and subsequent regulatory action, albeit with calls for further improvement in the process of data reporting, capture, and analysis.19,20 These signals are all part of ensuring that patients are protected and are given the benefit of the best long-term outcomes following implant surgery.
Disclosures
Professor Deva is a consultant and research coordinator for Abbvie (Chicago, IL) and a research coordinator for KCI (3M, St Paul, MN).
Funding
The author received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
Dr Deva is a professor, Department of Plastic and Reconstructive Surgery, Macquarie University, Sydney, Australia
Dr Deva an international editor for Aesthetic Surgery Journal.



