-
PDF
- Split View
-
Views
-
Cite
Cite
Mark L Jewell, Commentary on: Aspiration Before Tissue Filler—An Exercise in Futility and Unsafe Practice, Aesthetic Surgery Journal, Volume 42, Issue 1, January 2022, Pages 102–105, https://doi.org/10.1093/asj/sjaa408
Close - Share Icon Share
Safe injection practices regarding cosmetic injectable tissue fillers, both synthetic and autologous, offer patients optimal results with the least risk of adverse events (AEs). There is a range of AEs from ecchymosis to the most severe type of accidental intra-arterial injection that can produce tissue necrosis or vision loss. Even the most experienced injector can recall situations of moderate to severe ecchymosis that in retrospect was most likely caused by perforating a branch of the facial artery, yet filler material was not injected into the arterial lumen in any appreciable amount to cause harm. The bottom line here is that any agent, drug, or cosmetic injectable that is injected into the body has the potential to produce an adverse outcome. The importance of safe injection practices is paramount.
Given the sheer number of tissue filler injections performed, the incidence of accidental needle/cannula perforation through facial arteries is probably large (ecchymosis); fortunately, the accidental intra-arterial injection of tissue filler material is rare (catastrophic outcome). Although most of the published reports involve hyaluronic acid (HA) or fat, there is a single report of vision loss from an off-label neurotoxin injection into the masseter with a 30-gauge needle that was attributed to an ischemic retinopathy.1 This apparently involved a bolus injection of neurotoxin (25 units).
Although the network of ophthalmic arteries has been well-described, the exact location of its branches and cross-communication is, according to this article, confounded by variations in normal vascular anatomy and arterial depth. The authors are correct in stating that there is a higher risk of vision loss from glabellar injections, due to the short distance from the supratrochlear vessels to the retina (Figure 8 in the article).2
Therapeutic injections of medications below the skin (subcutaneous/intramuscular) have been taught with the technique of syringe plunger pullback to determine that the needle is not inside the lumen of an artery and hence that accidental intra-arterial injection cannot occur. Accidental intra-arterial injection of drugs such as promethazine can produce catastrophic tissue necrosis.3 There is some controversy regarding the value of aspiration before injection below the skin.4 However, syringe plunger pullback (aspiration) came to be regarded as a requisite step in safe injection practices for tissue fillers, based largely on traditional practice.
The authors have correctly questioned the value of aspiration as a safety maneuver, arguing that given the high viscosity of various fillers there is the potential for negative pressure in the syringe to suck the arterial lumen into the needle bevel and block aspiration. Their approach is juxtaposed to other reports in ASJ of positive aspiration events during tissue filler injection.5,6 How filler that is accidentally injected intra-arterially causes damage is also speculative. It could be either a column of filler that occludes the vessel, with loss of blood supply, or microemboli of filler causing injury in the eye or necrosis of facial tissues. The article is very well illustrated with regards to facial artery anatomy and potential situations of needle/cannula perforation of vessels.
A more in-depth look into syringes, needles, negative pressures, and tissue fillers, notably HA, is needed to understand the authors’ thought process leading to their concept of nonaspiration during tissue filler injections. I chose not to make this invited commentary a dissertation on microfluidics and rheology, but will cover these as they relate to flow, pressure, and viscosity because these important topics were not addressed in the article.
NEEDLE SIZE
Flow outward through a needle or cannula is a function of Poiseuille’s law: the flow rate Q is directly proportional to the pressure difference P2 − P1, and inversely proportional to the length l of the tube and the viscosity η of the fluid. Flow rate increases with r4, the fourth power of the radius of the lumen.7 Conversely, it diminishes by the same factor when going to a smaller needle/cannula.
Table 1 shows the known ISO Standard lumen diameters for 18- to 34-gauge needles. Most HA fillers are delivered via needles ranging from 27 to 32 gauge. Some variations in needle and cannula lumen, exterior dimensions, and surface coating exist as a proprietary feature from certain vendors (TSK) that may be beneficial in lessening the risk of arterial puncture (blunt tip) and reducing tissue resistance.8 Cannulas (18-30 gauge) are used for both synthetic filler and fat injections.
| Needle gauge (ISO) . | Outer diameter (mm) . | Lumen diameter (mm) . |
|---|---|---|
| 18 | 1.270 | 0.838 |
| 19 | 1.067 | 0.686 |
| 20 | 0.908 | 0.603 |
| 21 | 0.819 | 0.514 |
| 22 | 0.717 | 0.413 |
| 23 | 0.641 | 0.337 |
| 24 | 0.565 | 0.331 |
| 25 | 0.514 | 0.260 |
| 27 | 0.412 | 0.210 |
| 30 | 0.311 | 0.159 |
| 31 | 0.260 | 0.133 |
| 32 | 0.235 | 0.108 |
| 33 | 0.209 | 0.108 |
| 34 | 0.184 | 0.082 |
| Needle gauge (ISO) . | Outer diameter (mm) . | Lumen diameter (mm) . |
|---|---|---|
| 18 | 1.270 | 0.838 |
| 19 | 1.067 | 0.686 |
| 20 | 0.908 | 0.603 |
| 21 | 0.819 | 0.514 |
| 22 | 0.717 | 0.413 |
| 23 | 0.641 | 0.337 |
| 24 | 0.565 | 0.331 |
| 25 | 0.514 | 0.260 |
| 27 | 0.412 | 0.210 |
| 30 | 0.311 | 0.159 |
| 31 | 0.260 | 0.133 |
| 32 | 0.235 | 0.108 |
| 33 | 0.209 | 0.108 |
| 34 | 0.184 | 0.082 |
| Needle gauge (ISO) . | Outer diameter (mm) . | Lumen diameter (mm) . |
|---|---|---|
| 18 | 1.270 | 0.838 |
| 19 | 1.067 | 0.686 |
| 20 | 0.908 | 0.603 |
| 21 | 0.819 | 0.514 |
| 22 | 0.717 | 0.413 |
| 23 | 0.641 | 0.337 |
| 24 | 0.565 | 0.331 |
| 25 | 0.514 | 0.260 |
| 27 | 0.412 | 0.210 |
| 30 | 0.311 | 0.159 |
| 31 | 0.260 | 0.133 |
| 32 | 0.235 | 0.108 |
| 33 | 0.209 | 0.108 |
| 34 | 0.184 | 0.082 |
| Needle gauge (ISO) . | Outer diameter (mm) . | Lumen diameter (mm) . |
|---|---|---|
| 18 | 1.270 | 0.838 |
| 19 | 1.067 | 0.686 |
| 20 | 0.908 | 0.603 |
| 21 | 0.819 | 0.514 |
| 22 | 0.717 | 0.413 |
| 23 | 0.641 | 0.337 |
| 24 | 0.565 | 0.331 |
| 25 | 0.514 | 0.260 |
| 27 | 0.412 | 0.210 |
| 30 | 0.311 | 0.159 |
| 31 | 0.260 | 0.133 |
| 32 | 0.235 | 0.108 |
| 33 | 0.209 | 0.108 |
| 34 | 0.184 | 0.082 |
Little is known about the actual force required to penetrate an artery in subcutaneous tissue. It is reported that a force of somewhere between 0.81 and 3.70 newtons is required to pierce the skin with a 25-gauge hypodermic needle.9 Other approaches utilize silicone blocks as a skin substitute to measure penetration force and gliding.8
SYRINGE PLUNGER PRESSURE
Syringe plunger pressure has been studied in terms of the force required to achieve intravascular flow. Suffice it to say that this can be achieved at relatively low pressures with intravascular needle location.10 HA fillers are generally supplied in 1-mL syringes.
ASPIRATION
Aspiration is a similar function to injection: a stable pressure differential between normal atmospheric pressure on one side and negative pressure inside the syringe on the other when the plunger is withdrawn causes a backward flow through the needle. Negative pressure generated by a syringe is determined by the volume created in the syringe by pulling back the plunger.11 Syringe caliber is a secondary consideration. The pressure increase generated inside a syringe as the plunger is pulled back is not linear but parabolic in accordance with Boyle’s law (PV = K), with rapid increases in negative pressure generated initially at low volumes and leveling off after approximately 13 mL of plunger pullback. For practical purposes, the reported maximum negative pressure that can be produced by a 1-mL HA syringe is approximately –165 mm Hg or –165 torr.12 The force to generate a vacuum is expressed in negative torr-cm.3,13 The authors are correct in Figure 5B,C2 that it is possible to collapse a small artery with aspiration to the extent that blood would not flow retrogradely.
VISCOSITY
As well as needle lumen and pressure, fluid viscosity also affects flow. HA fillers exhibit a range of viscosities (η) and elastic moduli (G′) (resistance to deformation).14,15 They behave very differently to water or whole blood in terms of viscosity (water = 0.6922 mPa·s; whole blood at 37°C = 2.82 mPa·s). Given the higher reported viscosity of HA fillers, aspiration through a microneedle in any meaningful amount to signal intra-arterial location may not be possible.
However, once when I was personally performing a neurotoxin injection (0.05 mL volume) in the corrugator, I encountered a momentary whitish, linear discoloration along the course of the supratrochlear artery pathway up to the hairline due to an intra-arterial injection. Subsequently, blood flashed back into the low-loss 1-mL syringe with 32-gauge TSK needle that I was using (Figure 1).
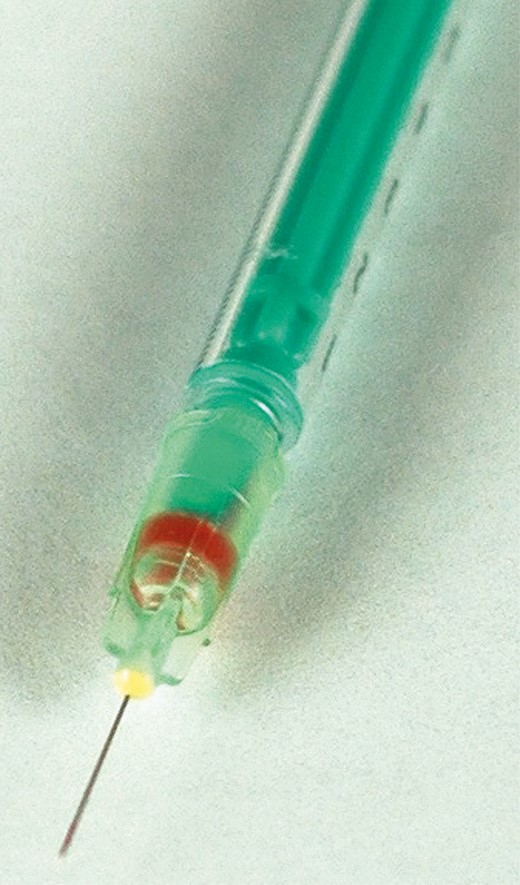
Blood backflow in 1-mL low-loss syringe with a 32-gauge needle from intra-arterial puncture.
The microneedle, which was filled with saline/reconstituted neurotoxin, offered minimal resistance to backflow when the supratrochlear artery was punctured. Fortunately, there was no AE from the small amount of neurotoxin that was injected intra-arterially. This was a totally different situation than trying to aspirate blood through a needle/cannula filled with highly viscous HA filler to confirm that the needle/cannula is not located in an artery.16 The glabella/corrugator region seems to be a safe zone for neurotoxins, yet a danger zone for tissue fillers. With something like 100 million vials of Botox Cosmetic (Allergan, Irvine, CA) purportedly sold in the United States since 2002,17 I conclude that there appears to be little risk of serious AEs from accidental intravascular injection of neurotoxin.
CONCLUSIONS
This usefully illustrated article is extremely well written and well reasoned. It references a useful consensus manuscript published in ASJ that deserves further reading in terms of anatomy and areas of high risk for intravascular injections (nose, glabella, pyriform fossa).18 It is deficient on the physics of syringe pressure and flow to explain the phenomena of intra-arterial injection, but the authors make the point correctly that aspiration during HA filler injections is not a useful practice for injection of HA fillers.
Each filler patient is unique and needs a requisite informed consent discussion that includes potential risk of serious AEs such as accidental intra-arterial filler injection and what exactly constitutes off-label product usage. The advice contained in the conclusions section of the article is particularly thoughtful and can be summarized as follows:
- •
Injectors should consider all mechanisms for avoiding intravascular complications.
- •
The choice of the implanting tool, either needle or cannula, would appear not to guarantee safety.
- •
It is also important to realize that aspiration may result in a false negative.
- •
Aspiration by its very nature precludes 2 other important safety measures—those of movement and avoidance of static bolus production.
- •
Recent literature would suggest that rather than relying on aspiration, avoidance mechanisms such as continuous movement when injecting, slow injection speed, low extrusion force, and small volumes, in conjunction with an in-depth understanding of the safer injection planes pertaining to vascular anatomy, may mitigate intravascular incidents.
I am in agreement with the additional practice, as mentioned in the Goodman et al18ASJ consensus manuscript, of administering epinephrine-containing local anesthesia to constrict local vessels as a way to reduce the risk of accidental intravascular injection. Small-volume injections for both tissue fillers and neurotoxins is a safe practice.
Safety with injectables was a major accomplishment during my terms as The Aesthetic Society’s president 2005-2006. I organized a coalition of core-trained specialties—plastic surgery, facial plastic, dermatology, dermatologic surgery, and oculoplastic—and promoted the benefits of safe injection practices. Additionally, a comprehensive workbook, Safety with Injectables Workbook, is a free download on The Aesthetic Society’s members-only website.19 This is a comprehensive workbook containing consents, a template, and information on management of filler/neurotoxin AEs. It is not as simple as opening a filler package and injecting without forethought concerning safe injectable practices.
The use of cosmetic injectables and autologous fat offers patients great opportunities to correct attritional volume loss and improve wrinkles. Safe injectable practices as emphasized by the authors and by Goodman et al are paramount to avoiding potentially catastrophic AEs. Aspiration should no longer be considered a practice that prevents intra-arterial filler AEs. It is time to stop aspirating before injecting facial fillers.
Along with facial vascular anatomy, the physics of injection and aspiration, combined with an understanding of the rheology of tissue fillers, are important concepts that every injector should grasp to develop safe injection practices. Similarly, an understanding of the physical characteristics of various breast implant silicone gels used for breast augmentation affects the outcome in terms of breast shape.20 It behooves plastic surgeons to take the time to understand the importance of these physical concepts when performing cosmetic injectables or selecting the optimal silicone gel formulation based on physical characteristics suited to the intended outcome.
Disclosures
Dr Jewell is a consultant for Allergan (Irvine, CA), Solta-Bausch Health (Bothell, WA, USA), Taproot Technologies (Johannesburg, South Africa), Silk Therapeutics (investigator and consultant) (Medford, MA, USA), and New Beauty Magazine (consultant) (New York, NY, USA).
Funding
The author received no financial support for the research, authorship, and publication of this article.
REFERENCES



