-
PDF
- Split View
-
Views
-
Cite
Cite
Eqram Rahman, Hitmi Khalifa Alhitmi, Afshin Mosahebi, Immunogenicity to Botulinum Toxin Type A: A Systematic Review With Meta-Analysis Across Therapeutic Indications, Aesthetic Surgery Journal, Volume 42, Issue 1, January 2022, Pages 106–120, https://doi.org/10.1093/asj/sjab058
Close - Share Icon Share
Abstract
Botulinum toxin A (BTX-A) is commonly employed as a neuromodulator in several neurological diseases and aesthetic indications. Formation of neutralizing antibodies (NAbs) after BTX-A injections may be responsible for treatment failure.
The authors sought to quantify the prevalence of NAbs following treatment with Abobotulinumtoxin A, Incobotulinumtoxin A, and Onabotulinumtoxin A for therapeutic indications.
An electronic systematic search (2000-2020) of PubMed, Scopus, Web of Science, and Embase was conducted. Original studies reporting prevalence of NAbs were included. Data analysis was carried out through open meta-analysis softwares.
Forty-three studies involving 8833 patients were included in this meta-analysis. The incidence of NAbs was 1.8% (summary estimate = 0.018, 95% CI [0.012, 0.023]); a meta-regression analysis revealed that BTX-A duration was significantly associated with increased incidence of NAbs (P = 0.007). Patients with dystonia had the highest incidence (7.4%) of NAbs against BTX-A (summary estimate = 0.074, 95% CI = [0.045, 0.103], I2 = 93.%, P < 0.00) followed by patients with spasticity (6.7%) and urological indications (6.2%). Abobotulinumtoxin A was associated with the highest incidence of NAbs (7.4%) (summary estimate = 0.074, 95% CI = [0.053, 0.096], I2 = 97.24%, P < 0.00) by the Incobotulinumtoxin A and Onabotulinumtoxin A 0.3% (summary estimate <0.003%, 95% CI = [−0.001, 0.007], P < 0.003).
Although the overall incidence of NAbs following BTX-A injections is relatively low, patients with secondary nonresponse to BTX-A with no apparent causes should be investigated for NAbs. A consensus needs to be developed for the optimal management of such patients.

From the first-ever documented food-borne botulism endemic “sausage poisoning” to the discovery of the molecular action of the botulinum toxin by Schievo in the 1990s, pioneering work by Kerner (1817), Ermengem (1895), Leuches (1910), Sommer (1920), Lamanna and Duff (1946), Burgen (1949), and finally Schantz and Scott (1968–1997) has caused botulinum toxin to be labelled as the “magic drug” that works across multiple therapeutic indications.1
Once started the journey as an orphan drug “Oculinum” (botulinum toxin A [BTX-A]), it’s small step into the neuromuscular junction and suppressing the presynaptic release of acetylcholine, purified botulinum toxin preparations reduce the hyperactivity of the muscle, thereby achieving a giant leap in the management of a wide range of muscle spasticity disorders, including blepharospasm, cervical dystonia, strabismus, and facial wrinkles. Other therapeutic areas such as hyperhidrosis, overactive bladder, chronic migraine, anal fissure are a few of the 150 different indications.
The main 3 commercially available (globally) botulinum toxin type A preparations are Botox (Onabotulinumtoxin A [ONA]; Allergan Inc., Irvine, CA), Dysport (Abobotulinumtoxin A [ABO]; Ipsen Biopharm Limited, Wrexham UK/Galderma LP, Fort Worth, TX), and Xeomin (Incobotulinum toxin A [INCO]; Merz Pharmaceuticals, Frankfurt, Germany).
Although the majority of patients have adequate therapeutic response following BTX-A treatment, a small number of patients may not benefit from initial BTX-A injections, constituting what is known as primary nonresponse. Other groups of patients may show initial adequate response; however, they lose the effect at subsequent injections, which is known as secondary nonresponse.2 Because the commonly utilized BTX-A preparations entail nonhuman proteins, they may influence the immune system to form antibodies against the foreign introduced antigens. The genesis of antibodies against BTX-A has been considered the main cause of secondary nonresponse.3 Other factors contributing to BTX-A secondary nonresponse include, but are not limited to, insufficient dosage and improper injection sites/methods.4
Antibodies directed against BTX-A are generally classified into neutralizing (NAbs) (formed against the binding site of heavy chain on core neurotoxin) and nonneutralizing antibodies (produced against accessory proteins or noneffective sites on the core part, which will not affect the BTX-A therapeutic effectiveness).5
Several laboratory assays were employed to identify BTX-A antibodies, including structural assays such as enzyme-linked immunosorbent assay and immunoprecipitation assays to detect the presence of antibodies with less specificity, and bioassays or functional assays such as mouse protection assay, mouse hemidiaphragm assay, unilateral brow injection test, the frontalis antibody test, sternocleidomastoid test, and the extensor digitorum brevis test to specifically look for NAbs.6 Of note, the NAbs can diminish after extended follow-ups. For instance, 1 study reported that the average period from antibody detection to their evanescence was 30 months.7 In another study, the antibodies’ titers reversed after 6 years in more than one-half of the included sample.8 However, the immunogenicity can be reactivated after reexposure to BTX-A. Given the fact that secondary nonresponse can be either partial or complete, some cases with partial response to BTX-A effects may regain therapeutic efficacy by increasing the dosing. One study has shown that the plasmapheresis may be effective in restoring BTX-A efficacy, yet implementation was limited because of high costs and increased risks.9
A handful of systematic reviews and meta-analyses have assessed the prevalence of antibodies formed in response to BTX-A injections for various clinical conditions. In a study by Lacroix-Desmazes et al, the overall rate of NAbs formation was 2.1%, and ABO and INCO did not differ.10 Another study revealed that the prevalence of antibody formation was approximately 1%, with no significant difference between ABO, INCO, and ONA.11 However, these studies had included literature published before 1998 and relied on evidence from case studies.9-11 It has been shown that newer BTX-A preparations introduced after 1998 have less antigenicity than earlier versions, which might have influenced the NAbs prevalence rates in the aforementioned reports.12
Therefore, the present systematic review and meta-analysis aimed to provide updated, robust evidence of the prevalence of antibodies formed in response to ABO, INCO, and ONA, and injections for approved therapeutic indications as well as investigate their potential determinants.
METHODS
The present systematic review (SR) and meta-analysis strictly adhered to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13 Each phase of this review was carried out in accordance with the guidelines of the Cochrane Handbook of Systematic Reviews and Meta-Analyses.14
Literature Search Strategy
An electronic literature search was conducted on February 2020 on the following medical databases: PubMed (United States National Library of Medicine [NLM], Bethesda, MD), Scopus (Elsevier, Amsterdam, the Netherlands), Web of Science (Clarivate, Philadelphia, PA), and Embase (Elsevier) from between January 2000 and January 2020 due to the fact that earlier studies were based on botulinum toxin formulations with high protein load. The following keywords were utilized in combination with Boolean logic for each database: botulinum toxin A (Abobotulinumtoxin A, ABO, Abobot*, Dysport; Incobotulinumtoxin A, INCO, Incobot*, Xeomin;Onabotulinumtoxin A, ONA, Onabot*, Botox;) AND (immunogenicity, neutralizing antibodies, antibod*, Nab or NAbs).
Eligibility Criteria and Study Selection
The present review included randomized controlled trials in addition to retrospective, cohort, and cross-sectional studies with the following criteria:
• Population: all therapeutic approved indications: individuals with blepharospasm; cerebral palsy; dystonia; forehead, glabellar, or crow feet lines; hyperhidrosis, limb spasticity; or urologic problems;
• Intervention: the utilization of BTX-A, ONA (Botox), ABO, or INCO (Xeomin);
• Outcomes: prevalence of NAbs among patients treated with BTX-A, assessed by structural assays such as immunoprecipitation assays and at least 1 bioassays such as mouse protection assay or mouse hemidiaphragm assay. We have also considered articles where mouse lethality assay was included.
Studies were excluded with the following criteria: single-arm studies, studies performed before 2000, review articles, case reports and series, non-English citations, letters, editorials, conference proceedings, and studies with unreliable data for extraction.
Screening and Study Selection
Two authors (E.R., H.H.) screened citations in 2 steps: (1) title and abstract screening, and (2) full-text screening. Additionally, we screened references to previous review articles not to miss any possible article. Any discrepancies between reviewers were solved by discussion and consensus in addition to consulting with a third, more experienced reviewer (A.M.).
Data Extraction
We assigned 2 authors (E.R., H.H.) to extract data from the included studies. Data extraction included baseline data of study personnel and risk of bias domains in addition to study outcomes.
Main Outcome Variables
The primary outcomes analyzed in this study included the incidence of NAbs across all botulinum toxin indications.
Risk of Bias Assessment
Two authors (E.R., H.H.) independently assessed the risk of bias domains among the included citations. For randomized controlled trials, we utilized the Cochrane risk of bias assessment tool.15 This tool can detect 5 types of bias: performance, selection, detection, reporting, and attrition. Included randomized controlled trials could be considered as high, unclear, or low bias source based on these domains. For cohort studies, we employed the Newcastle-Ottawa scale (NOS) for assessing bias sources.16 This tool screens for the selection of exposed and nonexposed participants, the comparability between study participants, the adequacy of the follow-up period, and the clarity of the definition of intended outcomes.
Data Analysis
Open Meta-analyst and Comprehensive Meta-analysis software were employed for meta-analysis and meta-regression of retrieved data from the included studies. The incidence of NAbs among all botulinum toxin indications was pooled as proportion. Besides, we performed a meta-regression analysis to explore the factors associated with the increase in NAbs. Moreover, a subgroup analysis was performed according to treatment indication, type of botulinum toxin, and whether the study was primarily designed to detect NAbs.
Assessment of Heterogeneity
The visual review of forest plots and the measurement of chi2 (a P value of 0.10 will be considered as statistically relevant heterogeneity) and I2 statistics were studied as a possible heterogeneity throughout studies. We investigated potential explanations utilizing sensitivity analysis where substantial heterogeneity (ie, I2 > 50%) was present.
Assessment of Reporting Biases
Potential bias in publication was evaluated utilizing funnel plots and if necessary corrected by trim and fill process.
Dealing With Missing Data
The study authors were contacted for the purpose of providing missing information or for clarifying the reason for the loss of data. There was believed to be negative data that remained incomplete.
RESULTS
Demographics and Baseline Characteristics
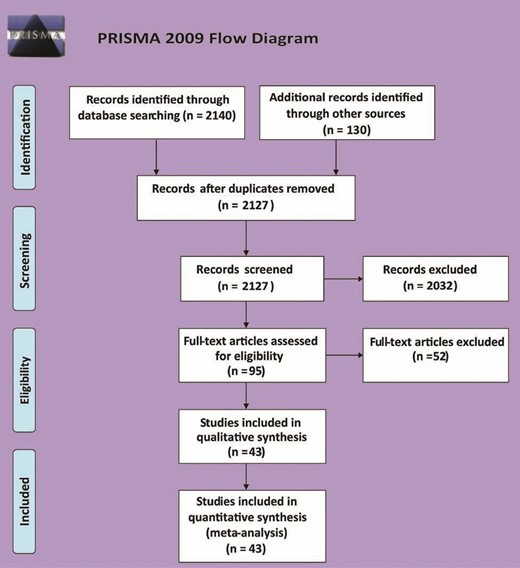
Database searching resulted in 2270 records. After duplicate removal, title/abstract screening, and full-text screening, 43 studies were finally included for this systematic review and meta-analysis (Figure 1).2,17-58 The included studies enrolled 8833 patients. Thirty studies were interventional, and 13 studies were an observational cohort design. A summary of the included studies and their baseline characteristics is presented in Table 1.
| Study ID . | Study design . | Age: mean (SD); range (y) . | Botulinum toxin type . | Indication . | Assay method . | Mean follow-up period (mo) . |
|---|---|---|---|---|---|---|
| Albrecht, 2019 | Cross-sectional | 65 (13) | ABO, ONA, and INCO | Dystonia, facial hemi spasm, spasticity and blepharospasm | ELISA and MDA | 67.2 |
| Bakheit, 2004 | Open label trial | 56.2 (11.5) | ABO | Spasticity | Mouse Lethality Assay | 5 |
| Bakheit, 2012 | Retrospective cohort | 46.6; 21-78 | ABO and ONA | Spasticity | MPA | 93 |
| Birklein, 2002 | Prospective cohort | 55; 36-69 | ABO | Dystonia | MDA and QSART | 72 |
| Brashear, 2002 | Double-blind RCT | 61; 23-88 | ONA | Spasticity | MPA | 3 |
| Brin, 2008 | Open-label, multi-center trial | 50.1; 20-82 | ONA | Dystonia | MPA and FTAT or UBI | 50.4 |
| Carruthers, 2015 | Double-blind RCT | 49.4 (9.3) | ONA | Glabellar lines | ELISA and MPA | 5 |
| Charles, 2012 | Double-blind RCT | 55; 29-77 | ONA | Dystonia | MPA | 2.5 |
| Coleman, 2012 | Double-blind RCT | 18 or older | ABO | Dystonia | MPA and RIPA | 12.9 |
| Cordivari, 2006 | Prospective cohort | — | ABO | Dystonia | EDBT, IPA, MBA | 14 |
| Elovic, 2008 | Open-label, multi-center trial | 58 (13) | ONA | Spasticity | MPA | 14 |
| Gordon, 2004 | Open-label trial | 61.5; 22.5-88.3 | ONA | Spasticity | MPA | 3 |
| Harii, 2008 | Multi-center, double-blind, randomized, placebo-controlled trial | 45.7 (±9.1) | ONA | Glabellar lines | MBA | 4 |
| Harii, 2017 | Double-blind RCT | 49.3 (6) | ONA | Crow’s feet lines | MPA | 11 |
| Hefter, 2012 | Prospective cohort | — | INCO | Dystonia | MDA | 50 |
| Hegele, 2011 | Prospective cohort | 63.5; 28-84 | ABO | Urological problems | MDA | 20 |
| Herrmann, 2004 | Retrospective cohort | 8 (4) | ABO or ONA | Spasticity | MDA | 30 |
| Imhof, 2011 | Open-label, multi-center, phase 3 trial | 45.7 (7.97) | INCO | Glabellar lines | FIA, MDA | 3 |
| Jankovic, 2011 | Double-blind RCT | 61.5 (11) | INCO | Blepharospasm | FIA, MDA | 3.4 |
| Kanovsky, 2009 | Double-blind RCT | 55.6 (12.1) | INCO | Spasticity | FIA, MDA | 5 |
| Kanovsky, 2011 | Open label trial | 55.7 (12.1) | INCO | Spasticity | MDA | 17 |
| Kawashima, 2009 | Open label trial | 46.9 (8.09) | ONA | Glabellar lines | MPA | 16 |
| Kranz, 2008 | Double-blind RCT | 52 (14) | ABO and ONA | Dystonia | MDT and NST | 3 |
| Lange, 2009 | Cross-sectional study | — | ABO and ONA | Dystonia, spasticity, and blepharospasm | MDA | 41 |
| Lawrence, 2009 | Prospective cohort | 40-58 | ABO | Glabellar lines | RIPA and MPA | 4 |
| Lowe, 2007 | Multi-center double-blind RCT | 33; 18-69 | ONA | Axillary hyperhydrosis | MPA | 13 |
| Moers Carpi, 2015 | Double-blind RCT | 50 (9.5) | ONA | Glabellar lines and glabellar + crow’s feet lines | ELISA and MPA | 7 |
| Mohammadi, 2009 | Retrospective cohort | 58 (27); 22-95 | ABO and ONA | Dystonia | MDA | 87.6 |
| Monheit, 2009 | Open label trial | 49.4 | ABO | Glabellar lines | MBA | 5 |
| Monheit, 2020 | Double-blind RCT | 44.7; 21-71 | ABO | Glabellar lines | MBA | 5 |
| Muller, 2009 | Retrospective cohort | 56.7 (11.9); 38-76 | ABO and ONA | Spasticity | MDA | 54 |
| Naumann, 2003 | Open label trial | 17-74 | ONA | Axillary hyperhydrosis | MPA | 16 |
| Oshima, 2017 | RCT | 2-16 | ONA | Spasticity | MPA and RIA | 26 |
| Schulte-Baukloh, 2008 | Prospective cohort | 48.3; 11-75 | ONA and INCO | Urological problems | MDA | 6 |
| Schulte-Baukloh, 2011 | Prospective cohort | 14.5; 6-22 | ONA | Urological problems | MDA | 71.6 |
| Schurch, 2005 | Double-blind RCT | 41; 20-72 | ONA | Urological problems | MPA | 6.5 |
| Truong, 2005 | Double-blind RCT | 53.4 (11.6) | ABO | Dystonia | Mouse Lethality Assay | 5 |
| Truong, 2010 | Double-blind RCT | 51.9 (13.4) | ABO | Dystonia | IPA then MDA | 3 |
| Truong, 2013 | Open label trial | 62.2 (10.3) | INCO | Blepharospasm | FIA then MDA | 17.2 |
| Voller, 2004 | Prospective cohort | 49.1 | ABO and ONA | Dystonia | MDT and NST | 58.8 |
| Wissel, 2017 | Prospective, single-arm, dose-titration study | 53.7 (13.1) | INCO | Spasticity | MDA | 12 |
| Yan Wu, 2009 | Prospective, double-blind, randomized, placebo-controlled, parallel-group comparative study | 42.3 | ONA | Glabellar lines | MBA | 4 |
| Yan Wu, 2019 | Multi-center, double-blind, randomized, parallel-group, placebo-controlled phase 3 study | 46.3 (9.64) | ONA | Crow’s feet lines | MBA | 5 |
| Study ID . | Study design . | Age: mean (SD); range (y) . | Botulinum toxin type . | Indication . | Assay method . | Mean follow-up period (mo) . |
|---|---|---|---|---|---|---|
| Albrecht, 2019 | Cross-sectional | 65 (13) | ABO, ONA, and INCO | Dystonia, facial hemi spasm, spasticity and blepharospasm | ELISA and MDA | 67.2 |
| Bakheit, 2004 | Open label trial | 56.2 (11.5) | ABO | Spasticity | Mouse Lethality Assay | 5 |
| Bakheit, 2012 | Retrospective cohort | 46.6; 21-78 | ABO and ONA | Spasticity | MPA | 93 |
| Birklein, 2002 | Prospective cohort | 55; 36-69 | ABO | Dystonia | MDA and QSART | 72 |
| Brashear, 2002 | Double-blind RCT | 61; 23-88 | ONA | Spasticity | MPA | 3 |
| Brin, 2008 | Open-label, multi-center trial | 50.1; 20-82 | ONA | Dystonia | MPA and FTAT or UBI | 50.4 |
| Carruthers, 2015 | Double-blind RCT | 49.4 (9.3) | ONA | Glabellar lines | ELISA and MPA | 5 |
| Charles, 2012 | Double-blind RCT | 55; 29-77 | ONA | Dystonia | MPA | 2.5 |
| Coleman, 2012 | Double-blind RCT | 18 or older | ABO | Dystonia | MPA and RIPA | 12.9 |
| Cordivari, 2006 | Prospective cohort | — | ABO | Dystonia | EDBT, IPA, MBA | 14 |
| Elovic, 2008 | Open-label, multi-center trial | 58 (13) | ONA | Spasticity | MPA | 14 |
| Gordon, 2004 | Open-label trial | 61.5; 22.5-88.3 | ONA | Spasticity | MPA | 3 |
| Harii, 2008 | Multi-center, double-blind, randomized, placebo-controlled trial | 45.7 (±9.1) | ONA | Glabellar lines | MBA | 4 |
| Harii, 2017 | Double-blind RCT | 49.3 (6) | ONA | Crow’s feet lines | MPA | 11 |
| Hefter, 2012 | Prospective cohort | — | INCO | Dystonia | MDA | 50 |
| Hegele, 2011 | Prospective cohort | 63.5; 28-84 | ABO | Urological problems | MDA | 20 |
| Herrmann, 2004 | Retrospective cohort | 8 (4) | ABO or ONA | Spasticity | MDA | 30 |
| Imhof, 2011 | Open-label, multi-center, phase 3 trial | 45.7 (7.97) | INCO | Glabellar lines | FIA, MDA | 3 |
| Jankovic, 2011 | Double-blind RCT | 61.5 (11) | INCO | Blepharospasm | FIA, MDA | 3.4 |
| Kanovsky, 2009 | Double-blind RCT | 55.6 (12.1) | INCO | Spasticity | FIA, MDA | 5 |
| Kanovsky, 2011 | Open label trial | 55.7 (12.1) | INCO | Spasticity | MDA | 17 |
| Kawashima, 2009 | Open label trial | 46.9 (8.09) | ONA | Glabellar lines | MPA | 16 |
| Kranz, 2008 | Double-blind RCT | 52 (14) | ABO and ONA | Dystonia | MDT and NST | 3 |
| Lange, 2009 | Cross-sectional study | — | ABO and ONA | Dystonia, spasticity, and blepharospasm | MDA | 41 |
| Lawrence, 2009 | Prospective cohort | 40-58 | ABO | Glabellar lines | RIPA and MPA | 4 |
| Lowe, 2007 | Multi-center double-blind RCT | 33; 18-69 | ONA | Axillary hyperhydrosis | MPA | 13 |
| Moers Carpi, 2015 | Double-blind RCT | 50 (9.5) | ONA | Glabellar lines and glabellar + crow’s feet lines | ELISA and MPA | 7 |
| Mohammadi, 2009 | Retrospective cohort | 58 (27); 22-95 | ABO and ONA | Dystonia | MDA | 87.6 |
| Monheit, 2009 | Open label trial | 49.4 | ABO | Glabellar lines | MBA | 5 |
| Monheit, 2020 | Double-blind RCT | 44.7; 21-71 | ABO | Glabellar lines | MBA | 5 |
| Muller, 2009 | Retrospective cohort | 56.7 (11.9); 38-76 | ABO and ONA | Spasticity | MDA | 54 |
| Naumann, 2003 | Open label trial | 17-74 | ONA | Axillary hyperhydrosis | MPA | 16 |
| Oshima, 2017 | RCT | 2-16 | ONA | Spasticity | MPA and RIA | 26 |
| Schulte-Baukloh, 2008 | Prospective cohort | 48.3; 11-75 | ONA and INCO | Urological problems | MDA | 6 |
| Schulte-Baukloh, 2011 | Prospective cohort | 14.5; 6-22 | ONA | Urological problems | MDA | 71.6 |
| Schurch, 2005 | Double-blind RCT | 41; 20-72 | ONA | Urological problems | MPA | 6.5 |
| Truong, 2005 | Double-blind RCT | 53.4 (11.6) | ABO | Dystonia | Mouse Lethality Assay | 5 |
| Truong, 2010 | Double-blind RCT | 51.9 (13.4) | ABO | Dystonia | IPA then MDA | 3 |
| Truong, 2013 | Open label trial | 62.2 (10.3) | INCO | Blepharospasm | FIA then MDA | 17.2 |
| Voller, 2004 | Prospective cohort | 49.1 | ABO and ONA | Dystonia | MDT and NST | 58.8 |
| Wissel, 2017 | Prospective, single-arm, dose-titration study | 53.7 (13.1) | INCO | Spasticity | MDA | 12 |
| Yan Wu, 2009 | Prospective, double-blind, randomized, placebo-controlled, parallel-group comparative study | 42.3 | ONA | Glabellar lines | MBA | 4 |
| Yan Wu, 2019 | Multi-center, double-blind, randomized, parallel-group, placebo-controlled phase 3 study | 46.3 (9.64) | ONA | Crow’s feet lines | MBA | 5 |
ABO, abobotulinum toxin a; EDBT, extensor digitorum brevis test; ELISA, enzyme-linked immunosorbent assay; FIA, fluorescence immune-assay; FTAT, frontalis antibody test; INCO, incobotulinum toxin a; IPA, immuno-precipitation assay; MBA, mouse bio assay; MDA, mouse diaphragm assay; MDT, mouse diaphragm test; MPA, mouse protection assay; NST, ninhydrin sweat test; ONA, onobotulinum toxin a; QSART, quantitative sudomotor axon reflex test; RCT, randomized controlled trial; RIA, radio-immune assay; RIPA, radio-immuno-precipitation assay; UBIT, unilateral brow injection test.
| Study ID . | Study design . | Age: mean (SD); range (y) . | Botulinum toxin type . | Indication . | Assay method . | Mean follow-up period (mo) . |
|---|---|---|---|---|---|---|
| Albrecht, 2019 | Cross-sectional | 65 (13) | ABO, ONA, and INCO | Dystonia, facial hemi spasm, spasticity and blepharospasm | ELISA and MDA | 67.2 |
| Bakheit, 2004 | Open label trial | 56.2 (11.5) | ABO | Spasticity | Mouse Lethality Assay | 5 |
| Bakheit, 2012 | Retrospective cohort | 46.6; 21-78 | ABO and ONA | Spasticity | MPA | 93 |
| Birklein, 2002 | Prospective cohort | 55; 36-69 | ABO | Dystonia | MDA and QSART | 72 |
| Brashear, 2002 | Double-blind RCT | 61; 23-88 | ONA | Spasticity | MPA | 3 |
| Brin, 2008 | Open-label, multi-center trial | 50.1; 20-82 | ONA | Dystonia | MPA and FTAT or UBI | 50.4 |
| Carruthers, 2015 | Double-blind RCT | 49.4 (9.3) | ONA | Glabellar lines | ELISA and MPA | 5 |
| Charles, 2012 | Double-blind RCT | 55; 29-77 | ONA | Dystonia | MPA | 2.5 |
| Coleman, 2012 | Double-blind RCT | 18 or older | ABO | Dystonia | MPA and RIPA | 12.9 |
| Cordivari, 2006 | Prospective cohort | — | ABO | Dystonia | EDBT, IPA, MBA | 14 |
| Elovic, 2008 | Open-label, multi-center trial | 58 (13) | ONA | Spasticity | MPA | 14 |
| Gordon, 2004 | Open-label trial | 61.5; 22.5-88.3 | ONA | Spasticity | MPA | 3 |
| Harii, 2008 | Multi-center, double-blind, randomized, placebo-controlled trial | 45.7 (±9.1) | ONA | Glabellar lines | MBA | 4 |
| Harii, 2017 | Double-blind RCT | 49.3 (6) | ONA | Crow’s feet lines | MPA | 11 |
| Hefter, 2012 | Prospective cohort | — | INCO | Dystonia | MDA | 50 |
| Hegele, 2011 | Prospective cohort | 63.5; 28-84 | ABO | Urological problems | MDA | 20 |
| Herrmann, 2004 | Retrospective cohort | 8 (4) | ABO or ONA | Spasticity | MDA | 30 |
| Imhof, 2011 | Open-label, multi-center, phase 3 trial | 45.7 (7.97) | INCO | Glabellar lines | FIA, MDA | 3 |
| Jankovic, 2011 | Double-blind RCT | 61.5 (11) | INCO | Blepharospasm | FIA, MDA | 3.4 |
| Kanovsky, 2009 | Double-blind RCT | 55.6 (12.1) | INCO | Spasticity | FIA, MDA | 5 |
| Kanovsky, 2011 | Open label trial | 55.7 (12.1) | INCO | Spasticity | MDA | 17 |
| Kawashima, 2009 | Open label trial | 46.9 (8.09) | ONA | Glabellar lines | MPA | 16 |
| Kranz, 2008 | Double-blind RCT | 52 (14) | ABO and ONA | Dystonia | MDT and NST | 3 |
| Lange, 2009 | Cross-sectional study | — | ABO and ONA | Dystonia, spasticity, and blepharospasm | MDA | 41 |
| Lawrence, 2009 | Prospective cohort | 40-58 | ABO | Glabellar lines | RIPA and MPA | 4 |
| Lowe, 2007 | Multi-center double-blind RCT | 33; 18-69 | ONA | Axillary hyperhydrosis | MPA | 13 |
| Moers Carpi, 2015 | Double-blind RCT | 50 (9.5) | ONA | Glabellar lines and glabellar + crow’s feet lines | ELISA and MPA | 7 |
| Mohammadi, 2009 | Retrospective cohort | 58 (27); 22-95 | ABO and ONA | Dystonia | MDA | 87.6 |
| Monheit, 2009 | Open label trial | 49.4 | ABO | Glabellar lines | MBA | 5 |
| Monheit, 2020 | Double-blind RCT | 44.7; 21-71 | ABO | Glabellar lines | MBA | 5 |
| Muller, 2009 | Retrospective cohort | 56.7 (11.9); 38-76 | ABO and ONA | Spasticity | MDA | 54 |
| Naumann, 2003 | Open label trial | 17-74 | ONA | Axillary hyperhydrosis | MPA | 16 |
| Oshima, 2017 | RCT | 2-16 | ONA | Spasticity | MPA and RIA | 26 |
| Schulte-Baukloh, 2008 | Prospective cohort | 48.3; 11-75 | ONA and INCO | Urological problems | MDA | 6 |
| Schulte-Baukloh, 2011 | Prospective cohort | 14.5; 6-22 | ONA | Urological problems | MDA | 71.6 |
| Schurch, 2005 | Double-blind RCT | 41; 20-72 | ONA | Urological problems | MPA | 6.5 |
| Truong, 2005 | Double-blind RCT | 53.4 (11.6) | ABO | Dystonia | Mouse Lethality Assay | 5 |
| Truong, 2010 | Double-blind RCT | 51.9 (13.4) | ABO | Dystonia | IPA then MDA | 3 |
| Truong, 2013 | Open label trial | 62.2 (10.3) | INCO | Blepharospasm | FIA then MDA | 17.2 |
| Voller, 2004 | Prospective cohort | 49.1 | ABO and ONA | Dystonia | MDT and NST | 58.8 |
| Wissel, 2017 | Prospective, single-arm, dose-titration study | 53.7 (13.1) | INCO | Spasticity | MDA | 12 |
| Yan Wu, 2009 | Prospective, double-blind, randomized, placebo-controlled, parallel-group comparative study | 42.3 | ONA | Glabellar lines | MBA | 4 |
| Yan Wu, 2019 | Multi-center, double-blind, randomized, parallel-group, placebo-controlled phase 3 study | 46.3 (9.64) | ONA | Crow’s feet lines | MBA | 5 |
| Study ID . | Study design . | Age: mean (SD); range (y) . | Botulinum toxin type . | Indication . | Assay method . | Mean follow-up period (mo) . |
|---|---|---|---|---|---|---|
| Albrecht, 2019 | Cross-sectional | 65 (13) | ABO, ONA, and INCO | Dystonia, facial hemi spasm, spasticity and blepharospasm | ELISA and MDA | 67.2 |
| Bakheit, 2004 | Open label trial | 56.2 (11.5) | ABO | Spasticity | Mouse Lethality Assay | 5 |
| Bakheit, 2012 | Retrospective cohort | 46.6; 21-78 | ABO and ONA | Spasticity | MPA | 93 |
| Birklein, 2002 | Prospective cohort | 55; 36-69 | ABO | Dystonia | MDA and QSART | 72 |
| Brashear, 2002 | Double-blind RCT | 61; 23-88 | ONA | Spasticity | MPA | 3 |
| Brin, 2008 | Open-label, multi-center trial | 50.1; 20-82 | ONA | Dystonia | MPA and FTAT or UBI | 50.4 |
| Carruthers, 2015 | Double-blind RCT | 49.4 (9.3) | ONA | Glabellar lines | ELISA and MPA | 5 |
| Charles, 2012 | Double-blind RCT | 55; 29-77 | ONA | Dystonia | MPA | 2.5 |
| Coleman, 2012 | Double-blind RCT | 18 or older | ABO | Dystonia | MPA and RIPA | 12.9 |
| Cordivari, 2006 | Prospective cohort | — | ABO | Dystonia | EDBT, IPA, MBA | 14 |
| Elovic, 2008 | Open-label, multi-center trial | 58 (13) | ONA | Spasticity | MPA | 14 |
| Gordon, 2004 | Open-label trial | 61.5; 22.5-88.3 | ONA | Spasticity | MPA | 3 |
| Harii, 2008 | Multi-center, double-blind, randomized, placebo-controlled trial | 45.7 (±9.1) | ONA | Glabellar lines | MBA | 4 |
| Harii, 2017 | Double-blind RCT | 49.3 (6) | ONA | Crow’s feet lines | MPA | 11 |
| Hefter, 2012 | Prospective cohort | — | INCO | Dystonia | MDA | 50 |
| Hegele, 2011 | Prospective cohort | 63.5; 28-84 | ABO | Urological problems | MDA | 20 |
| Herrmann, 2004 | Retrospective cohort | 8 (4) | ABO or ONA | Spasticity | MDA | 30 |
| Imhof, 2011 | Open-label, multi-center, phase 3 trial | 45.7 (7.97) | INCO | Glabellar lines | FIA, MDA | 3 |
| Jankovic, 2011 | Double-blind RCT | 61.5 (11) | INCO | Blepharospasm | FIA, MDA | 3.4 |
| Kanovsky, 2009 | Double-blind RCT | 55.6 (12.1) | INCO | Spasticity | FIA, MDA | 5 |
| Kanovsky, 2011 | Open label trial | 55.7 (12.1) | INCO | Spasticity | MDA | 17 |
| Kawashima, 2009 | Open label trial | 46.9 (8.09) | ONA | Glabellar lines | MPA | 16 |
| Kranz, 2008 | Double-blind RCT | 52 (14) | ABO and ONA | Dystonia | MDT and NST | 3 |
| Lange, 2009 | Cross-sectional study | — | ABO and ONA | Dystonia, spasticity, and blepharospasm | MDA | 41 |
| Lawrence, 2009 | Prospective cohort | 40-58 | ABO | Glabellar lines | RIPA and MPA | 4 |
| Lowe, 2007 | Multi-center double-blind RCT | 33; 18-69 | ONA | Axillary hyperhydrosis | MPA | 13 |
| Moers Carpi, 2015 | Double-blind RCT | 50 (9.5) | ONA | Glabellar lines and glabellar + crow’s feet lines | ELISA and MPA | 7 |
| Mohammadi, 2009 | Retrospective cohort | 58 (27); 22-95 | ABO and ONA | Dystonia | MDA | 87.6 |
| Monheit, 2009 | Open label trial | 49.4 | ABO | Glabellar lines | MBA | 5 |
| Monheit, 2020 | Double-blind RCT | 44.7; 21-71 | ABO | Glabellar lines | MBA | 5 |
| Muller, 2009 | Retrospective cohort | 56.7 (11.9); 38-76 | ABO and ONA | Spasticity | MDA | 54 |
| Naumann, 2003 | Open label trial | 17-74 | ONA | Axillary hyperhydrosis | MPA | 16 |
| Oshima, 2017 | RCT | 2-16 | ONA | Spasticity | MPA and RIA | 26 |
| Schulte-Baukloh, 2008 | Prospective cohort | 48.3; 11-75 | ONA and INCO | Urological problems | MDA | 6 |
| Schulte-Baukloh, 2011 | Prospective cohort | 14.5; 6-22 | ONA | Urological problems | MDA | 71.6 |
| Schurch, 2005 | Double-blind RCT | 41; 20-72 | ONA | Urological problems | MPA | 6.5 |
| Truong, 2005 | Double-blind RCT | 53.4 (11.6) | ABO | Dystonia | Mouse Lethality Assay | 5 |
| Truong, 2010 | Double-blind RCT | 51.9 (13.4) | ABO | Dystonia | IPA then MDA | 3 |
| Truong, 2013 | Open label trial | 62.2 (10.3) | INCO | Blepharospasm | FIA then MDA | 17.2 |
| Voller, 2004 | Prospective cohort | 49.1 | ABO and ONA | Dystonia | MDT and NST | 58.8 |
| Wissel, 2017 | Prospective, single-arm, dose-titration study | 53.7 (13.1) | INCO | Spasticity | MDA | 12 |
| Yan Wu, 2009 | Prospective, double-blind, randomized, placebo-controlled, parallel-group comparative study | 42.3 | ONA | Glabellar lines | MBA | 4 |
| Yan Wu, 2019 | Multi-center, double-blind, randomized, parallel-group, placebo-controlled phase 3 study | 46.3 (9.64) | ONA | Crow’s feet lines | MBA | 5 |
ABO, abobotulinum toxin a; EDBT, extensor digitorum brevis test; ELISA, enzyme-linked immunosorbent assay; FIA, fluorescence immune-assay; FTAT, frontalis antibody test; INCO, incobotulinum toxin a; IPA, immuno-precipitation assay; MBA, mouse bio assay; MDA, mouse diaphragm assay; MDT, mouse diaphragm test; MPA, mouse protection assay; NST, ninhydrin sweat test; ONA, onobotulinum toxin a; QSART, quantitative sudomotor axon reflex test; RCT, randomized controlled trial; RIA, radio-immune assay; RIPA, radio-immuno-precipitation assay; UBIT, unilateral brow injection test.
Risk of Bias Assessment
For interventional studies, low risk of bias was achieved by 12 studies regarding random sequence generation, 9 studies regarding allocation concealment, and 18 studies regarding blinding of participants, healthcare personnel, and outcome assessors. All the included studies were at low risk of bias regarding incomplete outcome data and selective reporting of outcomes (Figures 2, 3). Four of the included observational cohort studies had an NOS score of 9; 7 studies achieved a score of 8, and 2 studies scored 7 out of 9 points. With this, we conclude that the overall risk of bias in the included studies is moderate (Supplemental Tables 1, 2).
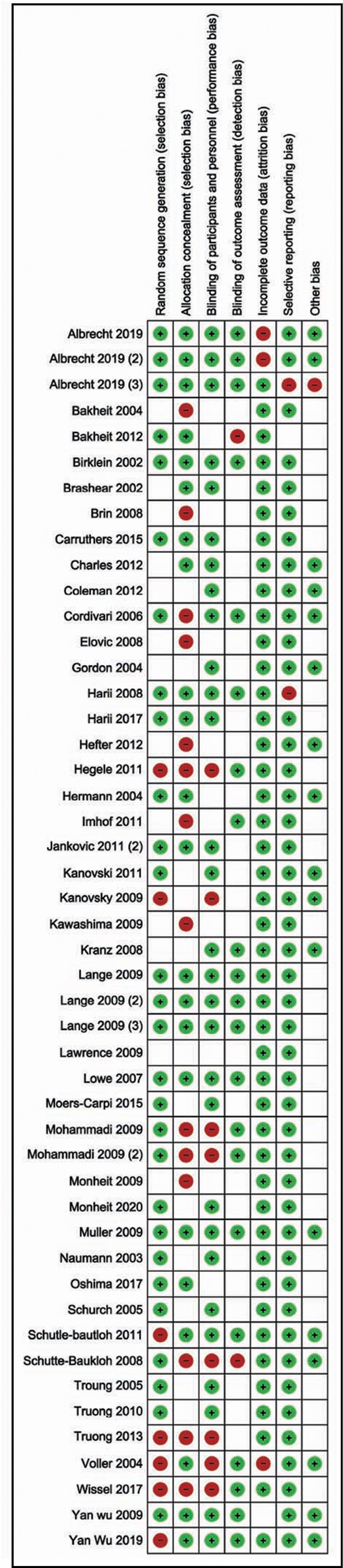
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
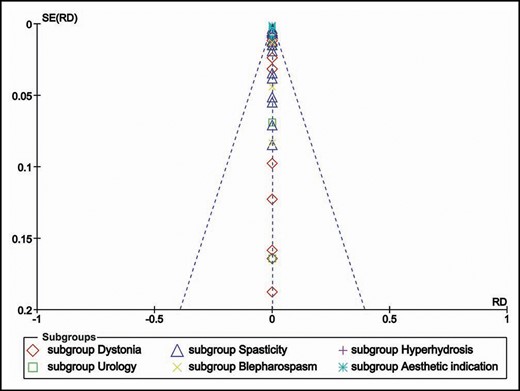
Publication Bias
The regression test for asymmetry of the funnel plot showed no publications bias of the included studies across the indications (Figure 4).
Incidence of NAbs Across all Botulinum Toxin-A Indications
Forty-three included studies provided data on the incidence of NAbs among patients injected with BTX-A for different indications. There was significant heterogeneity among these studies, and therefore we adopted the random-effects model for meta-analysis (I2 = 93.1%, P < 0.001). The summary pooled proportion indicated that the incidence of NAbs was 1.8% (summary estimate = 0.018, 95% CI [0.012, 0.023]); (Figure 5).
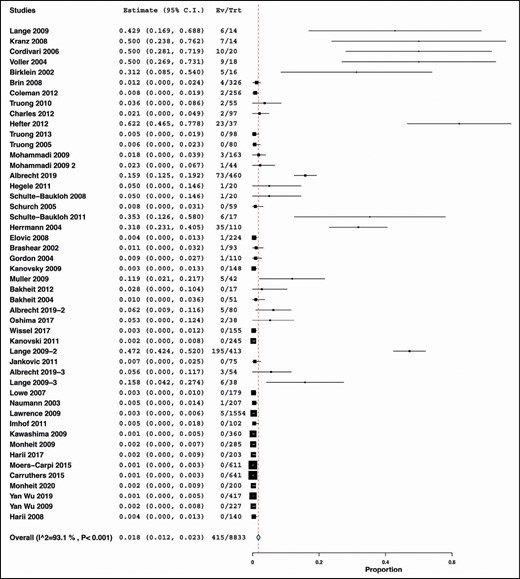
Prevalence of neutralizing antibodies across all indications and all Botox preparations.
Meta-regression analysis showed that treatment duration was significantly associated with increased incidence of NAbs (P = 0.007). (Figure 6) However, the number of injections (P = 0.14) and the dose of BTX-A (P = 0.23) were not significantly associated with the incidence of NAbs (Supplemental Figures 1 and 2).
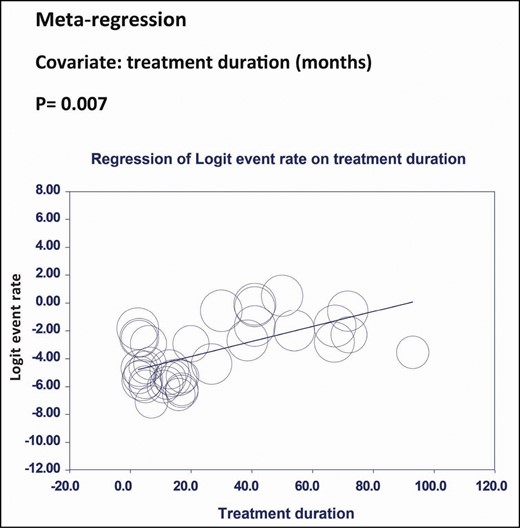
Meta-regression analysis of Logit event rate on treatment duration.
Subgroup Analysis
According to Indication
Stratifying data according to indication showed that patients with dystonia had the highest incidence (7.4%) of NAbs against BTX-A (summary estimate = 0.074, 95% CI = [0.045, 0.103], I2 = 93.%, P < 0.00). Patients with urological indications had an incidence of 6.2% (summary estimate = 0.062, 95% CI = [−0.017, 0.142], I2 = 69.55%, P = 0.02); spasticity patients had a similar incidence of 6.7% (summary estimate = 0.067, 95% CI = [0.041, 0.093], I2 = 97.17%, P < 0.00), and patients with blepharospasm developed NAbs with an incidence of 5.4% (summary estimate = 0.054, 95% CI = [−0.015, 0.123], I2 = 76%, P = 0.015). The incidence of NAbs was rare in both hyperhidrosis (summary estimate = 0.004, 95% CI = [−0.002, 0.010], I2 = 0%, P = 0.741) and aesthetic indications (summary estimate = 0.002, 95% CI = [0, 0.003], I2 = 0%, P = 0.984) (Figure 7).
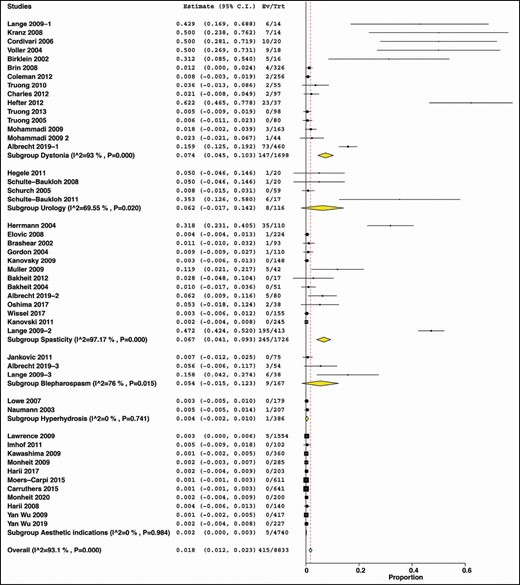
According to Botulinum Toxin Type
When subgrouping by the type of botulinum toxin, ABO was associated with the highest incidence (7.4%) of NAbs (summary estimate = 0.074, 95% CI = [0.053, 0.096], I2 = 97.24%, P < 0.00). INCO and ONA exhibited lower incidence of NAbs compared with ABO (INCO: summary estimate = 0.003, 95% CI = [−0.001, 0.007], I2 = 0%, P < 0.995); ONA: summary estimate = 0.003, 95% CI = [0.001, 0.006] I2 = 53.47%, P < 0.003). (Figure 8)
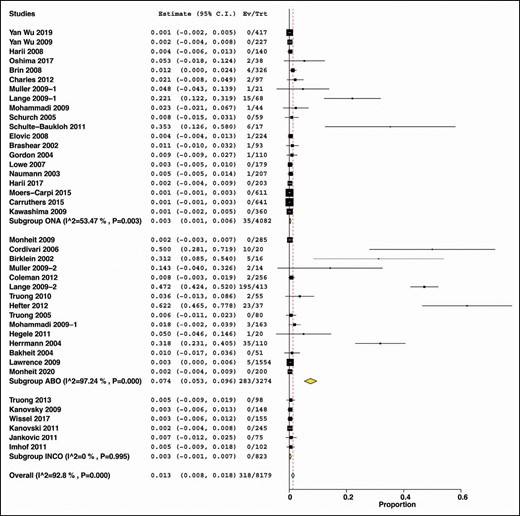
Subgroup analysis by the commercially available botulinum toxin type A.
According to Study Design
Studies primarily designed to detect NAbs in patients treated with botulinum toxin had a significantly higher incidence of NAbs (summary estimate = 0.166, 95% CI = [0.123, 0.209], I2 = 97.05%, P < 0.000) compared with studies not primarily designed to detect NAbs (summary estimate = 0.002, 95% CI = [0.001, 0.003], I2 = 0%, P = 0.671) (Figure 9).
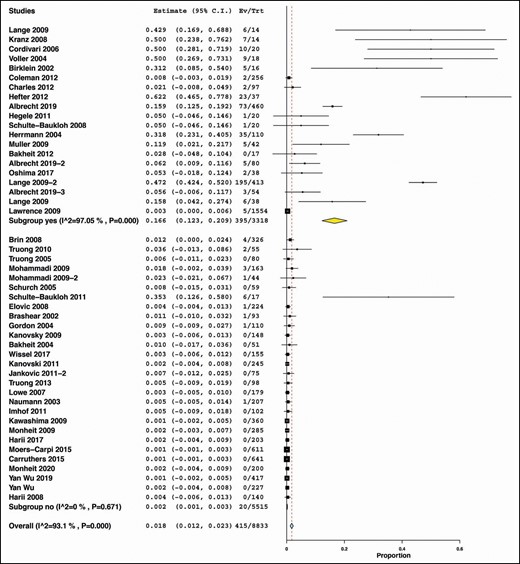
Subgroup analysis by whether the study primarily designed to detect neutralizing antibodies.
DISCUSSION
BTX-A has been shown to be effective for both short- and long-term management of dystonia, spasticity, neurogenic bladder, trigeminal neuralgia, migraine, and the cosmetic treatment of facial wrinkles. Although NAbs may affect BTX-A treatment outcomes, the present systematic review and meta-analysis of 43 studies—entailing the most extensive pooled analysis among published literature—showed that the incidence of NAb formation is only 2.4% across various therapeutic indications. In a previous meta-analysis by Fabbri et al, the prevalence of NAbs was 3.5% among clinically responsive patients and 53.5% among patients with secondary nonresponse.9 However, their study included articles published since 1991, and it is well-known that BTX-A preparations before 1998 were more antigenic, which may have influenced the authors’ inferences and statistics. Moreover, they did not provide the characteristics of the included studies so that future researchers can compare the data. In another recent meta-analysis, the authors included both old and new BTX-A generations with an overall prevalence of 1.9% and also provided statistics of each formulation. As anticipated, the prevalence of NAbs was higher for old BTX-A formulations compared with the presently available BTX-A forms.10 The meta-regression in our study has revealed that the treatment duration is significantly correlated with an increased incidence of NAbs. This is consistent with previous reports of ONA.59,60 Thus, it is recommended to consider a BTX-A regimen that is sufficient enough to attain therapeutic efficacy yet still below the duration at which antigenicity may occur with avoidance of the booster dose.61
In the present study, the greatest incidence of NAbs was reported with dystonia, followed by spasticity, urological indications, and blepharospasm. In the study mentioned above by Fabbri et al, the frequency of NAbs was 20% in patients with dystonia and 5.9% for patients with spasticity compared with 10% and 6% in our study, respectively. The history of BTX-A application for dystonia may explain the significant difference observed in the Fabbri et al study. It is worth noting that BTX-A was employed for the management of dystonia many years before administering it for limb spasticity; hence, the incidence of NAbs for patients with dystonia may be influenced by the old BTX-A included in the Fabbri et al analysis. Nonetheless, further studies are needed to clarify, because our analysis was solely based on newer preparations of BTX-A. Additionally, in a systematic review of 14 studies published between 2002 and 2018, the frequency of NAbs in patients with limb spasticity was approximately 1%, and the treatment duration was also associated with an increased incidence of NAbs.
It has also been suggested that a higher dosage and shorter interval of BTX-A especially in cervical dystonia and spasticity may contribute to the NAbs formation.2,62,63 However, in the present study, the meta-regression showed that the prevalence of NAbs is statistically significant for a longer duration (>10 years) than the dosage (mean dose per session in mouse unit [mU]: >389 for ABO, >120 mU for INCO, >145 mU for ONA) or the number of injections.64 This is most likely due to the fact that the dosage gradually increases over time to exert optimal therapeutic effect as a result of change in the afferent input, modification of the sensory afferent, and associated cortical plasticity.64-67
In comparing the 3 main commercially available products of BTX-A enrolled in our study, ABO was associated with the highest incidence of NAbs, followed by INCO, and ONA was associated with the lowest incidence. When NAbs are formed against specific a BTX-A formulation, it is recommended not to re-administer it, at least for the short term. Also, a possible therapeutic approach is switching to another BTX-A preparation; however, the available clinical evidence on this approach is not robust.11 A report of a 58-year-old man with spasticity and secondary nonresponse to ONA has shown clinical improvement after receiving INCO. However, no conclusion can be reached from this observation because NAbs were not assessed in the study.68 Due to the lack of quantitative, precise, and sensitive assays for checking NAbs, the relation between NAbs and BTX-A’s nonresponse remains a matter of concern. NAbs are only 1 potential reason for BTX-A’s lack of clinical efficacy. Other possible explanations includes BTX-A preparation and administration errors, inappropriate muscle selection due to inadequate understanding of the musculoskeletal anatomy, and insufficient dosage. Therefore, healthcare providers should consider these possible factors in case of treatment failure.8,69
Some notable observations were made while conducting the present systematic review. First, the majority of the studies related to the NAbs were commercially funded, and disclosure of financial conflicts of interest has limited effects and may not eliminate bias or its effects on practice.70 Moreover, incorporation of an inappropriate comparator and publication bias often favors the product linked with a funder.71 Second, although some authors suggested that the presence of the nontoxic clostridial proteins increased the chances of NAb formation—giving an example of an experiment with Botulinum toxin B in rabbits—neither claim provided robust argument, and no definite immunological pathway has been postulated.72 Third, the studies exploring NAb formation do not seem to follow the standard method of NAb detection, that is, structural assays followed by bioassays. Fourth, considering the number of BTX-A procedures vs NAb formation, a link to the patients’ genetic predisposition due to Human Major Histocompatibility Complex may be a possible explanation that requires further exploration.73 A clear and precise definition of the clinical efficacy of BTX-A and clinical nonresponse should be established. Furthermore, it is paramount to set a novel and highly sensitive diagnostic assay to assess the NAbs and make this widely accessed; hence, practitioners can readily monitor patients receiving BTX-A and take the proper measures to lessen BTX-A failure of treatment.
Although the overall number of included studies in the current systematic review remains respectable, quantifying the incidence of NAbs across all BTX-A indications has been limited by significant heterogeneity. Nonetheless, our study has several strengths. First, the evidence from the present meta-analysis is deemed considerable because it relied on data from randomized studies and observational cohort reports whereas case studies were excluded. Second, we limited the inclusion criteria to studies published between 2000 and 2020 as research showed that newer generations of BTX-A are less antigenic. Third, the risk of bias was evaluated employing the standardized Cochrane and NOS tools for randomized studies and observational studies, respectively.
A meta-analysis, often employed to combine the pooled studies’ effect size that certain respects are different, referred as “combining apples and oranges”. A meta-analysis may be invalidated by the ability to transcend substantial discrepancies between studies.74 Publication biases would likely influence the true effects of meta-analysis. These limitations are avoided by the robust inclusion and exclusion criteria. An effort was also made to execute a trial sequential analysis to substantiate the robustness of the meta-analysis. However, this was not possible due to the variability in the published data.
CONCLUSIONS
The pooled analysis in the present systematic review revealed that the overall incidence of NAbs following BTX-A therapy is relatively low. However, the incidence is much higher in specific conditions, such as dystonia, urological problems, and spasticity. ABO was associated with a high incidence of NAbs compared with INCO and ONA. Patients receiving BTX-A and exhibiting secondary nonresponse with no apparent causes should be investigated for NAbs. Further and more importantly, a consensus needs to be reached regarding the optimal management for those patients, such as introducing another type of BTX-A; however, this would require standard protocols and vigorous evidence from large randomized trials. Until then, “comparing the incomparables” may only lead to perplexion.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES



