-
PDF
- Split View
-
Views
-
Cite
Cite
Mingrui Jiang, Guangjun Zhong, Yichao Zhu, Liuhua Wang, Yuzhe He, Qiannan Sun, Xiaoqing Wu, Xiaolan You, Sujun Gao, Dong Tang, Daorong Wang, Retardant effect of dihydroartemisinin on ulcerative colitis in a JAK2/STAT3-dependent manner, Acta Biochimica et Biophysica Sinica, Volume 53, Issue 9, September 2021, Pages 1113–1123, https://doi.org/10.1093/abbs/gmab097
Close - Share Icon Share
Abstract
Dihydroartemisinin (DHA) is a semi-synthetic derivative and the main active metabolite of artemisinin. The purpose of this study was to investigate the effect of DHA on the ulcerative colitis (UC) in both in vivo and in vitro models. Weight, survival rate, colon length, and Disease Activity Index score were used to evaluate the severity of colitis. Reverse transcription quantitative polymerase chain reaction and enzyme-linked immunosorbent assay were used to detect the expressions of cytokines interleukin (IL)-1, IL-1β, IL-4, IL-6, IL-10, IL-12, and tumor necrosis factor-α (TNF-α). The expressions of Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3), and the phosphorylation of JAK2 (p-JAK2) and STAT3 (p-STAT3), were measured by western blot analysis. Western blot analysis and immunohistochemistry were used to detect the expressions of tight junction proteins. We found that the weights and colon lengths of mice in dextran sodium sulfate (DSS)+DHA group were significantly lower and longer than those in the DSS group, respectively. Compared with those in the DSS group, the expressions of IL-1β, IL-6, IL-17, and TNF-α in the DSS+DHA and DSS+5-aminosalicylic acid (5-ASA) groups were decreased, while the expressions of IL-4 and IL-10 were significantly upregulated. DHA largely increased the expressions of zonula occludens-1 and occludin. Western blot analysis and/or immunohistochemical staining analysis showed that the expressions of JAK2, STAT3, p-JAK2, and p-STAT3 in DSS+DHA and DSS+5-ASA groups were significantly lower than those in DSS group. DHA has a specific therapeutic effect on UC. The anti-inflammatory mechanism of DHA is related to the blockage of the JAK2/STAT3 signaling pathway. These findings provide evidence that DHA may be a useful drug and is expected to become a promising new treatment for human UC.
Introduction
Inflammatory bowel disease (IBD) is an immune-mediated disease characterized by chronic and recurrent inflammation, including ulcerative colitis (UC) and Crohn’s disease with the symptoms of abdominal pain, diarrhea, blood in the stool, and weight loss. Their incidence and prevalence are increasing globally [1]. Among them, UC is characterized by recurrence of mucosal inflammation, and the lesions are mainly located in the rectum and proximal colon. Current therapeutic interventions for UC focus on inducing and maintaining clinical remission. The exact cause of IBD is still unclear [2]. But it is known that intestinal bacteria, lifestyle habits, and genetic factors all contribute to the development of the disease [3]. The drugs currently used for the treatment of UC mainly include aminosalicylic acid (ASA), glucocorticoids, immunosuppressants, and biological products. Because of the long course of treatment, their treatment is usually limited by adverse events. Recurrence of the disease often occurs when the treatment is stopped [4]. Moreover, these drugs are not effective for all patients. This disease requires new and more effective treatments.
Dihydroartemisinin (DHA) is a semi-synthetic derivative and the main active metabolite of artemisinin, a natural product isolated from the Chinese herbal medicine Artemisia annua [5]. DHA is a recognized and well-tolerated drug with the advantages of highly curative effect, low side effects, and low cost. DHA is a derivative of the artemisinin family, and it is also one of the first-line antimalarial drugs recommended by the World Health Organization [6,7]. DHA has been proven to have antiviral and antibacterial effects, and it is also an effective immunomodulator [8].
DHA has anti-inflammatory and antitumor functions [7]. A previous report has shown that DHA inhibits the growth of melanoma cells, esophageal cancer cells, and other malignant tumor cells by regulating the signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa B (NF-κB) signaling pathways which activate the progression of IBD [9].
In the present study, we hypothesize that DHA may inhibit the progress of UC through a similar mechanism [10]. Although some treatments have been applied to control the development of IBD, some patients with IBD may still proceed to colorectal cancer. IBD is currently considered to be an autoimmune disease, and if it is not treated effectively, it may progress to colon cancer. Because the treatment of IBD requires long-term medication, DHA may be a promising drug candidate for the treatment of UC. Here, we investigated the effect of DHA on an in vivo model of UC induced by dextran sodium sulfate (DSS) and explored its underlying mechanism, which involves the Janus kinase 2 (JAK2)/STAT3 signaling pathways.
Materials and Methods
Materials
DHA (batch number: D7439), lipopolysaccharide (LPS) (batch number: L2880), and phorbol 12-myristate 12-acetate (PMA) (batch number: P1585) were purchased from Sigma (St Louis, USA), and sodium dextran sulfate (batch number: 160110) was purchased from MP Bio (Santa Ana, USA). 5-ASA (batch number: MB7539) was purchased from Meilun Biotech (Dalian, China). Interferon gamma (IFN-γ; batch number: HEIFP-0201) was purchased from Saiye Biotechnology (Suzhou, China). Anti-STAT3 antibody (batch number: 12640), anti-phospho-STAT3 (Tyr705) antibody (batch number: 9145s), anti-JAK2 antibody (batch number: 3230s), anti-phospho-JAK2 (Tyr1007/1008) antibody (batch number: 3776s), anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (batch number: 2118s), and horseradish peroxidase (HRP)-conjugated secondary antibody (batch number: 7074s) were purchased from Cell Signaling Technology (Danvers, USA). Anti-zonula occludens (ZO)-1 antibody (lot number: abs122482), anti-occludin antibody (batch number: abs136990), Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Assay kit (batch number: abs50001), Cell Counting Kit 8 (CCK-8; batch number: abs50003), mouse interleukin (IL)-1β enzyme-linked immunosorbent assay (ELISA) kit (batch number: abs520001), mouse IL-4 ELISA kit (batch number: abs520003), mouse IL-6 ELISA kit (batch number: abs520004), mouse IL-10 ELISA kit (batch number: abs520005), mouse IL-17 ELISA kit (batch number: abs520009), and mouse TNF-α ELISA kit (batch number: abs520010) were purchased from Absin (Shanghai, China). Bicinchoninic acid (BCA) Protein Assay Kit (batch number: WB6501) and enhanced chemiluminescence (ECL) Ultra Reagent A/B (batch number: P10100) were purchased from NCM Biotech (Suzhou, China). 5×FastKing-RT SuperMix (batch number: KR118) was purchased from Tiangen (Beijing, China). 2×Universal SYBR Green Fast quantitative polymerase chain reaction (qPCR) Mix (lot number: RK21203) was purchased from ABclonal (Wuhan, China).
Animals
Male C57BL/6J mice were obtained from the Animal Center of Translational Medical College of Yangzhou University (Yangzhou, China). All mice were 7–8 weeks old and caged under controlled conditions [light (12/12 h light/dark cycle), humidity (50%±5%), and temperature (20°C–22°C)]. Standard free eating and drinking were allowed during the experiment. Before the study, the animals were acclimated for 7 days. All animals were handled in accordance with the Animal Welfare Guidelines of the Institutional Animal Care and Use Committee of Yangzhou University. The protocol of this study was approved by the Medical Ethics Center, Northern Jiangsu People’s Hospital (permit number: 81972269).
Establishment of experimental colitis in mice
The mice were randomly assigned into four groups: control group (n=10), DSS group (n=10), DSS+5-ASA group (n = 10), and DSS+DHA group (n=10). After adaptive breeding for 7 days in an environment with a temperature of 20°C–22°C and 50% humidity, mice were provided with 2.5% DSS in drinking water for 7 days to induce acute colitis. Mice in chronic colitis model group were fed for three cycles with each cycle of 2.5% DSS for 7 days and distilled water for 10 days [11]. From the beginning of the third cycle, mice in the DSS+DHA group were fed with 20 mg/kg DHA daily for seven consecutive days. Similarly, mice in the DSS+5-ASA group were fed with 10 mg/kg 5-ASA for seven consecutive days as well. Mice in the control group and DSS group were fed with phosphate buffered saline (PBS) instead. The weight loss, stool characteristics, and blood in the stool were observed and recorded every day. The clinical course of colitis is evaluated by the daily Disease Activity Index (DAI), including three parameters: stool consistency, weight loss, and the presence of blood in stool and the anus. The score was determined as follows: change in body weight loss (0: none, 1: 1%–5%, 2: 5%–10%, 3: 10%–20%, and 4: >20%); stool blood (0: no blood, 2: blood trace in the stool clearly visible, and 4: gross rectal bleeding); and stool consistency (0: normal, 1–2: loose stool, and 3–4: diarrhea) [12]. On Day 51, mice were euthanized, and the colon and blood samples were collected. The length of the colon was measured, and the colons were saved for subsequent experiments [13].
Histological analysis of the colon
On Day 51, all mice were anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg). Subsequently, the entire length of the colon from the anus to the ileocecal area in each group (n = 10 per group) was measured and washed with PBS. The body weight was immediately assessed and the weight index of the colon was calculated as follows: colon weight/body weight×100%. Then, the colon segment was fixed in 4% formaldehyde solution for at least 7 days [14]. Subsequently, the colon tissues were dehydrated, embedded in paraffin, cut into 5-µm sections, and fixed on glass slides. These sections were stained with hematoxylin and eosin (H&E).
About 1-cm colon tissue, which is 0.5 cm away from the anal margin, was collected, and the ileocecal area was used for histopathological examination [15]. After H&E staining, the tissues were scored under single-blind conditions to evaluate the levels of inflammation and tissue damage in the distal and proximal colons [16]. The scores were based on Ameho criteria and were counted as follows: 0, no abnormal findings in texture; 1, mild mucosa and/or mucosa inflammatory infiltration (mixed neutrophils) and edema of the lower layer, punctate mucosal erosion, accompanied by capillary hyperplasia. The muscularis mucosa is intact; 2, 1-point change accounted for more than 50% of the specimen; 3, inflammatory infiltration and edema are obvious (neutrophils are often dominant); usually ulcers penetrate the muscularis mucosa to reach the submucosa. There is almost no inflammatory cell invasion in the muscularis propria and no muscle necrosis; 4, 3-point changes accounted for more than 50% of the specimen; 5, large sheet ulcers with coagulative necrosis, a large number of neutrophils infiltrated at the border, and fewer monocytes. The necrotic tissue penetrates the muscularis propria; and 6, 5-point changes account for more than 50% of the specimen [17].
Cell culture
Caco-2 cells (SCSP-5027; National Collection of Authenticated Cell Cultures, Shanghai, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM)-based cell culture medium (11054020; Gibco, Grand Island, USA) supplemented with 20% heat-inactivated fetal bovine serum (F8318; Sigma, St Louis, USA) and 1% penicillin/streptomycin (V900929; Sigma) in a cell incubator at 37°C with 5% CO2 [18].
On the apical side, Caco-2 cells inoculated for 18–21 days are cultured in DMEM, and the medium in the basolateral compartment is changed to Roswell Park Memorial Institute (RPMI)-based THP-1 medium (51536C; Sigma) without mercaptoethanol. Tohoku Hospital Pediatrics-1 (THP-1) cells obtained from the National Collection of Authenticated Cell Cultures (TCHu57) were seeded in a 25-cm2 flask (3×106 cells) and then differentiated with PMA (100 nM) for 24 h. Then, the cells were isolated using cell digestion solution (T4049; Sigma), plated on a suitable 6-well transwell plate at a density of 1.8×105 cells/well, and allowed to reattach for 1.5 h. To start the co-culture, the transwell filter containing Caco-2 cells was transferred to the well containing differentiated THP-1 cells and cultured for 24 h without any additional manipulation [19].
The modeled cells were set as follows: Caco-2+THP-1 co-culture blank control group, Caco-2+THP-1 co-culture+IFN-γ+LPS model group [20], Caco-2+THP-1 co-culture+IFN-γ+LPS+DHA (20 μM) group, and Caco-2+THP-1 co-culture+IFN-γ+LPS+DHA (40 μM) group [5]. The cells in the two DHA groups were treated with different concentrations of DHA for 24 h. At last, every group’s cells were stimulated with 100 ng/ml LPS for 6 h, and then cells from each group were placed in an Eppendorf tube, centrifuged at 7500 g for 5 min at 4°C [20], and the cells from each group were collected and cultured in DMEM.
Cell viability assay
Cell viability was measured using the CCK-8 method [21]. Briefly, cells were seeded in a 96-well plate with a density of 8000 cells per well in 0.2 ml DMEM and cultured for 24 h [22]. Then, cells were treated with different concentrations (0, 20, and 40 μM) of DHA for 24 h. After that, 20 μl CCK-8 was added into each well [23] and incubated for 4–6 h. Finally, the optical density of each well was measured at 450 nm with a multifunctional microplate reader (Thermo scientific, Waltham, USA), and cell viabilities were calculated.
Annexin V staining
Cells in the control group and the DHA treatment groups (0, 20, and 40 μM) were cultured for 48 h. Then, cells were resuspended in PBS in a test tube, and 5 μl of Annexin V-FITC and propidium iodide were added to each tube [21] and incubated for 10 min in the dark. The cell apoptosis was detected by flow cytometry as previously described [24].
Enzyme-linked immunosorbent assay
After the cell model was co-cultured for 24 h, the cell-free supernatant of the basolateral compartment was used to quantify the release of IL-lβ, IL-6, IL-17, IL-4, IL-10, and TNF-α. The absorbance was read spectrophotometrically at 450 nm [25].
The mouse serum was collected from the animal model, and the concentration levels of IL-1β, IL-6, IL-17, IL-4, IL-10, and TNF-α were measured using the corresponding ELISA kits according to the manufacturer’s instructions [26].
Immunohistochemical staining
Paraffin sections of colon tissue were dried in an oven at 60°C for 1 h and successively washed three times with xylene (5 min each), two times with 100% ethanol (10 min each), two times with 95% ethanol (10 min each), and two times with double-distilled water [27]. After being incubated for 5 min, the paraffin sections were placed in a medium intensity microwave oven for 8 min for antigen recovery, and nonspecific antigens were blocked using hydrogen peroxide (3%). Then, the sections were incubated with anti-JAK2 antibody or anti-ZO-1 antibody at 4°C overnight, followed by incubation with the HRP-conjugated secondary antibody and 3,3′-diaminobenzidine staining [28]. Finally, the sections were dehydrated and mounted, and observed under a microscope (Olympus, Tokyo, Japan). At least three immunohistochemical staining sections were examined in each group. The intestinal tissues of one clinical IBD patient with informed consent were collected for JAK2 and ZO-1 immunohistochemical staining as well. The study was approved by the Medical Ethics Center, Northern Jiangsu People’s Hospital (permit number: 81172279).
Western blot analysis
The protein concentration in the colon tissue supernatant was determined using the classic BCA Protein Assay Kit (Bio-Rad, Hercules, USA). Equal amount of protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, USA) [29]. After being blocked with 5% bovine serum albumin, the membrane was incubated with the following primary antibodies for 24 h at 4°C: anti-occludin (1:1000), anti-ZO-1 (1:1000), anti-JAK2 (1:1000), anti-p-JAK2 (1:1000), anti-STAT3 (1:1000), anti-p-STAT3 (1:2000), and anti-GAPDH (1:2000). Then, the membrane was incubated with the appropriate HRP-conjugated secondary antibody (1:2000–1:3000) and visualized using an ECL detection kit. ImageJ software was used to analyze the results [30]. Protein content=sample protein gray value/gray value of the internal reference of GAPDH in the same sample.
RNA extraction and real-time quantitative PCR
According to the manufacturer’s instructions, TRIzol reagent (15596026; Gibco) was used to extract total RNA from colon tissues [31]. FastKing-RT SuperMix and Universal SYBR Green Fast qPCR Mix reagents were used for RT-PCR. SYBR Green qPCR was performed on the OneStep real-time PCR system to detect the mRNA expressions of IL-lβ, IL-6, IL-10, TNF-α and ZO-1 in the colon tissue of each group of mice. Amplification conditions were 95°C for 3 min (1 cycle); 95°C for 5 s and 60°C for 34 s (40 cycles). After each cycling protocol, a melt curve analysis was generated by heating from 65°C to 95°C with 0.5°C increments for 5 s to confirm the absence of nonspecific products or primer dimers and define melting temperatures (Tm) for each amplicon. The primers were as follows: GAPDH forward: 5′-GAGTCAACGGATTTGGTCGT-3′, reverse: 5′-TTGATTTTGGAGGGATCTCG-3′;
IL-1β forward: 5′-GTAATGAAAGACGGCACACCC-3′, reverse: 5′-GTGCTGATGTACCAGTTGGG-3′; IL-6 forward: 5′-AGCCAGAGTCCTTCAGAGAG-3′, reverse: 5′-ACTCCTTCTGTGACTCCAGC-3′; IL-10 forward: 5′-GGTTGCCAAGCCTTATCGGA-3′, reverse: 5′-CTTCTCACCCAGGGAATTCA-3′; TNF-α forward: 5′-TCGAGTGACAAGCCTGTAGC-3′, reverse: 5′-GGAGGTTGACTTTCTCCTGG-3′; and ZO-1 forward: 5′-GGGCCATCTCAACTCCTGTA-3′, reverse: 5′-AGAAGGGCTGACGGGTAAAT-3′.
Statistical analysis
Data are expressed as the mean±standard deviation (SD). Data such as body mass index and DAI were evaluated by two-way analysis of variance (ANOVA). One-way ANOVA was used to compare data between groups. P<0.05 is considered statistically significant.
Results
DHA ameliorates DSS-induced colitis in vivo
To clarify the role of DHA in chronic colitis, we observed the effect of DHA on DSS-induced chronic colitis (Fig. 1A). Compared with mice with DSS-induced colitis, mice treated with DHA and 5-ASA showed significantly reduced inflammatory responses. The body weight of the DSS group mice was significantly lower than that of the control group. But the body weight of mice in the 20 mg/kg DHA and 10 mg/kg 5-ASA groups was significantly higher than that in the DSS group (Fig. 1B). Chronic colitis induced by DSS can shorten the colon of mice. DAI is an important indicator for evaluating disease activity. According to DAI obtained from weight loss, diarrhea, and bloody stool percentage, the DAI of the DSS group was significantly higher than that of the control group, while the DAI of the DSS+DHA group and DSS+5-ASA group was lower than that of the DSS group (Fig. 1C). The colon length in the DSS group was significantly shorter than that of the control group. The colon shortening of the DSS+DHA group and DSS+5-ASA group was significantly reduced (Fig. 1D,E).
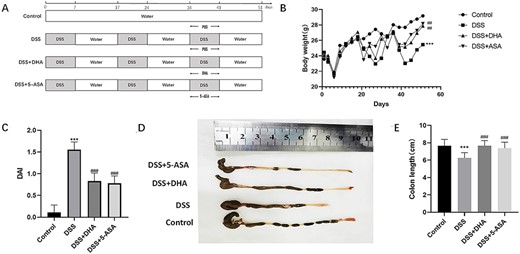
Effects of DHA on clinical signs in DSS-induced colitis (A) Animal model of DSS-induced chronic colitis in mice. (B) Body weight changes. (C) The DAI score was calculated as the average of weight loss score, diarrhea score, and fecal blood score on Day 51. (D) The colons collected in the UC model. (E) The difference of colon length among four groups. Data are presented as the mean±SD. ***P<0.001 vs control group. ##P<0.01, ###P<0.001 vs DSS group. n = 10.
Histological evaluation of colon sections of untreated colitis animals showed that DSS-induced colitis was characterized by loss of mucosal structure, thickening of the colon wall, ulcer formation, and extensive inflammatory cell infiltration of the colonic mucosa (Fig. 2A). DHA and 5-ASA treatment inhibited these pathological symptoms and the progressive recovery of tissues, reduced the infiltration of inflammatory cells in the mucosa and submucosa, and maintained the integrity of the colonic mucosa (Fig. 2A). Local colonic mucosal ulcers, congestion, and edema were observed with naked eyes in untreated colitis mice, while these symptoms were improved in colitis mice treated with DHA and 5-ASA (Fig. 2A). In addition, the histological scores in DSS+DHA group and DSS+5-ASA group were significantly lower than those in the DSS group (Fig. 2B). Overall, these results provide the first evidence for the protective effect of DHA in the treatment of UC in mice.
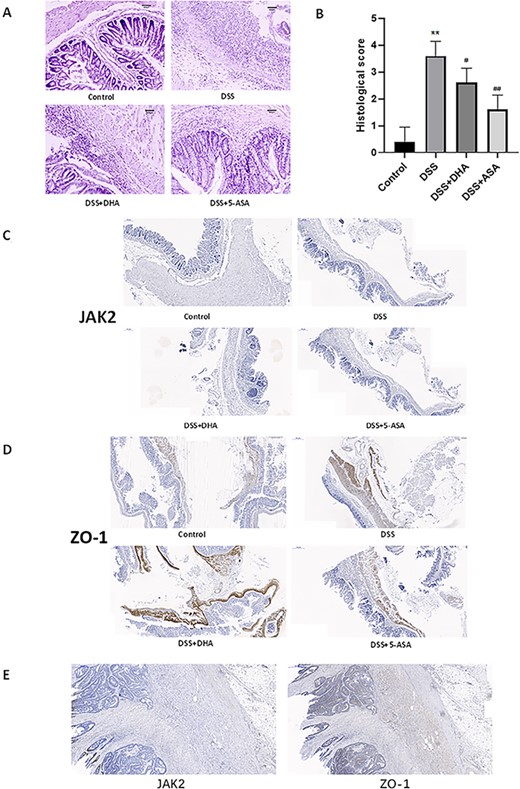
Effect of DHA on the colons in DSS-induced colitis in vivo (A) Representative H&E staining images of the colon sections from different groups. Scale bar: 200 μm. (B) Histology scores of different groups. (C) Immunohistochemical staining of JAK2 in colon sections from four groups of mice. (D) Immunohistochemical ZO-1 staining of in colon sections from four groups of mice. (E) Immunohistochemical staining of ZO-1 and JAK2 in colon biopsies of clinical IBD patients. Data are presented as the mean±SD. **P<0.01 vs control group. #P<0.05, ##P<0.01 vs DSS group. n = 5.
DHA increases the protein expressions of intestinal tight junction occludin and ZO-1
The expressions of occludin and ZO-1 in tight junction proteins are closely related to the occurrence and development of IBD [32]. To investigate whether DHA repairs the tight junction proteins that are damaged in the intestine of mice with chronic colitis, western blot analysis was used to detect the expressions of occludin and ZO-1. As shown in Fig. 3A, the protein expression levels of occludin and ZO-1 in the DSS group were significantly lower than those in the control group. The protein expression levels of occludin and ZO-1 in the DHA group and 5-ASA group were significantly increased compared with those in the DSS group (Fig. 3C,D). Reverse transcription (RT)-qPCR (Fig. 3B) and immunohistochemical staining (Fig. 2D) results showed that the ZO-1 expression in the DSS+DHA and DSS+5-ASA groups was stronger than that in the DSS group at both mRNA level and protein level. In order to verify the accuracy of the immunohistochemical results, we selected the intestinal tissues of a clinical IBD patient for ZO-1 immunohistochemical staining and obtained similar results. These results indicate that DHA increases the protein expressions of tight junction proteins occludin and ZO-1 in the intestine of mice with chronic colitis.
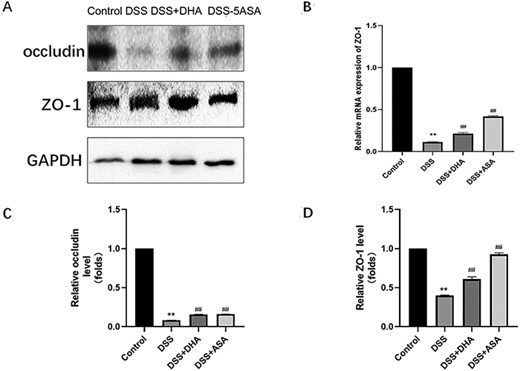
Effect of DHA on tight junction proteins in mice colon tissue in DSS-induced colitis (A) Western blot analysis of ZO-1 and occludin protein expressions. (B) Relative expression of ZO-1 mRNA in the four groups of mice colon tissue homogenates. (C,D) Quantitative analysis of ZO-1 and occludin proteins. Data are presented as the mean±SD. **P<0.01 vs control group. ##P<0.01 vs DSS group.
DHA protects the cell viability of Caco-2 cells in the inflammation model co-cultured with THP-1 cells
To explore whether DHA has a protective effect on the intestinal mucosa in an inflammatory environment, we established an in vitro co-culture model of differentiated Caco-2 cells and differentiated THP-1 cells in the intestine in either homeostatic or inflamed states. CCK-8 assay results showed that the viability of Caco-2 cells in the 20 or 40 µM DHA group was higher than that in the model group (Fig. 4C). The flow cytometry assay results showed that the apoptosis rate of Caco-2 cells in the 20 or 40 µM DHA group was not statistically significant compared with that of the model group (Fig. 4A,B). The viability of Caco-2 cells treated with DHA was higher than that in the model group, suggesting a protective effect of DHA on the intestinal mucosa simulated in vitro. However, the viability of Caco-2 cells in the 20 µM DHA group was higher than that in the 40 µM DHA group, indicating that 20 µM DHA protected Caco-2 cell models mimicking the intestinal mucosa more effectively than 40 µM DHA.
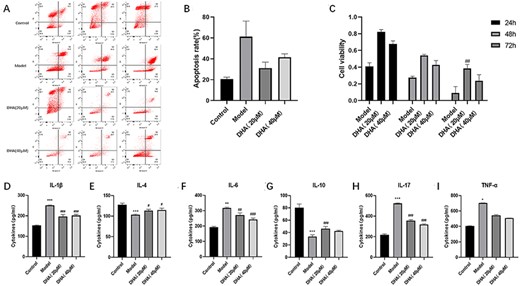
Effect of DHA on in vitro co-culture model (A,B) Apoptosis rate of Caco-2 cells in four groups of co-cultured inflammation models in vitro. (C) Cell viability of Caco-2 cells in four groups of co-cultured inflammation models in vitro. (D-I) The levels of IL-1β, IL-4, IL-6, IL-10, IL-17, and TNF-α in the basolateral side cell supernatant in four groups of co-cultured inflammation models in vitro. Data are presented as the mean±SD. *P<0.05, **P<0.01, ***P<0.001 vs control group. #P<0.05, ##P<0.01, ###P<0.001 vs Model group.
DHA inhibits the production of IL-1β, IL-6, and IL-17 by THP-1 macrophages in the in vitro co-culture inflammation model and promotes the production of IL-4 and IL-10
To explore whether DHA reduces the inflammatory response simulated in the co-culture model of Caco-2 cells and THP-1 cells in vitro, the production of IL-lβ, IL-6, IL-17, IL-4, IL-10, and TNF-α by basolateral side macrophages (differentiated THP-1 cells) co-cultured in an inflammation model in vitro was determined using ELISA kits. DHA (20 or 40 µM) pretreatment reduced the production of IL-lβ, IL-6, and IL-17 (Fig. 4D,F,H), while DHA increased the production of IL-4 and IL-10 (Fig. 4E,G). Interestingly, there was no significant difference in TNF-α levels on the basolateral side of the co-cultured inflammation model in each experimental group (Fig. 4I). These results showed that DHA increased the levels of anti-inflammatory cytokines and reduced the levels of pro-inflammatory cytokines in the in vitro co-culture inflammation model, suggesting that DHA can reduce the inflammatory response in the in vitro co-culture inflammation model.
Effect of DHA on the expressions of cytokines
To verify the effect of DHA on the inflammatory response of DSS-induced chronic colitis in mice, we determined the expressions of cytokines by ELISA and RT-qPCR. ELISA results showed that the levels of IL-1β, IL-6, IL-17, and TNF-α in the DSS group were higher than those in the control group, but they were significantly reduced by DHA treatment (Fig. 5A,C,E,F). The levels of IL-4 and IL-10 in the DSS+DHA group were significantly lower than those in the control group, but higher than those in the DSS group (Fig. 5B,D). RT-qPCR results showed that the mRNA levels of IL-1β, IL-6, IL-17, and TNF-α in DHA and 5-ASA groups were significantly higher than those in the control group but lower than those in the DSS group (Fig. 5G,H,J). On the other hand, DHA treatment significantly stimulated IL-10 mRNA expression compared with the DSS group (Fig. 5I). These results indicated that DHA inhibited the expressions of pro-inflammatory cytokines in UC and promoted the expressions of anti-inflammatory cytokines.
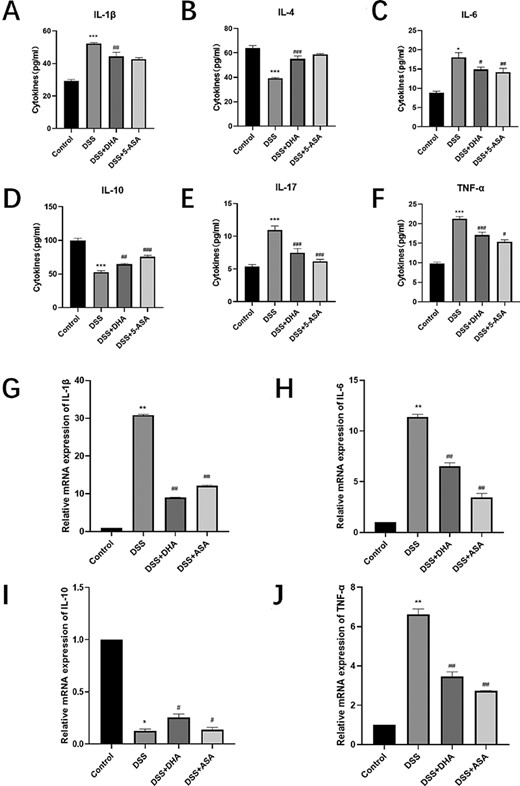
Effect of DHA on the production and expressions of DSS-induced colitis cytokines (A–F) Concentrations of IL-1β, IL-4, IL-6, IL-10, IL-17, and TNF-α in peripheral blood serum of four groups of mice. (G–J) The relative expression of IL-1β, IL-6, IL-10, and TNF-α mRNAs in the four groups of mice colon tissue homogenates. Data are presented as the mean±SD. *P<0.05, **P<0.01, ***P<0.001 vs control group. #P<0.05, ##P<0.01, ###P<0.001 vs DSS group.
Effect of DHA on the expressions of JAK2/STAT3 signal pathway-related proteins in DSS-induced chronic colitis in mice
As shown in Fig. 6A,B,E, compared with the DSS group, DHA significantly reduced the expressions of JAK2 and STAT3. In addition, we performed immunohistochemical staining on JAK2 and obtained similar results (Fig. 2C). In order to verify the accuracy of the immunohistochemical results, we selected the intestinal tissues of clinical IBD patients for immunohistochemical staining of JAK2 (Fig. 2E) and obtained similar results as the DSS group. The p-JAK2/JAK2 ratio was decreased after treatment in mice with experimental colitis (Fig. 6D). However, the levels of phosphorylated JAK2 (Fig. 6C) and phosphorylated STAT3 (Fig. 6F) in the colon mucosa of the DSS group mice were upregulated, which was opposite to the control group, DSS+DHA group, and DSS+5-ASA group. The ratios of p-JAK2/JAK2 (Fig. 6D) and p-STAT3/STAT3 (Fig. 6G), which reflect the levels of p-JAK2 and p-STAT3, indicated that DHA inhibited the phosphorylation of JAK2 and STAT3.
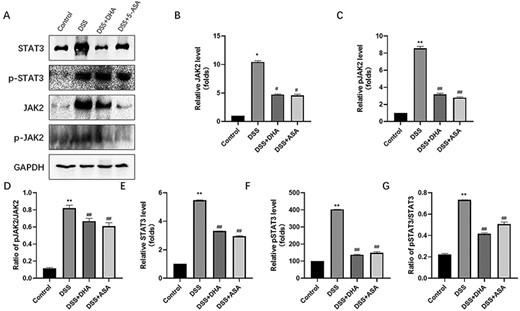
Effect of DHA on signal transducer and activator of transcription JAK2, p-JAK2, STAT3, and p-STAT3 (A) Western blot analysis of JAK2, p-JAK3, STAT3, and p-STAT3 proteins. (B) Quantitative analysis of JAK2 protein. (C) Quantitative analysis of p-JAK2 protein. (D) Ratio of p-JAK2/JAK2. (E) Quantitative analysis of STAT3 protein. (F) Quantitative analysis of p-STAT3 protein. (G) Ratio of p-STAT3/STAT3. Data are presented as the mean±SD. *P<0.05, **P<0.01 vs control group. #P<0.05, ##P<0.01 vs DSS group.
Discussion
UC is a chronic, relapsing-onset, and remission disease without permanent drug treatment, which may lead to significant long-term morbidity, irreversible course, and the risk of progression to malignant tumors. The incidence and prevalence of IBD are increasing worldwide. About 10 people per 100,000 people are diagnosed with UC each year. The incidence is rising in developing countries and is increasingly considered to be an emerging global disease. Although the pathogenesis of UC is still unclear, more and more studies have revealed that several factors, such as genetics, environmental factors, lifestyle habits, and increased production of inflammatory mediators, are possibly related to the pathogenesis of UC. An abnormal immune response is often observed in the colon, with different immune cells and cytokines.
The current common DSS-induced colitis method is to give mice DSS solution freely for 7 days. Although the mice will experience typical UC symptoms such as diarrhea, blood in the stool, and weight loss after 7 days of induction, the clinical manifestation like repeated attack and remission during the course of UC do not fully perform. Our 51-day intermittent use of DSS-induced colitis can preferably mimic the progression of UC. In this study, we objectively showed the effect of DHA on UC using the DSS-induced chronic colitis model. As a chemical agent used for colitis induction, DSS has the advantages of easy operation, convenient acquisition, high reproducibility, and causing similar symptoms to UC. The concentration of DSS used for colitis induction is usually 2%–5%, and a higher concentration of DSS may cause death in mice during colitis induction [33]. Therefore, in the process of establishing DSS-induced chronic colitis, we selected 2.5% DSS to induce chronic colitis. Mice induced with 2.5% DSS showed symptoms, such as thin stools, diarrhea, and weight loss, on the third day. With the increase in induction time, blood also appeared in the stool, which is a typical symptom of UC. In general, the chronic experimental colitis model we established has a higher success rate than common DSS-induced colitis model.
At present, the commonly used drugs for the treatment of UC include immunosuppressants, nonsteroidal anti-inflammatory drugs, and biological agents. However, these therapies may cause side effects such as vomiting, toxicity, systemic edema, and anemia. Therefore, people are becoming more and more interested in the use of natural compounds. These natural compounds with anti-inflammatory effects can be used to treat chronic inflammatory diseases.
DHA has been successfully used in the clinical treatment of malaria. DHA is an effective antimalarial drug, and it has been shown to exert anti-inflammatory and anticancer effects [34]. DHA inhibits IL-6 to restore the balance of Treg/Th17, thereby suppressing the occurrence of rheumatoid arthritis synovitis. DHA exerts anti-colitis effects by regulating the balance of Th17/Treg and also blocks the expressions and secretions of pro-inflammatory cytokines induced by DSS to inhibit phosphatidylinositol 3-kinase/AKT and NF-κB signaling pathways [34]. IBD, including UC, is generally regarded as an immune disease, and the risk of malignant tumors in the course of UC has been proven [29]. DHA’s anti-inflammatory, anti-cancer, and immune-regulating effects inspired us to explore whether DHA can be used as a new effective drug for the treatment of UC. In this study, the pre-established Caco-2+THP-1 stable co-cultured cell model was used to simulate the intestinal conditions of inflammation [19]. The results showed that DHA can increase the activity of Caco-2 cells that mimic the intestinal mucosa while inhibiting the THP-1 cells; produce cytokines IL-1β, IL-6, and IL-17; and promote IL-4 and IL-10 cytokine levels. Interestingly, the low dose of DHA (20 μM) showed stronger protection. This may suggest that the drug effect of DHA have a threshold, which is worthy of further exploration and verification in future studies.
DSS is a chemical reagent often used to simulate UC to induce mouse colitis [35]. Diarrhea and stool bleeding are the main symptoms of UC. UC is characterized by recurrence and remission [36]. After chronic colitis induction with DSS, the mice experienced weight loss, diarrhea, and fecal congestion. The total score of these three indicators directly reflects the severity of DAI or mouse disease. As DHA is a new extract, we referred to the results of the cell experiment and the principle of administration according to the body weight and used 20 mg/kg DHA in the DHA+DSS group mice. Our data indicated that DHA plays important roles in improving the clinical symptoms of UC, resulting in colon shortening and its pathological damage. We demonstrated that DHA promoted the productions of ZO-1 and occludin. Previous studies have shown that intestinal barrier dysfunction is related to the occurrence and development of IBD. Intestinal tight junction proteins including ZO-1 and occludin are important components of the intestinal barrier. The recovery of the intestinal barrier by DHA supplementation may be part of its anti-inflammatory effect. Whether DHA directly protects the intestinal barrier function or inhibits the progression of inflammation by inhibiting the JAK2/STAT3 pathway to protect the intestinal barrier function remains to be further studied. Although the pathogenesis of colon shortening in this field is still unclear, the improvement of colon shortening in mice may be related to the intestinal tight junction proteins ZO-1 and occludin. Our results suggest that DHA significantly retards the prognosis of UC.
The JAK family comprises four intracellular protein TYKs , i.e. JAK1, JAK2, JAK3 and TYK2. JAKs are associated with different cytokine receptors to recruit and phosphorylate other signalling molecules including members of the STAT family, i.e. STAT1, STAT2, STAT3, STAT4, STAT5A, and STAT5B [37], STAT6 and JAK1, JAK2, and TYK2 are commonly expressed cytokines [38], including interleukins and interferons, regulate intracellular signal transmission by inducing the JAK/STAT pathway and the mitogen-activated protein kinase pathway [31]. JAK protein binds to many types of cytokine receptors, especially the IL-6R family to induce signal transmission through JAK/STAT activation [39]. The polymorphisms of JAK2, TYK2, and STAT3 genes are associated with an increased risk of developing IBD [27]. Therefore, the role of JAK/STAT activation between acquired immunity and innate immunity of IBD has been studied. In particular, the JAK/STAT pathway has been shown to play a key role in maintaining a balance of inflammation, and excessive activation of STAT3 often leads to uncontrolled inflammation [3]. Disturbances in the IL-6/STAT3 signaling pathway are related to a variety of gastrointestinal and rheumatic diseases, including UC. The JAK/STAT pathway is usually involved in cytokine signal transduction; meanwhile, various cytokines, including IL-6 and IL-10, can activate STAT3 [40]. The binding of these ligands to their corresponding receptors leads to the activation of JAK. Activated JAK mediates the phosphorylation of STAT3 on Y705. Therefore, we measured the expressions of IL-1β, IL-4, IL-6, IL-10, IL-17, and TNF-α cytokines in mice and the levels of JAK2 and STAT3 in mice colon tissues. After 7 days of DHA treatment, we confirmed that DHA mainly improves the prognosis of UC by blocking the inflammation of the diseased animals. DHA reduced the protein levels of IL-1β, IL-6, IL-17, and TNF-α cytokines and their mRNA levels in the DSS-induced UC model, and increased the protein levels of IL-4 and IL-10 cytokines as well as their mRNA levels. After mice were induced with DSS, the levels of JAK2, STAT3, phosphorylated JAK2, and phosphorylated STAT3 in the colon tissue were significantly increased, suggesting that the phosphorylation of two members of the JAK/STAT signaling pathway (JAK2 and STAT3) was activated. After DHA treatment of DSS-induced mice for 7 days, the damage of the colonic mucosa was reduced, and the phosphorylation of JAK2 and STAT3 was lower than that in the colon of the DSS group mice. These results show that DHA inhibits the production of pro-inflammatory cytokines and promotes the expressions of protective cytokines, suggesting that DHA may inhibit the inflammation in UC by inhibiting IL-6, JAK2, and STAT3. These results suggest that DHA may be used in the treatment of IBD. Of course, these results need to be verified in the next step, and the specific mechanism of DHA in inhibiting the JAK2/STAT3 pathway needs to be further explored.
In summary, our study shows that DHA can alleviate colitis by protecting the intestinal tight junction proteins, inhibiting JAK2/STAT3 signaling pathway, inhibiting the production of pro-inflammatory cytokines, and promoting the expressions of protective cytokines. Our data may provide clues for the treatment and improvement of the prognosis of UC and indicate that DHA may be a promising option for the prevention and treatment of UC.
Funding
This work was supported by the grant from the State Key Laboratory of Cancer Biology (Air Force Medical University of PLA) (No. CBSKL201716).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
Author notes
Mingrui Jiang, Guangjun Zhong contributed equally to this work.



