-
PDF
- Split View
-
Views
-
Cite
Cite
Jinxin Wang, Wenlian Tang, Shiqi Chen, Juan Zhang, Jing Ji, Jingquan Dong, Gang Liu, Song Gao, Rapid and sensitive detection of Vibrio alginolyticus pathogenic strains by real-time recombinase polymerase amplification, Acta Biochimica et Biophysica Sinica, Volume 53, Issue 7, July 2021, Pages 950–954, https://doi.org/10.1093/abbs/gmab051
Close - Share Icon Share
Vibrio alginolyticus is a halophilic Gram-negative bacterium distributed widely in oceans and estuaries. In the mariculture industry, it is one of the most virulent Vibrio species that can infect fish, shrimp, and shellfish [1]. Its strong pathogenicity and quick onset can result in massive mortality of the aquatic animals [2]. Prevention of V. alginolyticus infection has become an urgent requirement in the mariculture industry to avoid economic losses.
Detection assays are fundamental tools for infectious disease control. For the detection of V. alginolyticus in aquatic animals, many molecular detection assays have been developed, including polymerase chain reaction (PCR)-based, quantitative PCR (qPCR)-based, and loop-mediated isothermal amplification (LAMP)-based assays [3–5]. These assays used a variety of virulence genes as the detection markers, including thermolabile hemolysin (tlh), thermostable direct hemolysin (tdh), cholera toxin transcriptional activator (toxR), heat stable heatstable enterotoxin (sto), cholera toxin A subunit (ctxA), and V. cholerae pathogenicity island (vpi) [6]. Targeting a virulence gene is important because there are nonpathogenic V. alginolyticus strains that should be avoided in the detection; on the other hand, a certain pathogenic strain may lack some of the virulence genes. It has been reported that, among these virulence genes, toxR has a good coverage for all the pathogenic strains [7].
The PCR-, qPCR-, and LAMP-based detection assays are valuable for the mariculture industry in preventing V. alginolyticus infections. But they are not suitable for on-site detection because of the dependence on equipment or laboratory settings. The LAMP assays are simpler than PCR/qPCR because thermocyclers are not required, but an accurate temperature-control device is still needed. A recombinase polymerase amplification (RPA) assay combined with lateral flow dipsticks (LFDs) has been developed for on-site detection of V. alginolyticus [7]. The assay is fast, simple, and highly specific, which is well suited for on-site detection. DNA amplification is achieved isothermally at 35–45°C by a combination of recombinase and polymerase activities [8]. Signal reading is simple with lines visualized on dipsticks. However, the LFD setting can introduce the risk of aerosol contamination. The amplification products are exposed to the environment during the lateral flow process and carry-over contamination in the subsequent assays may occur. For on-site detections where resources are usually limited, decontamination of the environment is difficult, and the false-positive risk from aerosol contamination is relatively high.
In this study, a real-time RPA assay combined with fluorescent signal was established for the detection of V. alginolyticus. This assay showed good specificity and sensitivity and was simple and fast. Importantly, the assay did not require the exposure of amplification products to the environment, and the fluorescent signals were directly read inside the reaction tubes. Thus, the false-positive risk from aerosol contamination was avoided. This real-time RPA assay has provided another choice for on-site detection of V. alginolyticus.
For the real-time RPA assay, the virulence gene toxR of V. alginolyticus (GenBank accession No. EU155543.1) was selected as the detection marker. The primer-probe set sequences were derived from the established RPA–LFD assay [7]. The forward and reverse primer sequences were 5ʹ-AGCCCATTTTCTACTGACTCTAACACTGAC-3ʹ and 5ʹ-GTTCGTGAATAACAATACGCAAACAGGAAG-3ʹ, respectively. The probe in the real-time RPA reaction was special because there was a tetrahydrofuran (THF) group at the middle, and the two T bases adjacent to the THF site were labeled with FAM (6-corboxy-fluorescein) and BHQ1 (Black Hole Quencher 1). During amplification, the probe would pair to one amplified strand, and the exonuclease III would cut at the THF site to separate FAM and BHQ1 to emit fluorescence. According to this design, the probe sequence in this study was 5ʹ-GAGCCGACTAAAACTCAGCCGAAGCCAGCA[FAM-dT][THF][BHQ1-dT]AACACGATTAACTG-3ʹ. The 3ʹ end of the probe was labeled with a C3 spacer to block unnecessary strand extension. The primers and probe were synthesized by General Biosystems (Chuzhou, China).
The real-time RPA assay conditions were set according to the manufacturer’s instructions of the TwistAmp DNA Amplification exo kit (TwistDx Inc, Maidenhead, UK). The 50 μl reaction system included 2.1 μl of each primer (10 μM), 0.6 μl of the probe (10 μM), 1 μl of the template, and other standard reaction components. Amplification was initiated by the addition of 2.5 μl of magnesium acetate (280 mM). Fluorescence was recorded on a qPCR machine (LightCycler 480 II; F. Hoffmann-La Roche AG, Basel, Switzerland) at 39°C in the FAM channel with signal reads at 13 s intervals for 25 min.
The specificity of the real-time RPA assay was tested with Vibrio species reference strains V. alginolyticus (ATCC 17749), V. parahaemolyticus (ATCC 17802), V. vulnificu (ATCC 27562), V. cholerae (ATCC 14100), V. harveyi (ATCC 43516), V. mimicus (MCCC 1A02602), V. rotiferianus (MCCC 1B00068) and V. ichthyoenteri (MCCC 1A00057), and Aeromonas species reference strains A. hydrophila (CICC 10500), A. veronii (BNCC 138468) and A. caviae (BNCC 139095). These strains were provided by Jiangsu Institute of Oceanology and Marine Fisheries (Nantong, China) and were confirmed by 16S rRNA sequencing [9]. Bacterial DNAs were extracted using TIANamp Bacterial DNA Kit (Tiangen Biotech, Beijing, China), quantified by Qubit 4 (Thermo Fisher Scientific, Wilmington, USA), normalized to 10 ng/μl, and used as the templates for the real-time RPA assay. Only the signal from the V. alginolyticus template was observed, while all the other bacteria were negative, suggesting good specificity of the real-time RPA assay (Fig. 1A).
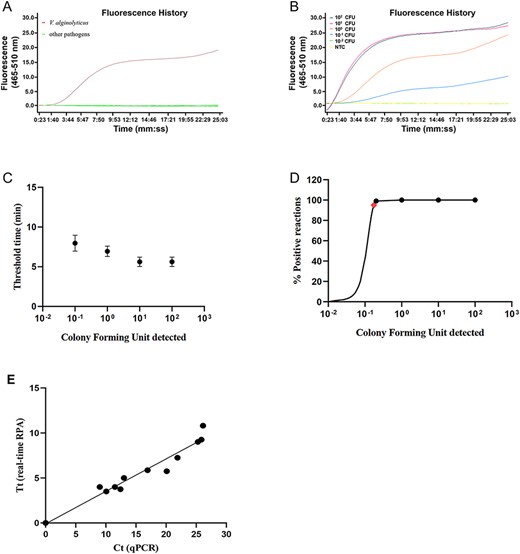
Detection of V. alginolyticus with real-time RPA (A) The fluorescence history diagram showing the results of real-time RPA with different templates including V. alginolyticus (brown line) and other pathogens (green lines; pathogens included: V. parahaemolyticus, V. vulnificu, V. cholerae, V. harveyi, V. mimicus, V. rotiferianus, V. ichthyoenteri, A. hydrophila, A. veronii, and A. caviae). All the other pathogens were negative; thus, the green lines were overlapping. The diagram is a typical representative of three independent experiments. (B) The fluorescence history diagram showing the results of real-time RPA with different amounts (in CFU) of V. alginolyticus. The amounts tested are indicated with different colors. The NTC is the no-template control. The diagram is a typical representative of eight independent experiments. (C) Semi-logarithmic regression of the data collected from the eight real-time RPA repeats in (B) using GraphPad Prism 8.0. (D) Probit regression analysis of the data collected from the eight real-time RPA repeats in (B) using SPSS software. The LOD at 95% probability (0.174 CFU/reaction) is depicted by a red rhomboid. (E) Comparisons between results of real-time RPA and qPCR on positive samples. Linear regression analysis of real-time RPA Tt (y axis) and qPCR Ct (x axis) were determined using GraphPad Prism 8.0. R2 = 0.9418.
Dilutions of October 2, 0102 colony forming units (CFUs)/μl of V. alginolyticus were used as the templates to evaluate the sensitivity of the real-time RPA assay. Diluted incubation cultures were boiled at 100°C for 10 min and directly used. The results showed that 10−1 CFU per reaction could be detected (Fig. 1B). A semi-log regression analysis of eight independent repeats (GraphPad Prism 8.0; GraphPad Software Inc, San Diego, USA) showed that the reaction time was 6–8 min for October 1, 0102 CFU per reaction (Fig. 1C). A probit regression analysis (SPSS software; IBM, Armonk, USA) of the results of the eight repeats suggested the limit of detection (LOD) to be 0.174 CFU per reaction in 95% of cases (Fig. 1D).
The real-time RPA assay was applied to the detection of V. alginolyticus in 80 clinical samples collected by the Jiangsu Institute of Oceanology and Marine Fisheries, and the results were compared with that from qPCR [5] (Table 1). The clinical samples were homogenized with a handheld 3rd Gen. TGrinder, and DNA was extracted from 1 g of each homogenate using a Magnetic Universal Genomic DNA kit (Tiangen Biotech). A total of 11 positive samples were detected by real-time RPA and qPCR with 100% consistency (Tables 1 and 2). The threshold time (Tt) of real-time RPA and cycle threshold (Ct) of qPCR of the positive samples were well correlated with an R2 value of 0.9418 (Fig. 1E). These results showed that the real-time RPA assay could accurately detect V. alginolyticus in clinical samples. It should be noted that, when sampling from shrimps for the detection of Vibrio species infections, the hepatopancreas and the intestine were the preferred sampling tissues, while sampling on the gill or the shrimp meat had the risk of false negative results. These tissues were included in this study solely for comparison between the real-time RPA assay and the qPCR method.
| No. . | Sample type . | Species . | Tissue . | Detection results . | |
|---|---|---|---|---|---|
| . | . | . | . | Real-time RPA (Tt, min) . | qPCR (Ct, n = 3) . |
| 1 | Shrimp | Exopalamon carinc auda | hepatopancreas | – | – |
| 2 | Shrimp | Exopalamon carincauda | hepatopancreas | – | – |
| 3 | Shrimp | Exopalamon carincauda | hepatopancreas | – | – |
| 4 | Shrimp | Exopalamon carincauda | hepatopancreas | 9 | 25.27 ± 0.23 |
| 5 | Shrimp | Exopalamon carincauda | gill | – | – |
| 6 | Shrimp | Exopalamon carincauda | gill | – | – |
| 7 | Shrimp | Exopalamon carincauda | gill | – | – |
| 8 | Shrimp | Exopalamon carincauda | gill | – | – |
| 9 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 10 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 11 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 12 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 13 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 14 | Shrimp | Litopenaeus vannamei | hepatopancreas | 7.25 | 21.89 ± 0.15 |
| 15 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 16 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 17 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 18 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 19 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 20 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 21 | Shrimp | Litopenaeus vannamei | hepatopancreas | 5 | 13.01 ± 0.22 |
| 22 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 23 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 24 | Shrimp | Litopenaeus vannamei | hepatopancreas | 4 | 8.99 ± 0.13 |
| 25 | Shrimp | Litopenaeus vannamei | hepatopancreas | 3.75 | 12.43 ± 0.12 |
| 26 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 27 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 28 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 29 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 30 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 31 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 32 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 33 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 34 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 35 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 36 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 37 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 38 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 39 | Shrimp | Litopenaeus vannamei | hepatopancreas | 3.5 | 10.08 ± 0.24 |
| 40 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 41 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 42 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 43 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 44 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 45 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 46 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 47 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 48 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 49 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 50 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 51 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 52 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 53 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 54 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 55 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 56 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 57 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 58 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 59 | Shellfish | ostrea gigas thunberg | meat | 5.75 | 20.11 ± 0.15 |
| 60 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 61 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 62 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 63 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 64 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 65 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 66 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 67 | Shellfish | ostrea gigas thunberg | meat | 4 | 11.51 ± 0.21 |
| 68 | Shellfish | ostrea gigas thunberg | meat | 5.86 | 16.92 ± 0.31 |
| 69 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 70 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 71 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 72 | Shellfish | ostrea gigas thunberg | meat | 9.25 | 25.85 ± 0.18 |
| 73 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 74 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 75 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 76 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 77 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 78 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 79 | Shellfish | Meretrix meretrix | meat | 10.8 | 26.13 ± 0.27 |
| 80 | Shellfish | Meretrix meretrix | meat | – | – |
| No. . | Sample type . | Species . | Tissue . | Detection results . | |
|---|---|---|---|---|---|
| . | . | . | . | Real-time RPA (Tt, min) . | qPCR (Ct, n = 3) . |
| 1 | Shrimp | Exopalamon carinc auda | hepatopancreas | – | – |
| 2 | Shrimp | Exopalamon carincauda | hepatopancreas | – | – |
| 3 | Shrimp | Exopalamon carincauda | hepatopancreas | – | – |
| 4 | Shrimp | Exopalamon carincauda | hepatopancreas | 9 | 25.27 ± 0.23 |
| 5 | Shrimp | Exopalamon carincauda | gill | – | – |
| 6 | Shrimp | Exopalamon carincauda | gill | – | – |
| 7 | Shrimp | Exopalamon carincauda | gill | – | – |
| 8 | Shrimp | Exopalamon carincauda | gill | – | – |
| 9 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 10 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 11 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 12 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 13 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 14 | Shrimp | Litopenaeus vannamei | hepatopancreas | 7.25 | 21.89 ± 0.15 |
| 15 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 16 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 17 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 18 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 19 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 20 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 21 | Shrimp | Litopenaeus vannamei | hepatopancreas | 5 | 13.01 ± 0.22 |
| 22 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 23 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 24 | Shrimp | Litopenaeus vannamei | hepatopancreas | 4 | 8.99 ± 0.13 |
| 25 | Shrimp | Litopenaeus vannamei | hepatopancreas | 3.75 | 12.43 ± 0.12 |
| 26 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 27 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 28 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 29 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 30 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 31 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 32 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 33 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 34 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 35 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 36 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 37 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 38 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 39 | Shrimp | Litopenaeus vannamei | hepatopancreas | 3.5 | 10.08 ± 0.24 |
| 40 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 41 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 42 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 43 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 44 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 45 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 46 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 47 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 48 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 49 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 50 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 51 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 52 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 53 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 54 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 55 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 56 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 57 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 58 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 59 | Shellfish | ostrea gigas thunberg | meat | 5.75 | 20.11 ± 0.15 |
| 60 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 61 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 62 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 63 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 64 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 65 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 66 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 67 | Shellfish | ostrea gigas thunberg | meat | 4 | 11.51 ± 0.21 |
| 68 | Shellfish | ostrea gigas thunberg | meat | 5.86 | 16.92 ± 0.31 |
| 69 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 70 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 71 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 72 | Shellfish | ostrea gigas thunberg | meat | 9.25 | 25.85 ± 0.18 |
| 73 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 74 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 75 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 76 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 77 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 78 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 79 | Shellfish | Meretrix meretrix | meat | 10.8 | 26.13 ± 0.27 |
| 80 | Shellfish | Meretrix meretrix | meat | – | – |
| No. . | Sample type . | Species . | Tissue . | Detection results . | |
|---|---|---|---|---|---|
| . | . | . | . | Real-time RPA (Tt, min) . | qPCR (Ct, n = 3) . |
| 1 | Shrimp | Exopalamon carinc auda | hepatopancreas | – | – |
| 2 | Shrimp | Exopalamon carincauda | hepatopancreas | – | – |
| 3 | Shrimp | Exopalamon carincauda | hepatopancreas | – | – |
| 4 | Shrimp | Exopalamon carincauda | hepatopancreas | 9 | 25.27 ± 0.23 |
| 5 | Shrimp | Exopalamon carincauda | gill | – | – |
| 6 | Shrimp | Exopalamon carincauda | gill | – | – |
| 7 | Shrimp | Exopalamon carincauda | gill | – | – |
| 8 | Shrimp | Exopalamon carincauda | gill | – | – |
| 9 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 10 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 11 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 12 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 13 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 14 | Shrimp | Litopenaeus vannamei | hepatopancreas | 7.25 | 21.89 ± 0.15 |
| 15 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 16 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 17 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 18 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 19 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 20 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 21 | Shrimp | Litopenaeus vannamei | hepatopancreas | 5 | 13.01 ± 0.22 |
| 22 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 23 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 24 | Shrimp | Litopenaeus vannamei | hepatopancreas | 4 | 8.99 ± 0.13 |
| 25 | Shrimp | Litopenaeus vannamei | hepatopancreas | 3.75 | 12.43 ± 0.12 |
| 26 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 27 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 28 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 29 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 30 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 31 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 32 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 33 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 34 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 35 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 36 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 37 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 38 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 39 | Shrimp | Litopenaeus vannamei | hepatopancreas | 3.5 | 10.08 ± 0.24 |
| 40 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 41 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 42 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 43 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 44 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 45 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 46 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 47 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 48 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 49 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 50 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 51 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 52 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 53 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 54 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 55 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 56 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 57 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 58 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 59 | Shellfish | ostrea gigas thunberg | meat | 5.75 | 20.11 ± 0.15 |
| 60 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 61 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 62 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 63 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 64 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 65 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 66 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 67 | Shellfish | ostrea gigas thunberg | meat | 4 | 11.51 ± 0.21 |
| 68 | Shellfish | ostrea gigas thunberg | meat | 5.86 | 16.92 ± 0.31 |
| 69 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 70 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 71 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 72 | Shellfish | ostrea gigas thunberg | meat | 9.25 | 25.85 ± 0.18 |
| 73 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 74 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 75 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 76 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 77 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 78 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 79 | Shellfish | Meretrix meretrix | meat | 10.8 | 26.13 ± 0.27 |
| 80 | Shellfish | Meretrix meretrix | meat | – | – |
| No. . | Sample type . | Species . | Tissue . | Detection results . | |
|---|---|---|---|---|---|
| . | . | . | . | Real-time RPA (Tt, min) . | qPCR (Ct, n = 3) . |
| 1 | Shrimp | Exopalamon carinc auda | hepatopancreas | – | – |
| 2 | Shrimp | Exopalamon carincauda | hepatopancreas | – | – |
| 3 | Shrimp | Exopalamon carincauda | hepatopancreas | – | – |
| 4 | Shrimp | Exopalamon carincauda | hepatopancreas | 9 | 25.27 ± 0.23 |
| 5 | Shrimp | Exopalamon carincauda | gill | – | – |
| 6 | Shrimp | Exopalamon carincauda | gill | – | – |
| 7 | Shrimp | Exopalamon carincauda | gill | – | – |
| 8 | Shrimp | Exopalamon carincauda | gill | – | – |
| 9 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 10 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 11 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 12 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 13 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 14 | Shrimp | Litopenaeus vannamei | hepatopancreas | 7.25 | 21.89 ± 0.15 |
| 15 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 16 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 17 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 18 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 19 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 20 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 21 | Shrimp | Litopenaeus vannamei | hepatopancreas | 5 | 13.01 ± 0.22 |
| 22 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 23 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 24 | Shrimp | Litopenaeus vannamei | hepatopancreas | 4 | 8.99 ± 0.13 |
| 25 | Shrimp | Litopenaeus vannamei | hepatopancreas | 3.75 | 12.43 ± 0.12 |
| 26 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 27 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 28 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 29 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 30 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 31 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 32 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 33 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 34 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 35 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 36 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 37 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 38 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 39 | Shrimp | Litopenaeus vannamei | hepatopancreas | 3.5 | 10.08 ± 0.24 |
| 40 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 41 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 42 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 43 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 44 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 45 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 46 | Shrimp | Litopenaeus vannamei | hepatopancreas | – | – |
| 47 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 48 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 49 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 50 | Shrimp | Litopenaeus vannamei | intestine | – | – |
| 51 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 52 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 53 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 54 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 55 | Shrimp | Litopenaeus vannamei | gill | – | – |
| 56 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 57 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 58 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 59 | Shellfish | ostrea gigas thunberg | meat | 5.75 | 20.11 ± 0.15 |
| 60 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 61 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 62 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 63 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 64 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 65 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 66 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 67 | Shellfish | ostrea gigas thunberg | meat | 4 | 11.51 ± 0.21 |
| 68 | Shellfish | ostrea gigas thunberg | meat | 5.86 | 16.92 ± 0.31 |
| 69 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 70 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 71 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 72 | Shellfish | ostrea gigas thunberg | meat | 9.25 | 25.85 ± 0.18 |
| 73 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 74 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 75 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 76 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 77 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 78 | Shellfish | ostrea gigas thunberg | meat | – | – |
| 79 | Shellfish | Meretrix meretrix | meat | 10.8 | 26.13 ± 0.27 |
| 80 | Shellfish | Meretrix meretrix | meat | – | – |
Comparison of the detection of V. alginolyticus in clinical samples by real-time RPA and qPCR
| Sample type . | Number of samples . | Number of positive samples detected . | |
|---|---|---|---|
| Real-time RPA . | qPCR . | ||
| Shrimp | 55 | 6 | 6 |
| Shellfish | 25 | 5 | 5 |
| Total | 80 | 11 | 11 |
| Sample type . | Number of samples . | Number of positive samples detected . | |
|---|---|---|---|
| Real-time RPA . | qPCR . | ||
| Shrimp | 55 | 6 | 6 |
| Shellfish | 25 | 5 | 5 |
| Total | 80 | 11 | 11 |
Comparison of the detection of V. alginolyticus in clinical samples by real-time RPA and qPCR
| Sample type . | Number of samples . | Number of positive samples detected . | |
|---|---|---|---|
| Real-time RPA . | qPCR . | ||
| Shrimp | 55 | 6 | 6 |
| Shellfish | 25 | 5 | 5 |
| Total | 80 | 11 | 11 |
| Sample type . | Number of samples . | Number of positive samples detected . | |
|---|---|---|---|
| Real-time RPA . | qPCR . | ||
| Shrimp | 55 | 6 | 6 |
| Shellfish | 25 | 5 | 5 |
| Total | 80 | 11 | 11 |
This real-time RPA assay could detect V. alginolyticus at an LOD of 0.174 CFU per reaction, which was comparable or better than the previously established RPA–LFD, qPCR, and LAMP assays [4,5,7]. The apparent LOD of lower than 1 CFU per reaction could possibly come from dead or non-culturable cells in the incubation culture. In the validation experiments using clinical samples, the detection accuracy of real-time RPA was as good as qPCR. The detection time of real-time RPA was 10 min or less, which was much faster than the previously reported assays. For template preparation, DNA extraction was conducted with a commercialized kit that required only a magnet to operate. Although a qPCR machine was used for fluorescent signal recording in this study, small and battery-powered portable tube scanners such as the Genie III scanner from Suntrap Science & Technology (Beijing, China) had been developed for on-site detections. According to the RPA reaction principle, the reaction temperature of 39°C does not have to be very accurate [8]. Thus, the real-time RPA assay was fast and accurate with minimum dependence on equipment or laboratory settings, which could be used to build a ‘mobile suitcase lab’ for on-site detections [10].
In conclusion, a real-time RPA assay for on-site detection of V. alginolyticus has been developed in this study. This assay has high sensitivity and specificity and can be completed within 10 min. More importantly, the portability of this assay is comparable to the RPA–LFD assay, while the false-positive risk from aerosol contamination is avoided. This real-time RPA assay provides an alternative for on-site detection of V. alginolyticus, which is of great significance for the mariculture industry in the prevention of infection outbreaks.
Acknowledgement
We thank Dr Hui Shen of the Jiangsu Institute of Oceanology and Marine Fisheries (Nantong, China) for providing the bacterial strains and clinical samples.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 31470275), the Key Natural Science Research Project of the Jiangsu Higher Education Institutions of China (No. 20KJA416002), the Lianyungang Science and Technology Project of China (No. SF2003), the Science and Technology Project of Lianyungang High-tech Zone of China (No. HZ201901), the Open-end Funds of Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening (Nos. HY202004, HY201703, and HY201805), the Postgraduate Research and Practice Innovation Program of Jiangsu Province (No. SJCX20_1228), and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
Author notes
Jinxin Wang, Wenlian Tang and Shiqi Chen contributed equally to this work.



