-
PDF
- Split View
-
Views
-
Cite
Cite
L i Dong, Yumin Zheng, Lianbo Gao, Xiaoguang Luo, lncRNA NEAT1 prompts autophagy and apoptosis in MPTP-induced Parkinson’s disease by impairing miR-374c-5p, Acta Biochimica et Biophysica Sinica, Volume 53, Issue 7, July 2021, Pages 870–882, https://doi.org/10.1093/abbs/gmab055
Close - Share Icon Share
Abstract
Long non-coding RNAs (lncRNAs) play biological roles in brain disorder and neurodegenerative diseases. As the functions of lncRNA NEAT1 in Parkinson’s disease (PD) remain unknown, in the present study, we aimed to explore the roles and underlying molecular mechanisms of NEAT1 in PD. A PD mouse model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and a cell model of SH-SY5Y induced by N-methyl-4-phenylpyridinium (MPP+) were established. The ratio of tyrosine hydroxylase (TH+) cells was determined by immunofluorescence assay, and the behavioral changes in mice were observed using pole tests and rotarod tests. The cellular viability and apoptosis of SH-SY5Y were detected by MTT assay and flow cytometric analysis, respectively, and the number of autophagosomes was subsequently measured by transmission electron microscopy. High-performance liquid chromatography was performed to detect the content of dopamine, and a dual-luciferase reporter assay was used to clarify the target of NEAT1 simultaneously. The results demonstrated that the level of NEAT1 was upregulated in the MPTP-induced PD mice, dopamine neurons, and the SH-SY5Y cells treated with MPP+, whereas the level of miR-374c-5p was downregulated. NEAT1 level was positively correlated with MPP+ in a concentration-dependent manner. NEAT1 inhibition efficiently facilitated cell proliferation but inhibited apoptosis and autophagy in the MPP+-treated SH-SY5Y cells. Additionally, silencing of NEAT1 increased the TH+ rate of neurons and suppressed autophagy greatly in PD mice. As a possible target of NEAT1, miR-374c-5p could impact on the apoptosis and autophagy of the SH-SY5Y cells. NEAT1 inhibition upregulated the expression of miR-374c-5p, enhanced SH-SY5Y cell viability, and repressed autophagy and apoptosis in MPTP-induced PD mice. These findings indicated a potential therapeutic role of NEAT1 in treating PD.
Introduction
Parkinson’s disease (PD) represents a progressive neurodegenerative disease that is characterized clinically by tremors at rest, movement delay, and less commonly cognitive impairment [1]. Although nearly 1.7% of the elderly in China suffer from this disorder, the etiopathogenesis remains unknown. Studies on the pathological mechanism of PD are urgently needed, which will benefit the patients in building up the confidence to overcome the disease [2]. Scholars have clarified the vital role of long non-coding RNAs (lncRNAs) in the neurodegenerative disorder of PD, Huntington’s disease, and Alzheimer’s disease, whereas the potential functions and mechanisms in these diseases have not been discovered yet [3–5].
Micro RNAs (miRNAs) are a crucial group of short non-coding RNA molecules that are closely associated with multiple biological processes, namely human neurodegenerative diseases [6]. They are also reported to be abnormally expressed in PD and essential in cellular apoptosis and autophagy. The expression of miRNAs can be affected by lncRNAs through a sponge effect, resulting in the development of PD [7,8]. Despite a hypothetical targeted relationship between miR-374c-5p and NEAT1, their interaction in PD remains to be explored.
In the present study, an in vivo model of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice and an in vitro model of the N-methyl-4-phenylpyridinium (MPP+)-treated SH-SY5Y cells were established. By detecting the contents of key apoptotic factors and autophagy-related proteins, we analyzed the mechanism of NEAT1 silencing in apoptosis and autophagy, clarified the role of NEAT1 in PD, and investigated the interplay between NEAT1 and miR-374c-5p in PD.
Materials and Methods
PD mice model establishment
All the animal experiments strictly followed the principles of the Care and Use of Laboratory Animals approved by the Ethics Committee of China Medical University (Shenyang, China). A total of 40 male C57BL/6 mice (aged 6 weeks) were obtained from the Laboratory Animal Center of China Medical University and randomly divided into a PD group and a control group. The mice in the PD group were intraperitoneally injected with 20 mg/kg of MPTP (Sigma, St Louis, USA) based on body weight, three times/day at intervals of 8 h, whereas the mice in the control group were injected with 0.9% normal saline intraperitoneally. The injections in both groups were continued for three successive weeks [9]. All experimental mice were anesthetized with pentobarbital (50 mg/kg) and sacrificed after the last injection on Day 21, and their midbrains were collected and cryopreserved at −80°C for further analyses. Two days before the mouse model establishment, 20 nmol of si-NEAT1-1 or si-NEAT1-2 were injected into the midbrain of PD mice to investigate the MPTP-induced effect of NEAT1 on PD by interfering with NEAT1 expressions, and 20 nmol of si-NC was injected as the negative control (NC). All the small interfering RNAs (siRNAs) were synthesized by GenePharma (Shanghai, China), and the sequence information is shown in Supplementary Table 1.
Pole test
The movement disorder of mice was evaluated by pole tests. The detailed procedures were described as follows. First, a 50-cm-long pole at 1-cm diameter was prepared, which was tightly wrapped in gauze, with a spherical protrusion on the top of the pole. Then, a timer was started immediately when the mice were placed on top of the pole following a 2-day induction with MPTP. Two indicators, i.e. T-turn time and T-descend time, were measured during the test. The T-turn time represents the time from which the mice started to move to the turning of its head downward, and the T-descend time is the time from the turning of the head downward to the natural arrival to the bottom of the pole. Moreover, all animals had 5-day training on pole climbing before the final test was performed. Each mouse was given two tests for pole climbing.
Rotarod test
A 3-day adaptive training was given to the mice before the experiment was carried out. All mice undergoing the test were placed on a spinning rod of a rotarod cylinder, and the duration time was subsequently recorded to measure how long the time could last following the mice was placed and stayed on the rod. The speed at the first five rounds of training was determined as 20 r/min and it was then reset to 30 r/min when the actual test was performed.
Cell culture
Human dopaminergic neuronal cell line SH-SY5Y was obtained from Beijing Beina Chuanglian Biotechnology Research Institute (Beijing, China) and cultured in DMEM supplemented with 10% FBS (foetal bovine serum; Solarbio, Beijing, China) at 37°C with 5% CO2. Cells were subsequently sub-cultured at a density of 90% confluence. To investigate whether and how NEAT1 affects PD, the SH-SY5Y cells were treated with MPP+ (Sigma) at 0, 100, 200, 400, and 800 μM concentrations, respectively, and cultured for 24 h to establish an in vitro cell model of PD [10].
Cell transfection
The SH-SY5Y cells were seeded in a 96-well plate and cultured at 37°C for 24 h following the treatment of MPP+. By overexpressing and interfering lncRNA and miRNA, respectively, the associated functions of NEAT1 and miR-374c-5p in the described cells were verified. The SH-SY5Y cells were incubated for 48 h before being transfected with NEAT1 overexpression, NEAT1-siRNAs, miR-374c-5p mimics, and miR-374c-5p inhibitor (GenePharma) using Lipofectamine® 3000 reagent (Guangzhou Jet Bio-Filtration, Guangzhou, China) according the manufacturers’ instruction. After all these transfected cells were harvested, the transfection efficiency was detected by quantitative real-time polymerase chain reaction (qRT-PCR). The full-length NEAT1 was synthesized by GenePharma and inserted into the pcDNA3.1 vector. The NC siRNA (Cat. No. A06001) and NC mimics and NC inhibitor (Cat. No. B04001; GenePharma) were also used to transfect SH-SY5Y cells as NCs.
Cell viability and apoptosis assay
MTT assay was performed to detect cell viability and detailed procedures were described as follows: The SH-SY5Y cells were transfected as previously described and subsequently incubated in 96-well plates. A total of 20 μl of MTT solution (5 mg/ml) was then added to each well at 0, 24, 48, and 72 h, and the absorbance was detected at a wavelength of 450 nm after 4 h of incubation in the dark.
Cell apoptosis was detected using the Annexin V-FITC cell apoptosis kit (Xiamen Lunchangshuo Biological Technology, Xiamen, China). Forty-eight hours after transfection, 1–5×105 cells were collected for re-suspension and mixed with propidium iodide and Annexin V-FITC for 10 min in the dark. A flow cytometer (Becton Dickinson, Sparks, USA) was used to detect cell apoptosis.
Luciferase activity assay
An online tool StarBase3.0 was used to predict whether there is a targeted correlation between NEAT1 and miR-374c-5p. Following insertion of the NEAT1 fragment with a putative binding site to miR-374c-5p into the pmirGLO luciferase vector (Promega, Madison, USA), a pmirGLO-wild type-NEAT1-3ʹ-UTR (NEAT1-Wt) was constructed. By inserting a mutant NEAT1 fragment into the pmirGLO vector, a pmirGLO-mutant-NEAT1-3ʹ-UTR (NEAT1-Mut) was established. In the luciferase assay, the cell transfection and stimulation processes were performed by co-transfecting either NEAT1-Wt or NEAT1-Mut with miR-374c-5p mimics and miR-374c-5p mimic NC into the SH-SY5Y cells, respectively. The Dual-Luciferase Reporter Assay System (Promega) was used to detect the luciferase activity following cell lysis.
RNA pull-down assay
After being transfected with biotinylated miR-374c-5p-Wt, miR-374c-5p-Mut, and miR-NC (GenePharma), the SH-SY5Y cells were divided into the following three groups: the Bio-NC, the Bio-miR-374c-5p-Wt, and the Bio-miR-374c-5p-Mut. Forty-eight hours after transfection, the transfected cells were lysed and incubated with Streptavidin agarose beads (Thermo Fisher, Waltham, USA) at 37°C for 1 h. The RNA levels were detected by qRT-PCR.
High-performance liquid chromatography
The level of dopamine in SH-SY5Y cells was measured by HPLC. At 48 h after transfection, the cells were cultured in the same way as before. Then, the cells were washed three times with PBS and added with 0.2% Triton. The cells were incubated at 37°C for 15 min to lyse. Cell lysate was collected and centrifuged at 4°C at 17,000 g for 20 min to measure dopamine (DA) content. The temperature of the chromatographic column (CAPCELL PAK C18 column, 5 μm, 4.6 mm×250 mm; Shiseido, Japan) was maintained at 30°C, the mobile phase consisted of methanol–water (15/85; v/v) with 0.1 M monobasic potassium phosphate (pH 4.6) and was delivered at a flow rate of 1 ml/min, and the optical density was detected at 354 nm. The injection volume was 10 μl. The prototypes were added in terms of the instructions. The column was balanced under the original condition for 10 min. Excitation/emission wavelength was scaled by fluorescence.
Immunofluorescence microscopy
The number of dopaminergic neurons was measured by TH expression in the midbrain of mouse. Briefly, the midbrain was fixed with 4% paraformaldehyde at 4°C overnight and then cut into sections to a thickness of 5 μm. The sections were washed with PBS and blocked with PBS containing 10% goat serum before incubating with 50 μl of rabbit anti-mouse TH antibody (PB9449; Boster, Wuhan, China) for 24 h. After being washed with PBS, the sections were incubated with FITC-conjugated secondary antibody (BA1031; Boster) at 37°C for 2 h. The nuclei were counterstained with 4,6-diamino-2-phenylindole dihydrochloride. The TH immunopositive dopaminergic neurons with red fluorescence were observed under an inverted fluorescence microscope (Olympus, Tokyo, Japan) at 400× magnification.
To measure the autophagy activity of SH-SY5Y cells, the microtubule-associated light chain 3 (LC3) protein expression was also detected by immunofluorescence staining. SH-SY5Y cells were cultured overnight on glass slides and fixed with 4% paraformaldehyde for 20 min. The subsequent procedures were the same as those used in the detection of TH expression, and the rabbit anti-LC3 antibody (ab192890; Abcam, Cambridge, UK) was used as the primary antibody.
TUNEL assay
The midbrain tissues from mice were fixed with 4% paraformaldehyde. The sections were treated with Protease K working solution (Solarbio) for 30 min at room temperature. After being rinsed with PBS, these sections were incubated with 100 μl of terminal transferase reaction solution (Solarbio) at 37°C for 1 h in the dark. Subsequently, 50 μl of HRP working solution (Solarbio) was added onto sections and the sections were incubated for 30 min at 37°C. Then, the sections were overlaid by using diaminobenzidine and counterstained with hematoxylin. After dehydration by gradient ethanol, the sections were treated with xylene and sealed in neutral resin. TUNEL-positive cells with green fluorescence were observed under a fluorescence microscope (Olympus).
Quantitative real-time PCR analysis
Total RNA was extracted from the brain tissues, DA neurons, or SH-SY5Y cells using TRIzol (Invitrogen, Carlsbad, USA); reverse-transcribed into cDNA; and analyzed by qRT-PCR using SYBR Green PCR kit (TaKaRa, Dalian, China). Primers used in this study were as follows: NEAT1 F: 5′-TTGGCAGACTCTTCTGGGTCTC-3′, R: 5′-TGTGTTTTGAAGGCATGGCTCA-3′; miR-374c-5p F: 5′-TCAGCGGATATAATACAACCTGC-3′, R: 5′-TATCGTTGTTCTCCACTCCTTCAC-3′; U6 F: 5′-CTCGCTTCGGCAGCACA-3′, R: 5′-AACGCTTC ACGAATTTGCGT-3′; p62 F: 5′-TCTTTGGACCCCCGTGTGA-3′, R: 5′-TCTCACAGATACCCCACGAC-3′; LC3 I F: 5′-TCCGACCGGCCTTTCAAGCAG-3′, R: 5′-GAGAACCTGACCAGAACTCCCAG-3′; LC3 II F: 5′-AAACGCATTTGCCATCACAGT-3′, R: 5′-GTGAGGACTTTGGGTGTGGTTC-3′; and β-actin F: 5′-ACACCTTCTACAATGAGCTG-3′, R: 5′-CTGCTTGCTGATCCACATCT-3′. NEAT1 was normalized to β-actin and miR-374 c-5p was normalized to U6. The relative expression levels were calculated using the 2−ΔΔCt method.
Western blot analysis
Total proteins were extracted from the samples using radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime, Beijing, China). Protein samples at 50 μg were subject to 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% skimmed milk and incubated with the following primary antibodies: anti-Bax antibody (1:500, BM19684), anti-caspase 3 antibody (1:1000, BM2156), anti-Bcl-2 antibody (1:1000, BM19693), anti-LC3 antibody (1:1000, BM19665), and anti-P62 antibody (1:1000, BM19700). All these antibodies were from IGEE (Chongqing, China). The anti-GAPDH antibody (1:1000, #5174) was from Cell Signalling Technology (Danvers, USA). Membranes were subsequently incubated with HRP-conjugated anti-rabbit IgG secondary antibody (1:5000; Life-iLab, Shanghai, China). Finally, protein bands were visualized by using an Enhanced Chemiluminescence Kit (Thermo Fisher) and the results were analyzed using Image Lab™ Software (Bio-Rad, Hercules, USA).
Statistical analysis
All data were shown as mean±standard deviation. Differences among the multiple treatment groups were calculated by one-way ANOVA followed by Tukey’s post hoc test. The comparison between the two groups was evaluated by the Student’s t-test and the data were analyzed using the SPSS 22.0 software. A P value of less than 0.05 was considered statistically significant.
Results
NEAT1 is highly expressed in PD mice and MPP+-treated SH-SY5Y cells
The T-turn time and T-descend time in the MPTP-induced PD mice were elongated (P<0.05; Fig. 1A,B) and the retention time was shortened (P<0.05; Fig. 1C) compared with the control group. The establishment of the PD mouse model was successful when the TH+ rate of cells in PD mice was markedly decreased (P<0.05; Fig. 1D). Additionally, qRT-PCR analysis showed that the expression of NEAT1 in the midbrain tissues of PD mouse model and in the DA neurons of SH-SY5Y cells was relatively higher than that in their corresponding controls (P<0.05; Fig. 1E,F). The level of NEAT1 in the SH-SY5Y cells was also upregulated in a concentration-dependent manner by treatment with MPP+ (P<0.05; Fig. 1G).
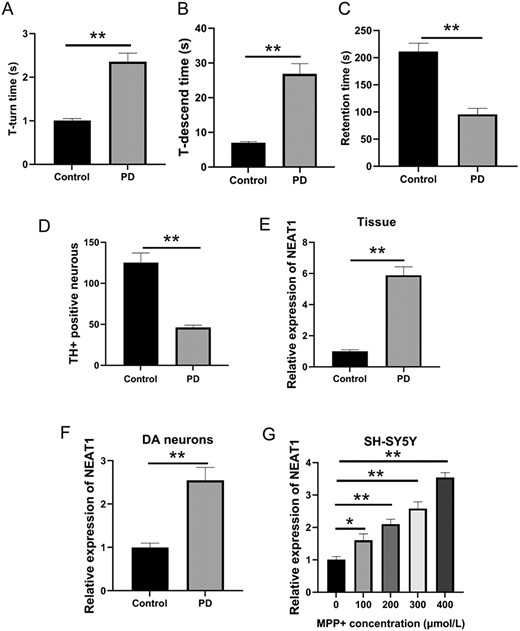
PD models in vivo and in vitro (A) T-turn time and (B) T-descend time were detected by the pole test. (C) Retention time was determined by the rotarod test. (D) Changes of TH-positive cells in the midbrain tissue were measured by immunohistochemistry. (E) NEAT1 expression in the brain tissues was determined by qRT-PCR. (F) NEAT1 expression in dopaminergic neurons of SH-SY5Y cells was detected by qRT-PCR. (G) Level of NEAT1 in SH-SY5Y cells treated with different concentrations of MPP+ was measured by qRT-PCR. *P<0.05, **P<0.01.
NEAT1 accelerates apoptosis of MPP+-induced SH-SY5Y cells
The levels of NEAT1 were greatly decreased in the MPP++si-NEAT1-1 group and the MPP++si-NEAT1-3 group compared with those in the MPP+ group (P<0.05; Fig. 2A). The si-NEAT1-1 was chosen for further experiments as it could downregulate the level of NEAT1. The MTT results indicated that the viability of SH-SY5Y cells was dramatically reduced in the MPP+ and the MPP++si-NC groups compared with the control group, whereas an increase was revealed in the MPP++si-NEAT1-1 group when compared with the MPP+ group (P<0.05; Fig. 2B). However, cell apoptosis assay showed an opposite result (Fig. 2D). Dopamine content in the MPP+ and MPP++si-NC groups was decreased in comparison to that in the control group (P<0.05), which was increased in the MPP++si-NEAT1-1 group compared with that in the MPP+ group (P<0.05; Fig. 2C). These findings also revealed that Bcl-2 expression was decreased in the MPP+ and MPP++si-NC groups (P<0.05), but the levels of Bax, cleaved caspase-3, and cleaved PARP were markedly upregulated compared with those in the control group. NEAT1 inhibition restored the abnormal expressions of Bcl-2, Bax, cleaved caspase-3, and cleaved PARP induced by MPP+ (P<0.05; Fig. 2E,F). In contrast to the NEAT1 silencing, prominently lower cell viability and higher apoptosis were observed in SH-SY5Y cells treated with MPP++NEAT1 overexpression (Supplementary Fig. S1).
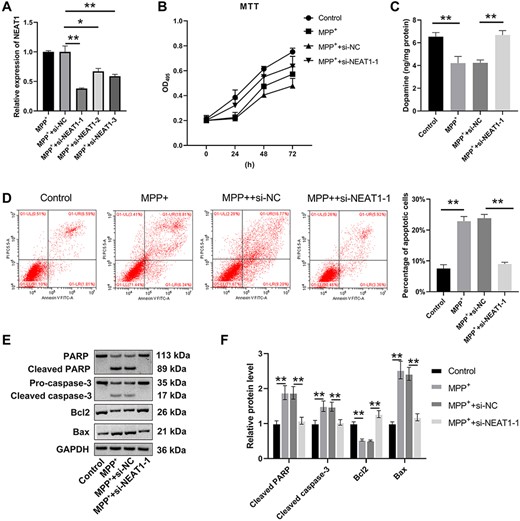
NEAT1 promotes MPP+-treated SH-SY5Y cell apoptosis (A) qRT-PCR was used to detect the expression of NEAT1 in SH-SY5Y cells. (B) MTT assay was used to detect the viability of SH-SY5Y cells. (C) Dopamine content was detected by HPLC. (D) Flow cytometry assay was carried out to analyze the apoptosis of SH-SY5Y cells. (E,F) Western blot analysis was used to measure the levels of Bcl-2, Bax, PARP, and caspase-3. *P<0.05, **P<0.01.
NEAT1 enhances autophagy of MPP+-induced SH-SY5Y cells
Results of qRT-PCR and western blot analysis indicated that the LC3 II/LC3 I level was upregulated in the MPP+ and MPP++si-NC groups compared with that in the control group (P<0.05), while the P62 level was decreased both in the MPP+ and MPP++si-NC groups (P<0.05; Fig. 3A). Conversely, when compared with that in the MPP+ group, the expression of LC3 II/LC3 I was inhibited in the MPP++si-NEAT1-1 group (P<0.05), whereas the level of P62 was elevated (P<0.05; Fig. 3A). Additionally, the fluorescence intensity of the LC3 cells in the MPP+ and MPP++si-NC groups was stronger than that in the control group (P<0.05; Fig. 3B), but weaker in the MPP++si-NEAT1-1 group compared with that in the MPP+ group (P<0.05). Results of NEAT1-1 overexpression showed that the level of P62 was decreased but the level of LC3 II/LC3 I was increased (Supplementary Fig. S2). These results demonstrated that NEAT1 enhanced autophagy of the SH-SY5Y cells induced by MPP+.

NEAT1 promotes MPP+-treated SH-SY5Y cell autophagy (A) Western blot analysis was conducted to detect the protein levels of LC3 I, LC3 II, and P62, respectively. (B) Changes of autophagosome were estimated by immunofluorescence microscopy. **P<0.01.
miR-374c-5p is a target of NEAT1
The StarBase3.0 database was used to predict the putative miR-374c-5p binding site in NEAT1 (Fig. 4A). As shown in Fig. 4B, the luciferase activity was greatly reduced after the addition of miR-374c-5p mimics to NEAT1-Wt (P<0.05), while no change was revealed in the NEAT1-Mut supplemented with miR-374c-5p mimics. Meanwhile, the RNA pull-down assay showed that the NEAT1 level was markedly increased by Bio-miR-374c-5p-Wt (P<0.05), while it did not work using Bio-miR-374c-5p-Mut (Fig. 4C). Our qRT-PCR results demonstrated that the expressions of miR-374c-5p in both the brain tissues and DA neurons were markedly reduced by MPTP (P<0.05; Fig. 4D,E). In addition, miR-374c-5p expression was also found to be greatly inhibited by MPP+ (P<0.05; Fig. 4F). In contrast to the MPP+ group, the miR-374c-5p expression in SH-SYSY cells was elevated in the MPP++si-NEAT1-1 group (P<0.05). The expression of miR-374c-5p was also increased in the miR-374c-5p mimics group when compared to that in the mimics NC group, but was decreased in the miR-374c-5p inhibitor group (P<0.05; Fig. 4F,G). Moreover, the expression of NEAT1 was lower in the miR-374c-5p mimics group but higher in the miR-374c-5p inhibitor group in comparison to that in the Mock group (P<0.05; Fig. 4H). All these results indicated that miR-374c-5p functioned as a target of NEAT1 and was negatively linked to NEAT1 in PD.
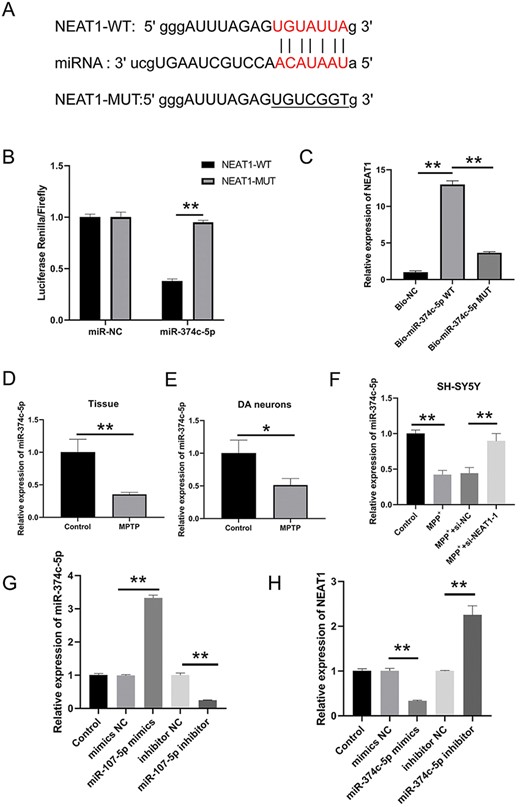
miR-374 c-5p is a target of NEAT1 (A) Binding site of miR-374 c-5p in NEAT1 was predicted by using StarBase3. (B) Dual-luciferase reporter assay was performed to measure the luciferase activity of NEAT1 vector. (C) Interplay between NEAT1 and miR-374 c-5p was verified by RNA pull-down assay. The expression of miR-374-5p in (D) brain tissue, (E) dopaminergic neuron, and (F) SH-SY5Y cells was measured by qRT-PCR. (G) miR-374 c-5p level in SH-SY5Y cells was quantified through qRT-PCR after various transfections. (H) NEAT1 level in SH-SY5Y cells was negatively correlated with the miR-374 c-5p. *P<0.05, **P<0.01.
NEAT1 accelerates apoptosis and autophagy of MPP+-induced SH-SY5Y cells by impairing the level of miR-374c-5p
MTT results demonstrated that the viability of the SH-SY5Y cells was markedly enhanced in the si-NEAT1-1+inhibitor-NC group in contrast to the si-NC+inhibitor-NC group (P<0.05), and the cell viability was inhibited in the si-NC+inhibitor group (P<0.05; Fig. 5A). SH-SY5Y cell viability in the si-NC+inhibitor-NC was higher than that in the si-NC+inhibitor group (P<0.05). There was no significant difference in cell viability between the si-NEAT1-1+inhibitor group and the si-NC+inhibitor-NC group (P>0.05; Fig. 5A). Apoptosis of the SH-SY5Y cells showed a sharp decrease in the si-NEAT1-1+inhibitor-NC group when compared with that in the si-NC+inhibitor-NC group (P<0.05) but increased in the si-NC+inhibitor group (P<0.05; Fig. 5B,C). Moreover, apoptosis of the SH-SY5Y cells in the si-NEAT1-1+inhibitor group was restored and identical to that of the si-NC+inhibitor-NC group (P<0.05; Fig. 5B,C). The results of western blot analysis indicated a lower expression of LC3 II/LC3 I in the si-NEAT1-1+inhibitor-NC group than in the si-NC+inhibitor-NC group (P<0.05), while the expression of P62 was increased in the si-NEAT1-1+inhibitor-NC group (P<0.05; Fig. 5D--F). When compared with that in the si-NC+inhibitor group, the expression of LC3 II/LC3 I was decreased and the expression of P62 was increased in the si-NEAT1+inhibitor group (P<0.05). As shown in Fig. 5G, dopamine content was elevated in the si-NEAT1-1+inhibitor-NC group (P<0.05) but declined in the si-NC+inhibitor group (P<0.05) compared with that in the si-NC+inhibitor-NC group. The dopamine content was elevated in the si-NEAT1-1+inhibitor group compared with that in the si-NC+inhibitor group. These findings suggested that NEAT1 play roles in promoting apoptosis and autophagy of MPP+-induced SH-SY5Y cells by impairing miR-374c-5p.
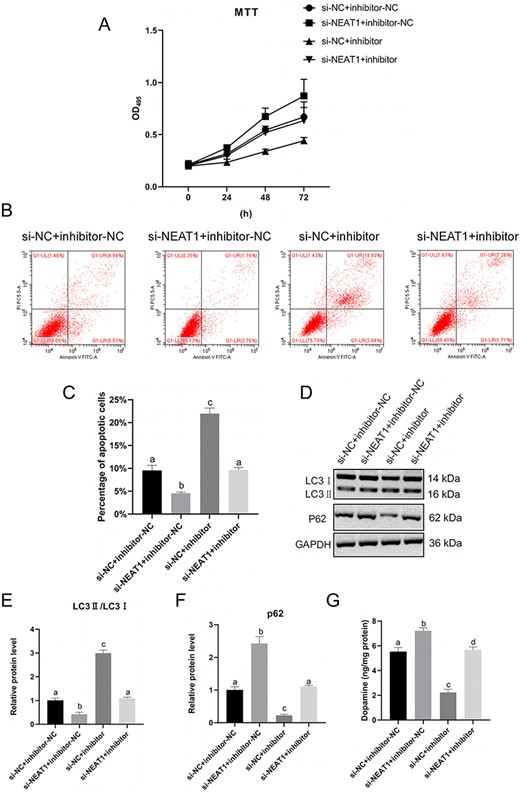
NEAT1 promotes apoptosis and autophagy of MPP+-induced SH-SY5Y cells by downregulating miR-374 c-5p (A) SH-SY5Y cells viability was evaluated by MTT assay. (B,C) SH-SY5Y cells apoptosis was analyzed by flow cytometry. (D–F) Protein levels of LC3 I, LC3 II, and P62 were determined by western blot analysis. (G) Dopamine content was measured by HPLC. *P<0.05 vs si-NC+inhibitor-NC group.
Next, autophagy initiation inhibitor 3-MA and autophagy inhibitor chloroquine (CQ) was used in subsequent experiments. As shown in Fig. 6A, cell viability was enhanced after 3-Methyladenine (3-MA) treatment (P<0.05) but weakened after CQ treatment (P<0.05). Moreover, 3-MA played a role in inhibiting cell apoptosis (P<0.05) while CQ played a role in promoting cell apoptosis (P<0.05; Fig. 6B). In the SH-SY5Y cells co-treated with MPP++si-NEAT1+3-MA, cell viability was increased and apoptosis was decreased compared with those in the MPP++3-MA group. In the MPP++si-NEAT1+CQ group, cell viability and apoptosis were restored. The expression of LC3 II/LC3 I was suppressed by 3-MA (P<0.05) but facilitated by CQ (P<0.05; Fig. 6C). Conversely, the expression of P62 was elevated by 3-MA (P<0.05) but suppressed by CQ (P<0.05; Fig. 6C). The results of NEAT1 overexpression were opposite to those of NEAT1 silencing (Supplementary Fig. S3). These results revealed that NEAT1 play a role in promoting apoptosis and autophagy of the SH-SY5Y cells induced by MPP+ through impairing miR-374c-5p.
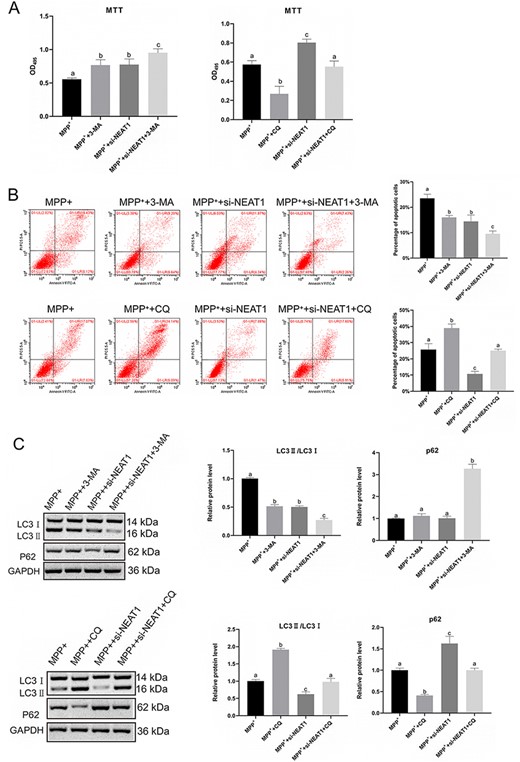
Effect of chloroquine and 3-MA on autophagy of MPP+-induced SH-SY5Y cells (A) SH-SY5Y cells viability was determined by MTT assay. (B) SH-SY5Y cell apoptosis was measured by flow cytometry. (C) Western blot analysis was carried out to detect the expressions of LC3 I, LC3 II, and P62. *P<0.05.
NEAT1 promotes cell autophagy in PD mice
Both the T-turn time and T-descend time were shortened in the si-NEAT1-1 group compared with those the Mock and si-NC groups (P<0.05; Fig. 7A,B), whereas the retention time was elongated (P<0.05; Fig. 7C). The TH+ rate of cells in the mouse midbrain was markedly increased in the si-NEAT1-1 group (P<0.05; Fig. 7D). The TUNEL assay showed decreased cell apoptosis in the midbrain tissue in the si-NEAT1-1 group (Fig. 7E). Furthermore, the level of NEAT1 in the midbrain tissue of the si-NEAT1-1 group was lower than that in the Mock and the si-NC groups (P<0.05; Fig. 7F), and the level of miR-374c-5p in the si-NEAT1-1 group was lower than that in the Mock and the si-NC groups (P<0.05; Fig. 7G). As shown in Fig. 7H,I, the LC3 II/LC3 I level was declined in the si-NEAT1-1 group compared with that in the mimics NC and si-NC groups (P<0.05), but the levels of P62 showed no significant difference. These results indicated that NEAT1 could promote cell autophagy in PD mice.
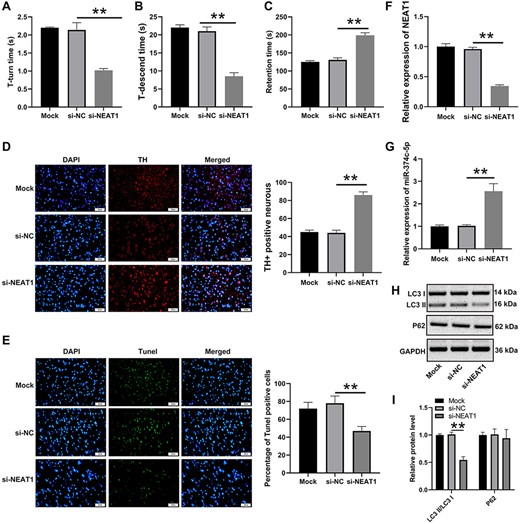
NEAT1 accelerates cell autophagy in PD mice A pole test was conducted to determine the (A) T-turn time and (B) T-descend time. (C) Retention time was measured by the rotarod test. (D) Changes in TH+ cells in mice midbrain tissue were determined by immunofluorescence microscopy. (E) The cell apoptosis of mice midbrain tissue was determined by TUNEL assay. The expressions of (F) NEAT1 and (G) miR-374 c-5p in brain tissues were detected by qRT-PCR. (H,I) Levels of autophagy-related factors were assessed by western blot analysis. **P<0.01.
Discussion
Little is known about the etiology and pathogenesis of PD in recent years. As a chemical contaminant producing neurotoxicity, MPTP can impair and aggravate the damage and apoptosis of dopaminergic neurons by inducing symptoms similar to PD [11]. MPTP is converted to its active metabolite MPP+, which can impair mitochondrial energy metabolism, induce ROS, and finally result in the death of dopaminergic neurons. Both MPTP and MPP are the active metabolites of PD and contribute to the establishment of PD models for further research [12,13]. Our present results demonstrated the successful establishment of the PD mouse model based on the following two factors: one was the elongated time spent in T-turn and T-descend but shortened time in retention, and the other was the declined TH+ cell rate in MPTP-induced PD mice.
PD is frequently encountered and pathologically characterized by a selective loss of dopaminergic neurons in the substantia nigra, as well as an intracellular accumulation of toxic proteins in the Lewy bodies [14]. Numerous reports indicated a relationship between PD pathogenesis and the processes of apoptosis and/or autophagy. Although neuronal autophagy appears primarily to be a protective process in the nervous system, impairment of basal autophagy results in neuronal death [15]. Moreover, many neurodegenerative diseases of the central and peripheral nervous systems arise from metabolic failure and toxic protein aggregation [16]. The alterations in the autophagic process are responsible for the aggregation of toxic proteins [17]. Numerous evidence demonstrated that abnormal expression of lncRNAs is correlated with the progression of this neurodegenerative disorder. Peng et al. [18] reported that lncRNA homeobox DNA-binding antisense growth-associated long noncoding RNA (HAGLROS) was upregulated in the PD mouse model induced by MPTP and in the SH-SY5Y cells treated with MPP+. Increasing studies have indicated the roles of lncRNA in PD. For example, lncRNA HAGLROS has been found to participate in regulating cellular apoptosis and autophagy in PD, and this process is realized by mediating the miR-100/ATG10 axis and the PI3K/Akt/mTOR signaling pathway [18]. lncRNA-p21 was also reported to suppress cell viability and promote cell apoptosis in PD by inhibiting miR-1277-5p [19]. The findings of our present study demonstrated the pathological role of NEAT1 in PD. By interfering NEAT1, apoptosis and autophagy of the SH-SY5Y cells were reduced, which is consistent with the results of a previous report that neuron apoptosis and autophagy are associated with the process of PD. Meanwhile, by interfering NEAT1, apoptosis and autophagy of the SH-SY5Y cells were inhibited due to its influence on the related proteins LC3 II/LC3 I and P62. Autophagosome was markedly reduced in the SH-SY5Y cells interfered by NEAT1. Interfering NEAT1 in mice robustly inhibited autophagy by decreasing the number of autophagosomes, thereby regulating the level of autophagy-related proteins. The results of the present study indicated that NEAT1 has the potential to be a novel diagnostic biomarker and therapeutic target in treating PD.
miRNAs are closely related to the pathogenesis of PD and play an essential role in the occurrence and development, diagnosis, and even treatment of PD. Taking miR-326 as an example, it is found that it acts as an apoptosis suppressor and promotes the proliferation of dopaminergic neurons in PD [20,21]. miR-505 can maintain the cytotoxicity of the SH-SY5Y cells induced by MPP+ in the PD mouse model in vitro [22,23]. Previous studies have confirmed that NEAT1 may function as a miRNA-competing endogenous RNA (ceRNA) and is indispensable in various diseases by antagonizing the function of miRNA and regulating the expression of miRNA target. By downregulating the target gene, NEAT1 can inhibit neuronal cell apoptosis in acute spinal cord injury [24–26]. There are also many differentially expressed miRNAs in MPTP-induced mouse midbrain tissues and control tissues, and miR-374C-5p is one of them (Supplementary Fig. S4). Our results suggested that miR-374c-5p serves as a direct target of NEAT1. We further identified the role of NEAT1 in PD and found that NEAT1 promoted MPP+-induced SH-SY5Y cell apoptosis and autophagy by impairing miR-374c-5p.
In summary, the findings of the present study demonstrated that NEAT1 was highly expressed in the MPTP-induced PD mouse model and the MPP+-treated SH-SY5Y cells. Meanwhile, miR-374C-5p, the target of NEAT1, was inhibited in MPTP-induced PD mouse model and the MPP+-treated SH-SY5Y cells. This study also revealed that NEAT1 was able to promote autophagy and apoptosis in MPTP-induced PD by downregulating the level of miR-374c-5p. We ultimately concluded that NEAT1 may be a potential player in the pathophysiological course of PD.
Supplementary Data
Supplementary data is available at Acta Biochimica et Biophysica Sinica online.
Funding
This work is supported by the grant from the National Natural Science Foundation of China (No. 81771375).
Conflict of Interest
The authors declare that they have no conflict of interest.



