-
PDF
- Split View
-
Views
-
Cite
Cite
Xianzheng Qin, Jiang Chen, Tian Zhou, 22q11.2 deletion syndrome and schizophrenia, Acta Biochimica et Biophysica Sinica, Volume 52, Issue 11, November 2020, Pages 1181–1190, https://doi.org/10.1093/abbs/gmaa113
Close - Share Icon Share
Abstract
22q11.2 deletion is a common microdeletion that causes an array of developmental defects including 22q11.2 deletion syndrome (22q11DS) or DiGeorge syndrome and velocardiofacial syndrome. About 30% of patients with 22q11.2 deletion develop schizophrenia. Mice with deletion of the ortholog region in mouse chromosome 16qA13 exhibit schizophrenia-like abnormal behaviors. It is suggested that the genes deleted in 22q11DS are involved in the pathogenesis of schizophrenia. Among these genes, COMT, ZDHHC8, DGCR8, and PRODH have been identified as schizophrenia susceptibility genes. And DGCR2 is also found to be associated with schizophrenia. In this review, we focused on these five genes and reviewed their functions in the brain and the potential pathophysiological mechanisms in schizophrenia, which will give us a deeper understanding of the pathology of schizophrenia.
Introduction
The chromosome 22q11.2 deletion syndrome (22q11DS) is a multisystem disease caused by a microdeletion in chromosome 22q11.2 region, which is the most common interstitial deletion in human, and has an incidence of 1 in 2000 to 4000 live births. About 87% of the 22q11.2 microdeletion is deletion of 3 megabases (Mb), a smaller percentage of about 8% is 1.5 Mb, and the rest of the deletion is nested deletion breakpoints [1–3]. There are approximately 60 known genes in the 3-Mb deletion region, and 35 known genes in the 1.5-Mb region. The clinical representations among these patients are diverse and complex. The changes of multiple organs are involved, including craniofacial defects and cardiovascular anomalies [4]. The severity of 22q11DS is independent of the size of the deletion, and only the deletion of 1.5 Mb affects the phenotypes [3]. It indicates that 35 known genes in the deletion of 1.5 Mb are crucial to the etiology of the syndrome. A previous study of brain structural alterations in 22q11DS has indicated that 22q11DS patients have a reduced cortical surface area. The expansion of cortical surface area is affected by the increased production of progenitor cells during embryonic development [5]. Therefore, the reduction of cortical surface area may suggest that 22q11DS is originated in the early stage of brain development. Compared with patients with 3-Mb deletion, 22q11DS patients with 1.5-Mb deletion have greater cortical surface area. It is the strong evidence to support the effect of deletion size on brain structure [6].
22q11DS contains the DiGeroge syndrome, velocardiofacial syndrome, Cayler syndrome, and conotruncal anomaly face syndrome, as well as other results of different syndromes [7]. It exhibits various syndromal features such as congenital heart defects (CHDs), cognitive impairments, hypoparathyroidism, immune deficiency, cleft palate, and a distinct facial appearance [8,9]. Especially the impartments of cognitive phenotype are associated with psychopathology such as the learning difficulties, autism spectrum disorder (ASD), cognitive deficits and early language delays, deficits in visuospatial abilities and arithmetic, attention deficit disorder (ADHD), and coordination deficits [10–13]. Furthermore, about 30% of patients with 22q11.2 deletion develop schizophrenia, with a risk of about 12 to 80 folds higher than the general population [4,14]. Early language delays and minor coordination deficits are some developmental abnormalities of the premorbid phase of schizophrenia [8]. Cognitive deficits in childhood are usually diagnosed as other psychiatric disorders like ADHD, ASDs, and generalized anxiety disorder [15,16]. In addition, about one-third of 22q11.2 deletion patients develop schizophrenia in late adolescence and early adulthood. Most importantly, the phenotype of schizophrenia has no significant difference between schizophrenia patients with 22q11.2 deletion or no deletion [17]. These features of 22q11DS mentioned above support the neurodevelopmental origins for schizophrenia [18].
Schizophrenia is a highly genetic and disabling mental disorder. By the research of the occurrence of schizophrenia in families, some genes have been speculated to be associated with schizophrenia. Although it has a stronger genetic component, the majority of patients with schizophrenia have no family history of this disorder [19,20]. There is mounting evidence that de novo copy number variants (CNVs) are becoming of great concern for genetic cause of schizophrenia, which include the 22q11.2 microdeletion [21]. 22q11.2 microdeletion accounts for 1% to 2% of adult schizophrenic patients [22].
Based on the behavioral similarity between 22q11DS and schizophrenia and the fact that individuals with 22q11DS have a high risk of developing schizophrenia, studying gene genetic variations associated with classical representations of schizophrenia by using 22q11DS as the model is the most effective solution to find its pathogenesis. In this review, we summarized the obtained evidences to support the association between 22q11DS and schizophrenia, and outline the roles of five 22q11DS genes COMT, zinc finger and DHHC domain-containing protein 8 (ZDHHC8), DiGeorge Critical Region 8 (DGCR8), proline dehydrogenase (PRODH), and DGCR2 in the brain and their possible pathophysiological mechanism underlying schizophrenia.
The Suggestive Linkage Analysis for Schizophrenia on Chromosome 22
The linkage study is usually used in families with two or more affected relatives. Schizophrenia acts as a disorder with a stronger genetic component, and it has a weak linkage with 22q12 region by studying the genetic linkage of schizophrenia [23]. The Johns Hopkins University/Massachusetts Institute of Technology (JHU/MIT) identified human chromosome 22 as an interest region with schizophrenia susceptibility genes [24]. A submission of parametric linkage analysis showed a dominant model with the marker IL2RB in chromosome 22ql3, which from 39 multiply affected families had a maximum logarithm of the odds (LOD) score of 2.85 [25]. However, there was no statistically significant linkage result. By sib pair analysis to detect the genotype of 495 markers throughout the genome in a JHU/MIT sample of 57 families and additional 9 multiply affected families, the result indicated locus CRYB2, which is a highly informative marker at chromosome 22q11.2-q12.1 region that has the highest overall LOD score 1.20 [26]. It indicated the association between schizophrenia and chromosome 22q region. By analyzing data with a lower maximum penetrance, researchers revealed that the chromosome 22q region is the most positive and refined map of the region, no region of 22q can be excluded from these samples, and the regions q11 to q12 are the most likely region for schizophrenia risk genes [25,26].
Individuals with 22q11DS are associated with a significantly increased risk of developing schizophrenia. The incidence of patients with 22q11DS who develop schizophrenia is up to around 25%, whereas the incidence in the general individuals is only 1% [27,28]. According to a previous study, in which authors used DSM-III-R criteria to detect the early adulthood with velocardiofacial syndrome, a representative syndrome of 22q11DS who are older than 17 years, about 30% individuals were diagnosed with schizophrenia [29].
22q11-Deletion Mouse Model
Although the arrangement of genes in chromosome is different, nearly all orthologs of the deleted genes in the region of human chromosome 22q11.2 can find analogs in mouse chromosome 16, except for clathrin heavy chain like 1 (CLTCL1) [30]. Mice (Df1) that have heterozygous deletion of the genes in about 1.5 Mb of chromosome 16qA13 have defects in learning and memory, especially in sensorimotor gating. Prepulse inhibition (PPI) of the startle reflex is a behavior paradigm to test the sensory-motor gating, which is often reduced in schizophrenics [14,31,32]. Compared with wild-type mice, Df1 mice reduced the response of PPI significantly [33]. Learning and memory can be measured by the Delayed Alternation Task [34] and the Latent Inhibition (LI) Assay [35]. The interruption of LI is deemed to be a cognitive impairment in the representation of schizophrenia [36]. The behavioral consistency between 22q11-deletion mice and individuals with schizophrenia suggests that mouse model is a relatively reliable model to study schizophrenia.
22q11-deletion mouse models are used not only in behavioral analysis, but also in cellular and molecular neuropathology. A microarray technology used to quantify the mRNA levels of the brain can identify the expressions of modified genes [37,38]. By investigating the 22q11-deletion mouse models, there are lots of differences in the expressions of genes in the deletion area during the development of brain [39–41]. For further analysis, this technology can identify the pathway of genes and localize the proteins that are encoded by genes and the interaction with other proteins in schizophrenia. It affords the possibility to intensively study the association between genes in 22q11DS and the pathogenesis of schizophrenia.
The Influence of Risk Genes in 22q11DS on Schizophrenia
Although 22q11DS increases the risk of schizophrenia, there is no correlation between the deletion size and schizophrenia. It has been reported that a schizophrenic patient with a smaller deletion region exhibited more severe schizophrenia symptoms than 22q11DS patients [22]. Genome-wide linkage studies indicated that the susceptibility locus for schizophrenia is located in chromosome 22q11, and the locus can be further refined to 1.5 Mb by analyzing these microdeletions with polymorphic markers [42]. In addition, the severity of 22q11DS is unrelated to the size of the deletion and only 1.5-Mb deletion affects the phenotypes [3]. Therefore, the genes within the 1.5-Mb region of 22q11DS may be responsible for the pathogenesis of schizophrenia. Genes in this region are, individually or in combination, possible causes of schizophrenia. It is worth mentioning that nondeletion variants of the approximately 35 genes in the 22q11.2 region might have more risk for developing schizophrenia than hemizygous deletion of these genes [14]. After years of efforts, most of the genes in this region are known, and the normal function of some genes is believed to be important for the prevention of the occurrence and development of schizophrenia. Some genes in 22q11.2 region are risk genes of schizophrenia and the abnormalities of them are associated with schizophrenia. Previous studies in mouse models have suggested several candidate genes in the 22q11.2 region, and one or more individual genes in the deleted region might increase the risk for schizophrenia [14,18,43]. The deficiency of one more genes can damage the function of synapses, and it is difficult to compensate this kind of damage [14]. Catechol-O-methyltransferase (COMT) gene was found to be the first gene to predict the development of schizophrenia in the patients with 22q11DS [43]. Beyond that, other risk genes in the 22q11.2 region include: DGCR8, DGCR2, PRODH, and ZDHHC8 (Fig.1). In the following section, we will describe in detail the most susceptible risk genes to schizophrenia.
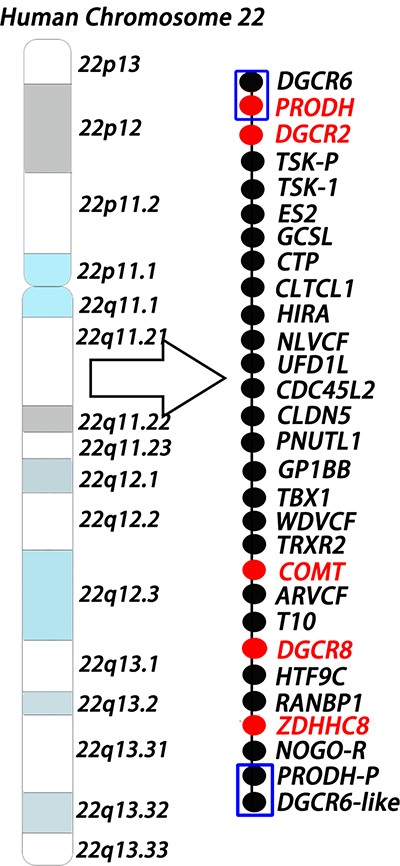
Human chromosome 22q11.2 microdeletion model The model shows the 1.5-Mb deletion region in human chromosome 22q11.2 locus. Each circle represents one gene, and the red color indicates risk genes for schizophrenia. The two ends of the 1.5-Mb region are low copy repeat sequences (shown as blue boxes), which make deletions more likely to happen.
COMT
COMT gene is a schizophrenia risk gene encodes COMT enzyme that plays an important role in degrading dopamine in the brain, and in particular, in the prefrontal cortex [44,45]. By investigating the human and animal models, the metabolic abnormalities of dopamine in the prefrontal cortex might change the synaptic plasticity and increase the risk of developing schizophrenia [46–48]. In COMT-knockout mice, removal rate of dopamine in the prefrontal cortex was slower and dopamine level was increased [49].
COMT gene has a functional single-nucleotide polymorphism (SNP), val158met. It makes a G to A mutation in the coding sequence, which causes a change of nucleotide from valine (Val) to methionine (Met) at codon 158 [50]. COMT val158met polymorphism has been shown to play an important role in the activity of the prefrontal cortex [51]. Compared with the Met allele, the activity of the Val allele of COMT is higher and the degradation of dopamine is more effective, which results in lower level of dopamine in the prefrontal cortex [52]. Wang et al. [53] found that compared with Met allele, schizophrenia patients with Val allele showed more negative symptoms like apathy, blunted affect and motor retardation. Previous studies in functional and anatomical aspects have suggested that the impairment of the prefrontal cortex is associated with schizophrenia [54,55]. In Gothelf’s study, they found that Met allele was associated with the decreased volume of the prefrontal cortex and further caused the damage of the prefrontal cortex, and it increased the probability of developing psychiatric symptoms [56,57].
It has been proposed that the Val allele may increase the risk for schizophrenia by affecting the dopamine metabolism and interfering the transmission of dopamine in the prefrontal cortex [58]. Since individuals with 22q11DS do not have an intact chromosome, patient only carries one copy of the COMT alleles. It leads to a speculation that the neurotransmission of dopamine is affected, especially in patients with the Met158 allele [59]. However, another report emphasized the association of schizophrenia with the low-activity Met allele [60]. Therefore, COMT may be involved in the pathogenesis of schizophrenia by affecting the metabolism of dopamine to further influence the synaptic plasticity and interfere with neurodevelopment.
However, a few studies revealed that the association between COMT polymorphism and schizophrenia is not obvious. Glatt et al. [61] indicated that the risk for schizophrenia in populations of European ancestry caused by COMT is extremely small. A meta-analysis showed that COMT Val allele has no association with schizophrenia [62]. Several pieces of evidence showed that the risk for COMT to schizophrenia may not just limit to the Val/Met polymorphism alone. It could involve other locus in the COMT gene and the interaction between gene and environment [63,64].
DGCR8
DGCR8 lies within the chromosome 22q11.2 region and encodes a component of the microprocessor complex, which plays an important role in miRNA-processing [65,66]. Increased DGCR8 expression and elevated miRNA level were observed in the postmortem prefrontal cortex of schizophrenia patients [67]. Several studies have shown that DGCR8 is directly associated with schizophrenia by using hemizygous deficient DGCR8 mice, which represents psychiatric and cognitive phenotypic deficits [32].
DGCR8 haploinsufficient mice (Dgcr8+/− mice) have been used extensively in experiments. The results indicated that the hemizygous expression of DGCR8 may not only reduce the biosynthesis of all miRNAs in the 22q11.2 region, but also involve the pathogenesis of 22q11DS-associated schizophrenia [32,66]. By observing the Dgcr8+/− mice and Df1 mice, researchers showed that the level of mature miRNA in hemizygous deficient mice is lower compared with control samples, and the miRNA expression is dysregulated in brain tissue and serum [68–71]. It strongly demonstrated the effects of hemizygous DGCR8 on altered miRNA expression [72]. The dysregulation of miRNA processing in 22q11DS that caused by hemizygous deficient DGCR8 may target multiple mRNAs and make these mRNAs to be dysregulated significantly, ultimately leading to the clinical phenotypes including schizophrenia [73].
Haploinsufficiency of DGCR8 leads to about 20% to 70% downregulation of a specific miRNA subset [65]. Among these miRNAs, the expressions of miR-185 in both the prefrontal cortex and the hippocampus were reduced, which led to a developmental deficit of dendritic and spines in the hippocampal neurons [32,74]. In the Dgcr8+/− mice, the number of cortical neurons was reduced and the structure of dendritic spines in the prefrontal cortex was also incomplete [75]. These results showed that DGCR8 plays a role in the development of circuitry in the prefrontal cortex and might influence the functional connectivity in the nervous system [76,77]. Heterozygous DGCR8 also decreases the proliferation of adult hippocampal progenitor cells (AHPs) in the hippocampal dentate gyrus, which will eventually affect the neurogenesis [78]. Defective adult neurogenesis is involved in the pathogenesis of the schizophrenia [79,80]. Interestingly, the expression of schizophrenia-associated gene insulin-like growth factor 2 (IGF2) was decreased in Dgcr8+/− mice, and rescued expression of IGF2 could restore the psychiatric and cognitive phenotypes of 22q11DS-associated schizophrenia [78].
Therefore, the association between miRNA dysregulation and haploinsufficiency of DGCR8 is a possible mechanism for the development of schizophrenia [32,74].
PRODH
PRODH gene encodes proline oxidase (POX), which is a mitochondrial membrane enzyme and associated with schizophrenia susceptibility [14,81]. POX catalyzes the metabolism of proline to glutamate that is of vital importance to transduce signals between neurons and to develop synaptic plasticity [82,83]. Proline is a modulator of glutamatergic and GABAergic neurotransmission, and the dysfunction of them involves in the development of schizophrenia [84]. Moreover, some evidence suggested that hyperprolinemia may lead to neurocognitive dysfunction, in both PRODH-deficient mice and Df1 mice that have an increased level of proline, and a mild form of hyperprolinemia has been found in individuals with 22q11DS and schizophrenia [81,85–88]. These results suggested that the deficiency of PRODH might be associated with schizophrenia.
There are several SNPs that are proposed to be associated with schizophrenia. SNPs with increased POX activity have a positive association with schizophrenia, and decreased enzyme activity has a negative association between SNPs and schizophrenia [89,90]. Substitutions in C757T, G1852A, and A1766G are the most common allelic variations in PRODH gene among which C757T and G1852A could decrease the activity of proline dehydrogenase, but A1766G increases its enzyme activity [42,89–91]. Based on the results of these polymorphisms in different populations, PRODH gene is a risk gene that is strongly associated with schizophrenia in the Iranian population but no significant association in European and Chinese population [92–94].
Mice with homozygous PRODH mutants had an increased level of proline and had a decreased PPI of the startle reflex, and schizophrenia patients also exhibited decreased PPI [88]. In PRODH-knockout mice, glutamatergic signaling was increased in the hippocampus [95]. It suggested the effects of neuromodulatory amino acids on schizophrenia.
In addition, gene–gene interaction between PRODH and COMT also increases susceptibility to schizophrenia [14]. In PRODH mutant mice, the level of COMT mRNA in the frontal cortex was increased, which was regarded as a compensatory response for abnormal expression of dopamine [96]. By supplementing tolcapone (COMT inhibitor) into PRODH-deficient mice, it badly damaged PPI and working memory [96]. Mice also showed altered transmission and catabolism of dopamine [4,97]. These studies suggested that the deficiency of more than one gene might more likely increase the risk of schizophrenia [14,96].
ZDHHC8
Some evidence suggested that ZDHHC8 acts as a schizophrenia risk gene [14]. It encodes a transmembrane palmitoyltransferase that plays an important role in the palmitoylation of protein, especially modifies neural proteins and affects neurotransmitter receptor function, neural development and synaptic transmission [98,99]. Early reports showed that the palmitoylation of postsynaptic density protein PSD95 could modulate the density of dendritic spines [100]. Decrease in dendritic spines has some signs that are associated with schizophrenia such as poor synaptic strength and disabled functional connectivity [101]. In addition, SNPs in ZDHHC8 are associated with schizophrenia and one of these SNPs is ZDHHC8 SNP rs175174 that has a stronger association with schizophrenia [89,102]. SNP rs175174 has a transformation of nucleotide between A and G, and in cell experiments, ZDHHC8 expression is modified through the effects of risk allele rs175174-A and it produces relatively higher level of the imperfectly-spliced form, which might encode an inactive truncated protein [14,103]. By analyzing the appropriate animal model, researchers demonstrated that allele A was overtransmitted significantly in female, but not significantly in male individuals with schizophrenia [102]. The above results support that the ZDHHC8 gene is associated with schizophrenia.
ZDHHC8 expression detection in adult mouse brain showed that ZDHHC8 is enriched in the cortex and hippocampus and these areas are more possible to be associated with the pathogenesis of schizophrenia [102]. In ZDHHC8-knockout mice, the brain morphology of homozygous mice was seemingly normal, but the degree of behavioral deficits varied due to sex differences. ZDHHC8-knockout mice showed a significant sexually dimorphic deficits and particularly homozygous mutant female mice presented a decline in locomotor activity, lower levels of PPI, and other features of mice with schizophrenia. Homozygous ZDHHC8 mutant male mice only had a slight deficit of locomotor activity, while heterozygotes were almost the same as wild-type mice [102].
Behavioral deficits may be due to the modification of neurotransmitter systems by the effects of the palmitoylation of neuronal proteins, and glutamatergic transmission is one of these changes of neurotransmitter systems [98,104]. By using psychomimetic dizocilpine (MK801), which increases the excretion of glutamate and thereout activates glutamatergic neurotransmission in both homozygous ZDHHC8 mutant mice and wild-type control, MK801-induced locomotor activity in homozygous ZDHHC8 mutant female mice showed a less sensitive presentation than control mice [102,105]. It is believed that the mutation of ZDHHC8 affects partial behaviors through the interference of glutamatergic transmission.
ZDHHC8-deficient mice are reported to have a decreased dendritic arborization and low spine densities in the hippocampal neurons during neurodevelopment, which is the same as Df1 mice, while administration of active ZDHHC8 protein can rescue these deficits [100]. Schizophrenic patients have a decreased density of dendritic spines in the cortex and hippocampus, where ZDHHC8 is highly expressed [106]. Defect of ZDHHC8 affects the palmitoylation and causes the decrease of dendritic spines, which may be a possible mechanism of schizophrenia. Furthermore, the effect of ZDHHC8 associated Akt and CDC42 signaling pathway on schizophrenia has been got further studied [101,107–109].
DGCR2
DGCR2 is a gene that causes the 22q11DS, and it encodes a novel putative adhesion receptor protein and also has effects on the neuronal migration and cortical projection neurons (PNs) migration from the intermediate zone of cortical layers toward upper during this process [110–112]. It has been reported that the haploinsufficiency of DGCR2 may contribute to the increased risk of schizophrenia [113,114]. Therefore, the differential expression of DGCR2 between patients with schizophrenia and normal individuals may be a possible explanation. The abnormal expression of DGCR2 might change synaptic plasticity and interfere with the transduction of signals between neurons through abnormal dopamine pharmacological metabolism [101,115,116]. The individuals with schizophrenia showed a reduced expression level of DGCR2 [113,115]. Interestingly, the expression of DGCR2 in the dorsolateral prefrontal cortex is increased by treatment with antipsychotic drugs [113]. A reasonable speculation is that low expression of DGCR2 increases the possibility of schizophrenia and antipsychotic drugs could help relieve some symptoms by elevating DGCR2 expression.
An Ashkenazi Jewish population study revealed that several SNPs within DGCR2 are associated with the development of schizophrenia, and the risk alleles of SNPs are associated with reduced expression of DGCR2 gene in the brain [117]. A rare de novo DGCR2 mutation (P429R mutation), which makes a rare missense mutation of the 429th amino acid proline (P) in DGCR2 to arginine (R) in the primary structure, was identified from the patients with schizophrenia [114]. It is an important SNP for the development of schizophrenia.
Recently, it was proposed that DGCR2 short hairpin RNA (sh-DGCR2) that reduces the expression of DGCR2 and P429R mutation could influence the migration of PNs. The results showed that DGCR2 was expressed in the late embryonic phase of corticogenesis and DGCR2 regulated the radial-guided locomotion and terminal translocation of PNs [115]. Compared with mice with P429R mutation, wild-type DGCR2 mice could rescue the laminar positioning of PNs [115]. It suggested that P429R mutation is a pathological mutation that affects migration of PNs. Reelin is a cell-extrinsic regulator that regulates inside-out lamination of cortical PNs, and DGCR2 could bind with Reelin to regulate the phosphorylation of downstream effectors [118]. Reelin-dependent phosphorylation targets, such as DAB1, AKT, and ERK1/2, are involved in controlling the migration of PNs, and the expressions of these targets were found to be decreased in DGCR2-knockdown mice [115,119–121]. Schizophrenia is considered to be a neurodevelopmental disorder, and the above evidence strongly support that DGCR2 is a risk gene for schizophrenia by affecting the migration of PNs in the development of cortical circuit [115].
Conclusions and Future Directions
22q11.2 deletion can cause a series of developmental defects, which include 22q11DS, and about 30% of patients with 22q11.2 deletion develop schizophrenia. There is a deletion of 1.5 Mb in the 22q11.2 region, and it approximately contains 35 known genes. Some of the genes in this region play an important role in preventing the development of schizophrenia, and deletion of one or more of these genes may increase the risk of schizophrenia.
Table1 gives a brief summary of these risk genes, and it emphasizes the function of risk genes and their possible pathological changes. COMT acts as the first gene to find the association between schizophrenia and 22q11DS; the defect of COMT could affect the degradation of dopamine and interfere with the transmission of dopamine in the prefrontal cortex. These effects alter synaptic plasticity and damage the prefrontal cortex and further influence the neurodevelopment. DGCR8 is involved in the processing of miRNAs, and knockout of DGCR8 could reduce the expression of miR-185 and lead to a developmental defect in dendrites and spines in the hippocampal neurons. In addition, it also influences the functional connectivity in the nervous system. PRODH catalyzes proline catabolism and involves glutamatergic signaling, and deficiency of PRODH will affect the development of synaptic plasticity. It also interacts with COMT and changes the release and clearance of dopamine. In PRODH-knockout mice, COMT level is increased to compensate the changes of dopamine. Therefore, gene–gene interactions increase the risk for schizophrenia. ZDHHC8 shows sexually dimorphic deficits in behaviors, and the symptoms of schizophrenia in female are more significant. Defect of ZDHHC8 inhibits the palmitoylation and affects the function of neural development and synaptic transmission. The expression of DGCR2 in schizophrenia patients is reduced, and DGCR2 could interfere with the formation of synapses and influence the transduction of signals through altered dopamine metabolism. Furthermore, DGCR2 has a de novo P429R mutation, which affects the migration of PNs in the development of cortical circuit.
| Risk gene . | Full name . | Normal function . | Defective expression and its change . | Follow-up effects . | Risk for the development of schizophrenia . |
|---|---|---|---|---|---|
| DGCR8 | DiGeorge Critical Region 8 | Encodes a component of the microprocessor complex, which plays an important role in miRNA processing | 1. Decrease the number of cortical neurons and change the structure of dendritic spines in prefrontal cortex | 1. Have effects on the development of circuitry in the prefrontal cortex and might influence the functional connectivity in the nervous system | The dysregulation of mRNAs leads to clinical phenotype included schizophrenia. Defective adult neurogenesis is also sufficient for schizophrenia. |
| 2. Decrease the proliferation of AHPs in the hippocampal dentate gyrus | 2. Eventually affect the neurogenesis | ||||
| 3. Alter processing of miRNA, which may target multiple mRNAs | 3. Cause dysregulation of target mRNAs and affect the plasticity of the prefrontal cortex | ||||
| DGCR2 | DiGeorge Critical Region 2 | Encodes a novel putative adhesion receptor protein, which has effects on the neuronal migration and the migration of PNs | 1. Interfere the radial-guided locomotion and terminal translocation of PNs | 1. Affect the migration of PNs in the development of cortical circuit | Altered cortical circuit is associated with schizophrenia. Changed synaptic plasticity increases the risk for schizophrenia. |
| 2. Change dopamine pharmacological metabolism | 2. Influence the transduction of signals between neurons | ||||
| COMT | Catechol-O-methyltransferase | Encoded enzyme COMT plays an important role in degrading dopamine, the prefrontal cortex in particular | 1. Increase dopamine level and influence the transmission of dopamine | 1. Abnormal dopamine metabolism changes the synaptic plasticity | Changes in synaptic plasticity increase the risk for schizophrenia. Impairment of the prefrontal cortex and influences of transmission of dopamine are also associated with schizophrenia. |
| 2. Decrease volume of the prefrontal cortex | 2. Damage the prefrontal cortex and cause the neurotransmission of dopamine that is affected | ||||
| PRODH | Proline dehydrogenase | Encodes proline dehydrogenase, which catalyzes the metabolism of proline to glutamate and provides energy, maintains homeostasis, and produces ROS by proline oxidation | 1. Interferes proline catabolism and increases the level of proline | 1. Increased proline level could develop for hyperprolinemia and leads to neurocognitive dysfunction | Gene–gene interaction between PRODH and COMT increases the risk for schizophrenia. Hyperprolinemia has been recorded in patients with schizophrenia. |
| 2. Change the release and clearance of dopamine | 2. Change synaptic plasticity | ||||
| ZDHHC8 | Zinc finger and DHHC domain-containing protein 8 | Encodes a transmembrane palmitoyltransferase that plays an important role in the palmitoylation of protein, especially neural proteins | 1. Show sexually dimorphic deficits in behaviors, especially in female | 1. The degree of behavioral deficits is variant due to the number of functional ZDHHC8 gene | Impaired synaptic strength and disabled functional connectivity are the signs associated with schizophrenia. ZDHHC8-enriched brain areas like cortex and hippocampus are more possible for pathogenesis of schizophrenia. |
| 2. Interferes glutamatergic neurotransmission through altered palmitoylation | 2. Reduced palmitoylation affects the function of neurotransmitter receptor, neural development and synaptic transmission | ||||
| 3. Decrease dendritic arborization and spine densities | 3. Impair synaptic strength and affects functional connectivity | ||||
| Risk gene . | Full name . | Normal function . | Defective expression and its change . | Follow-up effects . | Risk for the development of schizophrenia . |
|---|---|---|---|---|---|
| DGCR8 | DiGeorge Critical Region 8 | Encodes a component of the microprocessor complex, which plays an important role in miRNA processing | 1. Decrease the number of cortical neurons and change the structure of dendritic spines in prefrontal cortex | 1. Have effects on the development of circuitry in the prefrontal cortex and might influence the functional connectivity in the nervous system | The dysregulation of mRNAs leads to clinical phenotype included schizophrenia. Defective adult neurogenesis is also sufficient for schizophrenia. |
| 2. Decrease the proliferation of AHPs in the hippocampal dentate gyrus | 2. Eventually affect the neurogenesis | ||||
| 3. Alter processing of miRNA, which may target multiple mRNAs | 3. Cause dysregulation of target mRNAs and affect the plasticity of the prefrontal cortex | ||||
| DGCR2 | DiGeorge Critical Region 2 | Encodes a novel putative adhesion receptor protein, which has effects on the neuronal migration and the migration of PNs | 1. Interfere the radial-guided locomotion and terminal translocation of PNs | 1. Affect the migration of PNs in the development of cortical circuit | Altered cortical circuit is associated with schizophrenia. Changed synaptic plasticity increases the risk for schizophrenia. |
| 2. Change dopamine pharmacological metabolism | 2. Influence the transduction of signals between neurons | ||||
| COMT | Catechol-O-methyltransferase | Encoded enzyme COMT plays an important role in degrading dopamine, the prefrontal cortex in particular | 1. Increase dopamine level and influence the transmission of dopamine | 1. Abnormal dopamine metabolism changes the synaptic plasticity | Changes in synaptic plasticity increase the risk for schizophrenia. Impairment of the prefrontal cortex and influences of transmission of dopamine are also associated with schizophrenia. |
| 2. Decrease volume of the prefrontal cortex | 2. Damage the prefrontal cortex and cause the neurotransmission of dopamine that is affected | ||||
| PRODH | Proline dehydrogenase | Encodes proline dehydrogenase, which catalyzes the metabolism of proline to glutamate and provides energy, maintains homeostasis, and produces ROS by proline oxidation | 1. Interferes proline catabolism and increases the level of proline | 1. Increased proline level could develop for hyperprolinemia and leads to neurocognitive dysfunction | Gene–gene interaction between PRODH and COMT increases the risk for schizophrenia. Hyperprolinemia has been recorded in patients with schizophrenia. |
| 2. Change the release and clearance of dopamine | 2. Change synaptic plasticity | ||||
| ZDHHC8 | Zinc finger and DHHC domain-containing protein 8 | Encodes a transmembrane palmitoyltransferase that plays an important role in the palmitoylation of protein, especially neural proteins | 1. Show sexually dimorphic deficits in behaviors, especially in female | 1. The degree of behavioral deficits is variant due to the number of functional ZDHHC8 gene | Impaired synaptic strength and disabled functional connectivity are the signs associated with schizophrenia. ZDHHC8-enriched brain areas like cortex and hippocampus are more possible for pathogenesis of schizophrenia. |
| 2. Interferes glutamatergic neurotransmission through altered palmitoylation | 2. Reduced palmitoylation affects the function of neurotransmitter receptor, neural development and synaptic transmission | ||||
| 3. Decrease dendritic arborization and spine densities | 3. Impair synaptic strength and affects functional connectivity | ||||
| Risk gene . | Full name . | Normal function . | Defective expression and its change . | Follow-up effects . | Risk for the development of schizophrenia . |
|---|---|---|---|---|---|
| DGCR8 | DiGeorge Critical Region 8 | Encodes a component of the microprocessor complex, which plays an important role in miRNA processing | 1. Decrease the number of cortical neurons and change the structure of dendritic spines in prefrontal cortex | 1. Have effects on the development of circuitry in the prefrontal cortex and might influence the functional connectivity in the nervous system | The dysregulation of mRNAs leads to clinical phenotype included schizophrenia. Defective adult neurogenesis is also sufficient for schizophrenia. |
| 2. Decrease the proliferation of AHPs in the hippocampal dentate gyrus | 2. Eventually affect the neurogenesis | ||||
| 3. Alter processing of miRNA, which may target multiple mRNAs | 3. Cause dysregulation of target mRNAs and affect the plasticity of the prefrontal cortex | ||||
| DGCR2 | DiGeorge Critical Region 2 | Encodes a novel putative adhesion receptor protein, which has effects on the neuronal migration and the migration of PNs | 1. Interfere the radial-guided locomotion and terminal translocation of PNs | 1. Affect the migration of PNs in the development of cortical circuit | Altered cortical circuit is associated with schizophrenia. Changed synaptic plasticity increases the risk for schizophrenia. |
| 2. Change dopamine pharmacological metabolism | 2. Influence the transduction of signals between neurons | ||||
| COMT | Catechol-O-methyltransferase | Encoded enzyme COMT plays an important role in degrading dopamine, the prefrontal cortex in particular | 1. Increase dopamine level and influence the transmission of dopamine | 1. Abnormal dopamine metabolism changes the synaptic plasticity | Changes in synaptic plasticity increase the risk for schizophrenia. Impairment of the prefrontal cortex and influences of transmission of dopamine are also associated with schizophrenia. |
| 2. Decrease volume of the prefrontal cortex | 2. Damage the prefrontal cortex and cause the neurotransmission of dopamine that is affected | ||||
| PRODH | Proline dehydrogenase | Encodes proline dehydrogenase, which catalyzes the metabolism of proline to glutamate and provides energy, maintains homeostasis, and produces ROS by proline oxidation | 1. Interferes proline catabolism and increases the level of proline | 1. Increased proline level could develop for hyperprolinemia and leads to neurocognitive dysfunction | Gene–gene interaction between PRODH and COMT increases the risk for schizophrenia. Hyperprolinemia has been recorded in patients with schizophrenia. |
| 2. Change the release and clearance of dopamine | 2. Change synaptic plasticity | ||||
| ZDHHC8 | Zinc finger and DHHC domain-containing protein 8 | Encodes a transmembrane palmitoyltransferase that plays an important role in the palmitoylation of protein, especially neural proteins | 1. Show sexually dimorphic deficits in behaviors, especially in female | 1. The degree of behavioral deficits is variant due to the number of functional ZDHHC8 gene | Impaired synaptic strength and disabled functional connectivity are the signs associated with schizophrenia. ZDHHC8-enriched brain areas like cortex and hippocampus are more possible for pathogenesis of schizophrenia. |
| 2. Interferes glutamatergic neurotransmission through altered palmitoylation | 2. Reduced palmitoylation affects the function of neurotransmitter receptor, neural development and synaptic transmission | ||||
| 3. Decrease dendritic arborization and spine densities | 3. Impair synaptic strength and affects functional connectivity | ||||
| Risk gene . | Full name . | Normal function . | Defective expression and its change . | Follow-up effects . | Risk for the development of schizophrenia . |
|---|---|---|---|---|---|
| DGCR8 | DiGeorge Critical Region 8 | Encodes a component of the microprocessor complex, which plays an important role in miRNA processing | 1. Decrease the number of cortical neurons and change the structure of dendritic spines in prefrontal cortex | 1. Have effects on the development of circuitry in the prefrontal cortex and might influence the functional connectivity in the nervous system | The dysregulation of mRNAs leads to clinical phenotype included schizophrenia. Defective adult neurogenesis is also sufficient for schizophrenia. |
| 2. Decrease the proliferation of AHPs in the hippocampal dentate gyrus | 2. Eventually affect the neurogenesis | ||||
| 3. Alter processing of miRNA, which may target multiple mRNAs | 3. Cause dysregulation of target mRNAs and affect the plasticity of the prefrontal cortex | ||||
| DGCR2 | DiGeorge Critical Region 2 | Encodes a novel putative adhesion receptor protein, which has effects on the neuronal migration and the migration of PNs | 1. Interfere the radial-guided locomotion and terminal translocation of PNs | 1. Affect the migration of PNs in the development of cortical circuit | Altered cortical circuit is associated with schizophrenia. Changed synaptic plasticity increases the risk for schizophrenia. |
| 2. Change dopamine pharmacological metabolism | 2. Influence the transduction of signals between neurons | ||||
| COMT | Catechol-O-methyltransferase | Encoded enzyme COMT plays an important role in degrading dopamine, the prefrontal cortex in particular | 1. Increase dopamine level and influence the transmission of dopamine | 1. Abnormal dopamine metabolism changes the synaptic plasticity | Changes in synaptic plasticity increase the risk for schizophrenia. Impairment of the prefrontal cortex and influences of transmission of dopamine are also associated with schizophrenia. |
| 2. Decrease volume of the prefrontal cortex | 2. Damage the prefrontal cortex and cause the neurotransmission of dopamine that is affected | ||||
| PRODH | Proline dehydrogenase | Encodes proline dehydrogenase, which catalyzes the metabolism of proline to glutamate and provides energy, maintains homeostasis, and produces ROS by proline oxidation | 1. Interferes proline catabolism and increases the level of proline | 1. Increased proline level could develop for hyperprolinemia and leads to neurocognitive dysfunction | Gene–gene interaction between PRODH and COMT increases the risk for schizophrenia. Hyperprolinemia has been recorded in patients with schizophrenia. |
| 2. Change the release and clearance of dopamine | 2. Change synaptic plasticity | ||||
| ZDHHC8 | Zinc finger and DHHC domain-containing protein 8 | Encodes a transmembrane palmitoyltransferase that plays an important role in the palmitoylation of protein, especially neural proteins | 1. Show sexually dimorphic deficits in behaviors, especially in female | 1. The degree of behavioral deficits is variant due to the number of functional ZDHHC8 gene | Impaired synaptic strength and disabled functional connectivity are the signs associated with schizophrenia. ZDHHC8-enriched brain areas like cortex and hippocampus are more possible for pathogenesis of schizophrenia. |
| 2. Interferes glutamatergic neurotransmission through altered palmitoylation | 2. Reduced palmitoylation affects the function of neurotransmitter receptor, neural development and synaptic transmission | ||||
| 3. Decrease dendritic arborization and spine densities | 3. Impair synaptic strength and affects functional connectivity | ||||
The duplication of 22q11.2 region is a possible protective mechanism for schizophrenia. It is due to the reciprocal homologous recombination of 22q11.2 region but fewer such events have been identified. 22q11.2 duplication has been reported to be associated with behavioral and psychiatric abnormalities, but compared with schizophrenia, the phenotype is often milder [122]. In addition, the genetic component of 22q11.2 duplication is not obvious, and 70% of cases are inherited from unaffected parents. According to the studies of Rees et al. [122], the duplications of one or more genes in 22q11.2 region could reduce the risk of developing schizophrenia. Therefore, identification and duplication of risk genes of schizophrenia are necessary and might be used to promote targeted therapy.
22q11DS patients have a reduced cortical surface area, and the decrease of cortical surface area may suggest that 22q11DS originates in the early stage of brain development. Therefore, it is very necessary to investigate the relationship between children brain structure and 22q11DS patients. The application of 22q11-deletion mouse models is also important for the research of human psychiatric anomalies.
In summary, abnormal changes in the metabolism of transmitters, the function of synapses and nerval development will have an impact on the progression of schizophrenia. Interactions between genes like COMT and PRODH, and the influence of environmental factors have also been reported to be associated with schizophrenia. The above results also revealed that schizophrenia is a complex neurodevelopmental disorder caused by multiple factors. Further studies of 22q11DS are helpful to solve the problems related to schizophrenia. The investigation of the risk genes in the 22q11.2 region plays an important role in exploring the pathogenesis of schizophrenia and effective targeted treatments.
Funding
This work was supported by the grant from the National Natural Science Foundation of China (No. 31860268).
References
Author notes
Xianzheng Qin and Jiang Chen contributed equally to this work.



