-
PDF
- Split View
-
Views
-
Cite
Cite
Søren Vinther Larsen, Anja Poulsen, Low-Cost Bubble-CPAP Treatment of a Severe Case of Pneumonia in a Low- and Middle-Income Country: A Case Report on the Importance of Optimizing Bubble-CPAP Setup, Journal of Tropical Pediatrics, Volume 67, Issue 3, June 2021, fmaa049, https://doi.org/10.1093/tropej/fmaa049
Close - Share Icon Share
Abstract
A homemade low-cost bubble continuous positive airway pressure (bCPAP) setup can be created using resources available at most secondary healthcare facilities in low- and middle-income countries (LMICs). This setup has the potential of saving millions of children’s lives worldwide; however, treatment failure due to bCPAP setup insufficiencies and lack of educated staff remains a significant obstacle. Here, we report a first-hand experience on the use of an established low-cost bCPAP setup to be used in LMICs and how optimization of two parameters was critical to effectively treat a severe case of lower respiratory disease in a 6-month-old infant in Tanzania. We report this case to strengthen that reducing the resistance in the tube system and minimizing air leakage at the nasal interface are crucial for efficient delivery of the CPAP therapy.
Lay Summary
This case report describes how it is possible to successfully treat a 6-month-old infant with severe pneumonia in a low resource setting at a district hospital in Tanzania with a homemade low-cost oxygen therapy system called bubble continuous positive airway pressure (bCPAP). The construction was made of an oxygen concentrator, found in most secondary healthcare facilities in Africa, a nasal prong and a sterile water bottle. When optimized, this setup has the potential to mimic the therapeutic effect of an expensive and very effective therapy used in developed countries. The focus of this case report is how two adjustments of the bCPAP setup were necessary to achieve effective and safe treatment; however, we experienced that this homemade setup was very time-demanding and the quality of the treatment critically depended on the clinician to monitor and constantly optimize the equipment. So for this to be an effective and reliable treatment in secondary healthcare facilities in Tanzania, it requires sufficient staff education and nasal prongs that are suited for the purpose.
INTRODUCTION
More than 80% of global under-5-mortality (U5M) occurs in low- and middle-income countries (LMICs) [1]. One of the largest culprits is lower respiratory disease [2]. Continuous positive airway pressure (CPAP) has proven an effective treatment of lower respiratory diseases [3]. It works by applying constant positive pressure which prevents small airways from collapsing during exhalation. This improves gas exchange and minimizes work of breathing (WOB) preventing respiratory collapse [3]. It is established that the therapy can be achieved using a bubble CPAP (bCPAP) setup in low-resource settings [3]. However, it is crucial that the system is optimized in order to achieve effective therapy. We report how a low-cost bCPAP setup was optimized to effectively treat a 6-month-old infant with severe lower respiratory disease at a district hospital in Tanzania.
CASE REPORT
A previously healthy 6-month-old girl was admitted to Magunga District Hospital, Korogwe, Tanzania, with cough and difficulty in breathing (DIB). On presentation, she was lethargic, had a respiratory rate (RR) of 108 breaths/min, and chest indrawing. Oxygen saturation was 84%, heart rate (HR) was 190 beats/min and temperature was 39.8°C. Bilateral crepitation and wheezing were heard on auscultation. The infant was well-nourished, non-anemic and point of care tests for HIV and malaria were negative. Clinical diagnosis was pneumonia.
Treatment included intravenous ampicillin and gentamicin and bCPAP therapy. bCPAP was created using an oxygen concentrator, nasal prong, and a sterile water bottle, inspired by Trevor Duke’s CPAP guide (Fig. 1) [3]. The CPAP level was set to 5 cm H2O by submerging the expiratory tube 5 cm beneath the water surface.
![bCPAP setup inspired by Trevor Duke’s CPAP guide [8]. It consists of an oxygen concentrator, nasal prong and a sterile water bottle. The nasal prong has one of the tubes cut and tied at one end, while the other end is submerged 5 cm into sterile water where continuous bubbles will appear.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/tropej/67/3/10.1093_tropej_fmaa049/2/m_fmaa049f1.jpeg?Expires=1748278647&Signature=a09aCBQAgoLQ~U~oJIyRVm1uXUyfWdJKdy673p4mgJZtsloe5V5NoApNsdwcLe1VijkwE1u~X~I~59N02pvHi3TZbmiz5aSBQr3cnqV69CUKRj31hKP9IoGyY97tfTS3fS-AmREAlRMhhElZ7GIK1PW~8zE3GoLEuODWdMCXskcItT-~sd1XRmqwYWr1AjlfeRYSw9BLJXKE9NKLDab20EHbv0C-2PJuYREtx4DMZnt8Mf-xdt7EHHvHfYAHQoZyr94Z9xwHqwWILLxZU5ZdfRxtM-Ew~u1uvw3CGOFTSiYVZZ43LhKC582V1XyQs52QTQYw2dvPUxwptCzBiEKxNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
bCPAP setup inspired by Trevor Duke’s CPAP guide [8]. It consists of an oxygen concentrator, nasal prong and a sterile water bottle. The nasal prong has one of the tubes cut and tied at one end, while the other end is submerged 5 cm into sterile water where continuous bubbles will appear.
To avoid adverse effects, such as mucosal irritation or pneumothorax [3], the air was humidified at room temperature (about 28°C) and airflow was delivered at maximum 8 l/min. The mouth was closed, but no pressure was applied, thus, in case of too high pressure, the mouth may work as a safety pop-off valve [4]. The infant was breastfeeding during which we paused bCPAP to avoid aspiration. No nasogastric tube was inserted due to lack of space at the nasal interface, so we closely monitored for gastric distension. After initiation of the therapy, the infant stabilized slightly, shown by the improvement in vital signs (Fig. 2, day 1 at 4 PM); however, the WOB was still high interpreted by the relatively high RR and HR.
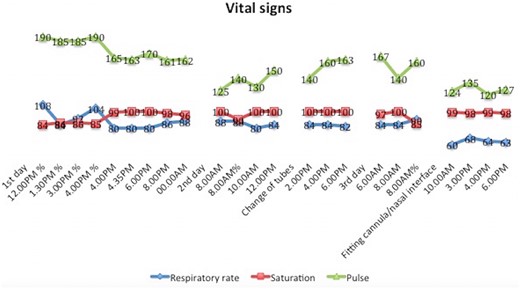
Vital signs (respiratory rate, oxygen saturation, and heart rate) during 3 days of bubble continuous positive airway pressure treatment. Two adjustments were implemented; (i) On the second day, the tube in use was replaced with one with larger diameter, thus reducing air resistance. (ii) On the third day, the nasal interface was optimized with tape to minimize air leakage. The 2 consecutive days of the treatment are not shown, as they resemble the last 4 data points. % = No supplemental oxygen; this was turned off once each day to assess the infant’s response without supplement. Note that no oxygen concentrator was available for the first 4 h following admission.
Two adjustments were implemented to improve the quality of the treatment: (i) The infant nasal cannula was switched to a child size with a larger internal diameter (ID) of the expiratory tube to reduce air resistance. (ii) The nasal interface was sealed with two pieces of tape wrapped around each cannula to minimize air leakage to a degree that was sufficient to transfer positive pressure to the lungs but not completely occlude the interface. Prior to this, a piece of tape was pierced by the cannulas with the adhesive side toward the nose to secure correct intranasal placement (Fig. 3). The second adjustment improved RR and HR, reducing WOB. After 5 days of treatment, the infant was able to be discharged, afebrile and without DIB.
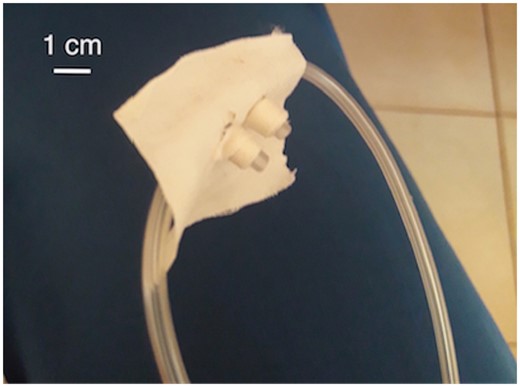
Optimized nasal interface. Three pieces of tape were used to seal the area for reduced air leakage; implemented on day 3.
DISCUSSION
We present how it is possible to treat a 6-month-old infant with severe lower respiratory disease with low-cost bCPAP in an LMIC, with emphasis on the importance of optimizing the bCPAP setup to achieve effective and safe CPAP therapy.
Two adjustments were implemented during the treatment. The first, increasing the ID of the cannula reduced the air resistance. This had no noticeable clinical effect qua the stationary vital signs. However, by minimizing the contribution of the tube system to the positive airway pressure, the CPAP setting was closer to the warranted pressure of 5 cm H2O to avoid lung damage and increase in WOB. The reduction in air resistance was confirmed by the appearance of continuous bubbles at the expiratory limb before connecting to the infant. An in vitro study shows that ID < 4 mm may introduce unpredictable increases in the lung pressure [5, 6]. An ID of the expiratory tube of 10 mm is recommended, but these are seldom available in LMIC secondary healthcare facilities. Another way of reducing the air resistance is to shorten the expiratory tube. This is, however, limited by the setup demanding a range of ∼20–30 cm.
The second adjustment, sealing the nasal interface with tape, drastically decreased RR and HR, thus, the WOB. This improvement was sufficient to prevent respiratory collapse. Before this adjustment, the air leakage reduced system pressure so the treatment was comparable only to standard oxygen therapy. More than 50% sealing at the interface is demanded for CPAP effect [7]; however, if completely occluded, we may fail to support the inspiratory flow demand which may lead to treatment failure [7]. Since low-cost bCPAP setup does not provide insight in patient flow requirement, vital signs and clinical presentation are central for monitoring treatment failure; hence, close monitoring and qualified staff is required. In this case, the improvement in RR and HR were proof of treatment success.
Literature, though still emphasizing the need for more robust studies on implementation in LMICs [7], generally favors bCPAP compared with standard oxygen therapy [8]. Therefore, it should be a serious asset in decision making to improve the high U5M in LMICs, where it has the potential to be as effective and cheap per life saved as the pneumococcal vaccine [9]. The big challenge lies in implementation in LMICs given limited resources, high maintenance and lack of training [10, 11]. Treating this infant was time-demanding as equipment required frequent adjustments to obtain sufficient quality. This is a particular challenge in settings where nurse-to-patient ratios are low.
CONCLUSION
This case report shows how it is possible to create a bCPAP system with the resources available in a LMIC secondary healthcare facility and the importance of optimizing and monitoring the treatment to achieve safe and efficient bCPAP therapy. This case report calls for nasal prongs with ID ≥ 10 mm and adaptable nasal cannulas able to achieve sufficient nasal occlusion and simultaneously be compatible with the oxygen concentrator’s ¼-inch barbed fitting [12], as these are abundant in most LMIC’s secondary healthcare facilities [13].
ACKNOWLEDGEMENT
The line drawing was created by Andrea Hilden Honoré.
FUNDING
This work was supported by Faculty of Health, Copenhagen University Fond for medical students [A5614].
REFERENCES
UNICEF, WHO, World Bank Group and United Nations. Levels & Trends in Child Mortality Report 2017. UNICEF,



Comments