-
PDF
- Split View
-
Views
-
Cite
Cite
Chengfeng Yang, Response to Concerns about Arsenic-Induced Epithelial to Mesenchymal Transition and Malignant Transformation of Human Bronchial Epithelial Cells, Toxicological Sciences, Volume 122, Issue 2, August 2011, Pages 607–609, https://doi.org/10.1093/toxsci/kfr151
Close - Share Icon Share
We thank Dr Samuel M. Cohen for his interest in reading our recent article in which we investigated the role of microRNA-200b in arsenic-induced epithelial to mesenchymal transition (EMT) and malignant transformation of human bronchial epithelial cells. We understand Dr Cohen’s concern that the pathology of subcutaneous xenograft tumors that grew in the nude mice upon inoculation of arsenic-transformed human bronchial epithelial cells did not represent the major kinds of human lung tumors. However, as described in our article and further explained below, the purpose of using the mouse subcutaneous xenograft tumor model in our study was not intended for characterizing the pathology of arsenic exposure–caused human lung tumors but rather for providing an evidence demonstrating cell malignant transformation by arsenic. Mouse subcutaneous xenograft (ectopic) tumor models have been widely used in toxicology and cancer research, although they have significant limitations mainly because of lack of organospecific tumor stromal interactions (Killion et al., 1999; Turk et al., 2011). It is now believed that the organospecific tumor stromal interactions play a crucial role in tumor cell growth, differentiation, and tumor pathology. Therefore, the histopathology of human tumor cells mouse subcutaneous xenograft tumors does not well represent the kinds of tumors that occur in their original sites in humans. To overcome these limitations and produce mouse xenograft tumors that have more features comparable with human situations, it has been suggested to transplant tumor cells into their original location, designated as the orthotopic site (Killion et al., 1999; Turk et al., 2011). Unfortunately, many orthotopic xenograft tumor models have not been well developed because of the technical difficulties.
The tumorigenicity of arsenic-treated cells when subcutaneously injected into immunocompromised mice along with their anchorage-independent growth in soft agar have been frequently used to determine whether arsenic exposure causes cell malignant transformation (Bredfeldt et al., 2006; Sens et al., 2004; Zhao et al., 1997). However, previous studies showed that the pathology of subcutaneous xenograft mouse tumors produced by injection of arsenic-transformed cells did not represent the main kinds of tumors occurring in their original sites in humans or animals exposed to arsenic. For example, Zhao et al. (1997) reported that mouse xenograft tumors resulting from subcutaneous inoculation of rat liver epithelial TRL 1215 cells malignantly transformed by arsenic showed fibrosarcomatous and undifferentiated areas with multiple mitotic features. In contrast, the liver tumors in mice exposed to arsenic were liver adenomas and carcinomas (Waalkes et al., 2003). Although Sens et al. (2004) and Bredfeldt et al. (2006) found that subcutaneous inoculation of arsenic-transformed human bladder urothelial cells into immunocompromised mice grew tumors with a prominent squamoid differentiation, these authors pointed out that the overwhelming majority of bladder cancers in general patient populations were transitional cell carcinomas with little or no evidence of squamous differentiation. Clearly, mouse ectopic xenograft tumor models have their limitations in representing the pathology of tumors that actually occur in humans or animals resulting from arsenic exposure. Nevertheless, these limitations did not hamper using the tumorigenicity of arsenic-treated cells when subcutaneously inoculated into immunocompromised mice as an indicator of malignant transformation by arsenic (Wnek et al., 2010). In our study, arsenic exposure–caused cell malignant transformation was initially assessed using soft agar colony formation assay, followed by examining tumorigenicity of cells when subcutaneously injected into nude mice. Due to aforementioned limitations of mouse ectopic xenograft tumor models, we do not think it is appropriate to compare the pathology of mouse ectopic xenograft tumors with that of human lung tumors. However, per Dr Cohen’s suggestion, additional immunofluorescence staining for our mouse xenograft tumor tissue section is now provided. Figure 1 shows extensive positive staining of thyroid transcription factor-1 (TTF-1), which is present in the epithelium of the lung and used as a marker of human primary lung tumors (Reis-Filho et al., 2000), in xenograft tumor tissues produced by subcutaneous injection of arsenic-transformed human bronchial epithelial cells into the nude mice. Moreover, immunofluorescence stainings of these xenograft tumor tissue sections with inflammatory cell marker CD3 (T-cell marker) and CD20 (B-cell marker) were negative (data not shown). These findings along with previous results showing extensive positive staining of Turbo green fluorescent protein that we introduced into arsenic-transformed cells indicate that the xenograft tissues produced by injection of arsenic-transformed human bronchial epithelial cells were lung epithelial–derived tumors but not inflammatory tissues. We are now exploring the possibility of establishing an orthotopic xenograft mouse tumor model using our arsenic-transformed human bronchial epithelial cells.
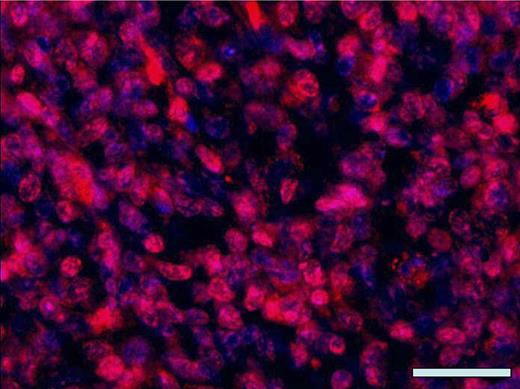
Representative immunofluorescence staining with anti-TTF-1. Tissue sections from nude mouse xenograft tumors produced by subcutaneous injection of arsenic-transformed human bronchial epithelial cells (As-p53lowHBEC-GFP) were stained with anti-TTF-1 (sc-13040; Santa Cruz Biotechnology Inc.) as previously described (Zhao et al., 2010). Nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) (blue). The overlaid fluorescence image was made from anti-TTF-1 staining (red color) and nucleus DAPI staining (blue color). Scale bar: 100 μm.
EMT is now widely viewed as a key event in tumor progression and is usually associated with tumor invasion and metastasis. However, as pointed out in our article, it has not been studied and it is not known whether EMT also plays a role in carcinogen-induced malignant transformation, the initial step of tumorigenesis. Based on our findings, we proposed that “in addition to promoting tumor invasion and metastasis, ZEB1-mediated EMT may also be critically involved in the initiation of tumorigenesis by promoting cell malignant transformation.” Shortly after our article was published, another article examining the role of EMT in tobacco carcinogens–caused premalignant transformation of immortalized human bronchial epithelial cells was published (Tellez et al., 2011). The authors concluded as follows: “Our findings extend present concepts of how EMT participates in cancer pathophysiology by showing that EMT induction can participate in cancer initiation to promote the clonal expansion of premalignant lung epithelial cells” (Tellez et al., 2011). It is anticipated that more evidence supporting a role for EMT in tumor initiation will be emerging.
It has been shown that arsenic acts as a cocarcinogen (Rossman et al., 2001), which may partially explain the reason why cell transformation by arsenic only occurred in p53-deficient cells. Although “individuals who develop tumors in response to arsenic exposure are usually not p53 deficient nor are many of the tumors that are induced,” this does not exclude the possibility that p53 function was inactivated, but its expression level was not changed in human tumors resulting from arsenic exposure. Recent studies have shown that carcinogens such as arsenic can inactivate p53 function but yet not change p53 protein levels (Komissarova and Rossman, 2010; Li et al., 2010).
Funding
National Institutes of Health (1R01ES017777).




Comments