-
PDF
- Split View
-
Views
-
Cite
Cite
Jing Peng, Lin Yang, Long Liu, Renyuan Zhou, Jihong Liu, Ningchen Li, Liming Li, Yongguang Jiang, Yuqiang Liu, Zhaohui Zhu, Xiaodong Zhang, Guowei Shi, Suyog Jain, Emmanuele A. Jannini, Zhichao Zhang, Safety and Effectiveness of Dapoxetine On Demand in Chinese Men With Premature Ejaculation: Results of a Multicenter, Prospective, Open-Label Phase IV Study, Sexual Medicine, Volume 9, Issue 2, April 2021, Page 100296, https://doi.org/10.1016/j.esxm.2020.100296
Close - Share Icon Share
Abstract
Dapoxetine on demand has been approved for premature ejaculation (PE) management in China; however, studies on the efficacy and safety of this treatment in the Chinese population are scarce.
The aim of this study was to evaluate the safety and effectiveness of dapoxetine 30 mg and 60 mg on demand in Chinese men with PE.
Phase IV real-world study on 1,252 patients with PE. If men reported no response to dapoxetine 30 mg after 4 weeks treatment, dapoxetine has been uptitrated at 60 mg for 4 weeks more.
Self-reported data were collected for demographics, general and sexual health characteristics, PE severity, and treatment safety and effectiveness, as measured by the PE profile questionnaire.
Adverse events (AEs), such as nausea, thirst, headache, and dizziness, similarly to previous literature, were detected. The treatment-emergent AEs rate was higher in the patients treated with 30 and 60 mg (n = 192) compared with those treated with the dapoxetine 30 mg only (n = 1060) (34.4% vs 15.8%, respectively). No new safety concerns were observed. The overall effectiveness rates were 88.2% in subjects using 30 mg of dapoxetine, whereas a rescue from the previous failure was in 55.7% in the patients who received 60 mg after the initial 30 mg. Overall, 83.2% responded to dapoxetine at dosages equal to or lower than 60 mg.
The results in this study demonstrated in a large Chinese population that on-demand dapoxetine is a safe and effective symptomatic treatment in patients with PE.
J Peng, L Yang, L Liu, et al. Safety and Effectiveness of Dapoxetine On Demand in Chinese Men With Premature Ejaculation: Results of a Multicenter, Prospective, Open-Label Phase IV Study. Sex Med 2021;9:100296.
Introduction
Premature ejaculation (PE) is one of the most common male sexual complaints, with a reported prevalence of 21–33% in some populations,1,2 such as China.3,4 PE has been defined by the International Society of Sexual Medicine in a tridimensional perspective encompassing the time from penetration (measured as intravaginal ejaculatory latency time), feeling of control over ejaculatory mechanism and distress provoked by the condition (both measured as patient-reported outcomes, PROs).5,6 PE can interfere with sexual satisfaction, leading to poor self-image, thus adversely impacting the overall quality of life among affected men7–10 and their partners.11
Selective serotonin reuptake inhibitors (SSRIs), which were developed to treat depression and other psychiatric disorders, are being used increasingly as an off-label treatment option for PE because of the finding that delayed ejaculation is a commonly reported side effect.12–16 The mechanism of action of SSRIs is thought to be related to inhibition of neuronal reuptake of serotonin and subsequent potentiation of serotonin activity in the synaptic cleft. The pharmacokinetics of these compounds is designed for a long-acting action. For this reason, they are not ideal for on-demand use. Moreover, the use of SSRIs in PE is to be considered off-label.6
Dapoxetine is a compound belonging from the SSRI family, but, for the very short half-life, without lesser effects on the mood. Because of the peculiar pharmacokinetics, dapoxetine has not been classified within the antidepressants but instead approved in many countries as the first oral drug for on-demand treatment of PE.17 The clinical benefit of dapoxetine has been demonstrated in a pooled analysis of 5 randomized, placebo-controlled, phase 3 clinical trials (N = 6,081). Dapoxetine delays ejaculation approximately 2.5- to 3.0-fold in the overall population (vs 1.6-fold with placebo; P < .0001).18 Dapoxetine has received approval for the treatment of PE in over 50 countries worldwide,5 including China since 2013.19 Most frequent treatment-emergent adverse events (TEAEs) reported in the clinical trials were nausea, dizziness, diarrhea, insomnia, and headache.19 Despite the well-known peculiarities of the Chinese population, the safety and effectiveness of dapoxetine have not been studied systematically yet. Therefore, we designed a phase IV study on the safety and effectiveness of dapoxetine in routine clinical practice in Chinese men with PE.
Methods
Study Design
This study was a phase IV, prospective, multicenter, open-label study to determine the safety and effectiveness of dapoxetine administered 1-3 hours before intercourse to Chinese men with PE. The study was registered in the Chinese Clinical Trial Registry (Registration number: CTR20140887). Patients were enrolled in the study between December 2014 and May 2017, provided that they met the following inclusion criteria. Briefly, the patients included Chinese Han men aged ≥18 years and <64 years, with an abridged International Index of Erectile Function total score ≥21 and fulfilling ISSM definition.20 Exclusion criteria included the following: (i) men who had not been diagnosed with PE by a medical care provider; (ii) men below 18 years old or above 64 years old; (iii) men with other conditions, clinically assessed by the investigator, such as an allergy to dapoxetine or any other components of dapoxetine preparation; hypogonadism21 or any other overt endocrine disease,22 such as hyperthyroidism23,24; prostate infection/inflammation,25 heart problems, for example, heart failure or arrhythmia; severe liver or kidney problems; depression or other abnormal mental conditions; significant orthostatic hypotension; (iv) assumption of certain drugs such as monoamine oxidase inhibitors or other antidepressants; thioridazine (for treating schizophrenia); certain drugs used to treat fungal infection; certain drugs used to treat human immunodeficiency virus; certain antibiotics used to treat infection; lithium (for treating bipolar disorder); tryptophan (for inducing sleep); St John's wort (a type of herbal medicine); tramadol (for treating severe pain); drugs for treating migraine; (v) history of or current major psychiatric disorder or severe renal impairment; (vi) history of alcohol or substance abuse; (vii) other sexual dysfunction disorders; (viii) patients who were using other herbal, pharmacological or psychosexological treatments for PE.26 All patients provided written informed consent, and the study was conducted following the Declaration of Helsinki. An ethical review board at each of the 10 sites approved the study protocol.
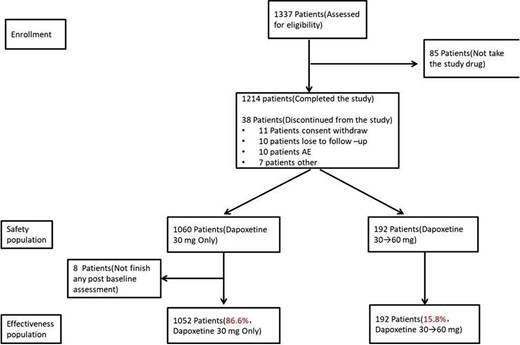
The study was conducted in 2 phases: all enrolled patients received dapoxetine 30 mg, taken as needed approximately 1 to 3 hours before sexual activity, being instructed to use the drug at least 6 times per month. After 4 weeks, if the response, defined as later described, was not satisfactory and the adverse effects were tolerated, the dose has been titrated up to 60 mg for another 4 weeks treatment. Thus, in the end, there were 2 treatment groups (30 mg only and 30 to 60 mg). A telephone follow-up was conducted 2 weeks after the end of the observational treatment period (week 6 or 10) to record any AEs. Figure 1 displays the study design.

As this study was a part of the risk management plan for dapoxetine required by the Chinese medicine agency (NMPA), a sample size of 3,000 patients was given by the same NMPA. This study of 3,000 patients was split into 2 different center clusters with 1,500 patients each. This publication shares the results of the first cluster of centers.
The primary end point of the study was safety at 4 weeks or 8 weeks. Safety was evaluated throughout the study by examining the incidence, severity, and type of adverse events (AEs), physical examination results, and vital sign measurements. If a patient experienced an AE during the study, the event was managed at the discretion of the investigator. The patient was followed up until the outcome of the AE was determined.
The key purpose of the safety observation was to collect adverse drug reactions.
The secondary end points were measured by the self-filled Premature Ejaculation Profile (PEP) Questionnaire. The results of the PEP Questionnaire are classified as PROs, assessing the subjective components of PE. A baseline PEP was completed by each patient at entry into the study. Effectiveness was defined as at least 1 point improvement in at least 2 measures, control AND satisfaction, at the end of the study compared with the baseline.27,28 The 4 items in PEP were additional secondary end points.
Safety Analyses
The safety population included all enrolled patients who received at least one dose of dapoxetine, and patients without follow-up data were excluded. Safety was analyzed via the proportion of patients at 4 or 8 weeks who experienced at least one TEAE (serious or non-serious). A TEAE was defined as an event that first occurred or worsened after baseline. Treatment-related AEs were defined as events the investigator indicated to be related to treatment.
Statistical Analyses
SAS Version 9.3 (SAS Institute, Inc) was used for statistical analyses. Continuous variables were summarized using the descriptive statistics: number of patients (n), mean, standard deviation, median, minimum (min), and maximum (max). Percentages were based on the number of patients in the respective analysis set and, unless specified otherwise, included patients with non-missing values only. Categorical variables were summarized by number and percentage of patients in each category, n (%).
Patient data listings were based on all enrolled patients, unless specified otherwise, and were sorted by dose levels, site, and patient. All summary analyses were performed by dose levels and total enrolled patients. Nominal visits were used for the analysis. Change from the baseline was computed as the value at each postbaseline visit - baseline result. Unless otherwise stated, the baseline was defined as the value obtained at the consultation visit. All statistical tests were performed as 2-sided at the 5% significance level. Point estimates were accompanied with 2-sided 95% confidence intervals (CI) where applicable. No adjustment for multiplicity was made.
Results
Of the 1,500 target, a total of 1,337 patients were enrolled into the study, of which, 1,214 (90.8%) patients completed the study, 85 (6.4%) patients did not assume dapoxetine for various reasons, and 38 (2.8%) patients discontinued from the study. The main reasons for discontinuation from the study included withdrew consent (n = 11), loss to follow-up (n = 10), AE (n = 10), and other (n = 7). During the study, most patients (1,060 patients) took dapoxetine 30 mg only and 192 patients had their dapoxetine doses up-titrated from 30 mg to 60 mg (Figure 2 ). Baseline characteristics of patients are displayed in Table 1 .
| Characteristics . | Dapoxetine 30 mg only (N = 1,052) n (%) . | Dapoxetine 30→60 mg (N = 192) n (%) . | Total (N = 1,244) n (%) . |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 32.43 (7.08) | 33.58 (7.24) | 32.61 (7.12) |
| Weight (kg) | |||
| Mean (SD) | 71.37 (10.41) | 73.77 (11.44) | 71.74 (10.60) |
| Height (cm) | |||
| Mean (SD) | 172.57 (5.13) | 173.34 (4.56) | 172.69 (5.06) |
| BMI (kg/m2) | |||
| Mean (SD) | 23.95 (3.21) | 24.51 (3.38) | 24.04 (3.25) |
| IIEF-5 score | |||
| Mean (SD) | 23.18 (1.42) | 22.82 (1.40) | 23.12 (1.42) |
| Characteristics . | Dapoxetine 30 mg only (N = 1,052) n (%) . | Dapoxetine 30→60 mg (N = 192) n (%) . | Total (N = 1,244) n (%) . |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 32.43 (7.08) | 33.58 (7.24) | 32.61 (7.12) |
| Weight (kg) | |||
| Mean (SD) | 71.37 (10.41) | 73.77 (11.44) | 71.74 (10.60) |
| Height (cm) | |||
| Mean (SD) | 172.57 (5.13) | 173.34 (4.56) | 172.69 (5.06) |
| BMI (kg/m2) | |||
| Mean (SD) | 23.95 (3.21) | 24.51 (3.38) | 24.04 (3.25) |
| IIEF-5 score | |||
| Mean (SD) | 23.18 (1.42) | 22.82 (1.40) | 23.12 (1.42) |
IIEF = International Index of Erectile Function; BMI = body mass index, N/n = number of patients, SD = standard deviation.
Age was derived as (date of informed consent–date of birth)/365.25.
The distribution of demographic and baseline characteristics among 2 treatment groups was analyzed using chi-square or Fisher's exact test for categorical variables and t-test for continuous variables.
Baseline was defined as the value obtained at the consultation visit.
| Characteristics . | Dapoxetine 30 mg only (N = 1,052) n (%) . | Dapoxetine 30→60 mg (N = 192) n (%) . | Total (N = 1,244) n (%) . |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 32.43 (7.08) | 33.58 (7.24) | 32.61 (7.12) |
| Weight (kg) | |||
| Mean (SD) | 71.37 (10.41) | 73.77 (11.44) | 71.74 (10.60) |
| Height (cm) | |||
| Mean (SD) | 172.57 (5.13) | 173.34 (4.56) | 172.69 (5.06) |
| BMI (kg/m2) | |||
| Mean (SD) | 23.95 (3.21) | 24.51 (3.38) | 24.04 (3.25) |
| IIEF-5 score | |||
| Mean (SD) | 23.18 (1.42) | 22.82 (1.40) | 23.12 (1.42) |
| Characteristics . | Dapoxetine 30 mg only (N = 1,052) n (%) . | Dapoxetine 30→60 mg (N = 192) n (%) . | Total (N = 1,244) n (%) . |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 32.43 (7.08) | 33.58 (7.24) | 32.61 (7.12) |
| Weight (kg) | |||
| Mean (SD) | 71.37 (10.41) | 73.77 (11.44) | 71.74 (10.60) |
| Height (cm) | |||
| Mean (SD) | 172.57 (5.13) | 173.34 (4.56) | 172.69 (5.06) |
| BMI (kg/m2) | |||
| Mean (SD) | 23.95 (3.21) | 24.51 (3.38) | 24.04 (3.25) |
| IIEF-5 score | |||
| Mean (SD) | 23.18 (1.42) | 22.82 (1.40) | 23.12 (1.42) |
IIEF = International Index of Erectile Function; BMI = body mass index, N/n = number of patients, SD = standard deviation.
Age was derived as (date of informed consent–date of birth)/365.25.
The distribution of demographic and baseline characteristics among 2 treatment groups was analyzed using chi-square or Fisher's exact test for categorical variables and t-test for continuous variables.
Baseline was defined as the value obtained at the consultation visit.
Safety
At the end of the treatment period, no deaths were reported. Serious AEs included salivary gland cyst and cholecystitis, which were not considered related to study drug. Overall, 262 (20.9%) patients reported AEs and 233 (18.6%) patients reported TEAEs during the study which was mostly mild (Table 2 ). The most frequent TEAEs included dizziness (8.8%, 19.8%), nausea (5.8%, 12.5%) and headache (1.8%, 2.6%) respective in 30 mg and 30 mg to 60 mg group. The proportion of patients reporting both AEs and TEAEs were greater in the dapoxetine 30 to 60 mg group compared with the dapoxetine 30 mg only group (39.6% vs 17.5% and 34.4% vs 15.8%). No syncope was reported during this study. All TEAEs were considered related to the study drug. Mean treatment compliance was 85.28% in total, 85.08% in dapoxetine 30 mg only group, and 86.39% in dapoxetine 30 to 60 mg group.
| Type of AE . | Dapoxetine 30 mg only (N = 1,060) n (%) . | Dapoxetine 30→60 mg (N = 192) n (%) . | Total (N = 1,252) n (%) . |
|---|---|---|---|
| Number of patients with: | |||
| AEs | 186 (17.5) | 76 (39.6) | 262 (20.9) |
| SAEs∗ | 1 (0.1) | 3 (1.6) | 4 (0.3) |
| Drug-related AEs | 172 (16.2) | 71 (37.0) | 243 (19.4) |
| Drug-related SAEs | 0 | 0 | 0 |
| Number of patients with: | |||
| TEAEs | 167 (15.8) | 66 (34.4) | 233 (18.6) |
| Serious TEAEs | 0 | 0 | 0 |
| Drug-related TEAEs | 167 (15.8) | 66 (34.4) | 233 (18.6) |
| Drug-related serious TEAEs | 0 | 0 | 0 |
| Dizziness | 93 (8.8) | 38 (19.8) | 131 (10.5) |
| Nausea | 62 (5.8) | 24 (12.5) | 86 (6.9) |
| Headache | 19 (1.8) | 5 (2.6) | 24 (1.9) |
| Somnolence | 9 (0.8) | 4 (2.1) | 13 (1.0) |
| Thirst | 5 (0.5) | 3 (1.6) | 8 (0.6) |
| Erectile dysfunction | 2 (0.2) | 3 (1.6) | 5 (0.4) |
| Type of AE . | Dapoxetine 30 mg only (N = 1,060) n (%) . | Dapoxetine 30→60 mg (N = 192) n (%) . | Total (N = 1,252) n (%) . |
|---|---|---|---|
| Number of patients with: | |||
| AEs | 186 (17.5) | 76 (39.6) | 262 (20.9) |
| SAEs∗ | 1 (0.1) | 3 (1.6) | 4 (0.3) |
| Drug-related AEs | 172 (16.2) | 71 (37.0) | 243 (19.4) |
| Drug-related SAEs | 0 | 0 | 0 |
| Number of patients with: | |||
| TEAEs | 167 (15.8) | 66 (34.4) | 233 (18.6) |
| Serious TEAEs | 0 | 0 | 0 |
| Drug-related TEAEs | 167 (15.8) | 66 (34.4) | 233 (18.6) |
| Drug-related serious TEAEs | 0 | 0 | 0 |
| Dizziness | 93 (8.8) | 38 (19.8) | 131 (10.5) |
| Nausea | 62 (5.8) | 24 (12.5) | 86 (6.9) |
| Headache | 19 (1.8) | 5 (2.6) | 24 (1.9) |
| Somnolence | 9 (0.8) | 4 (2.1) | 13 (1.0) |
| Thirst | 5 (0.5) | 3 (1.6) | 8 (0.6) |
| Erectile dysfunction | 2 (0.2) | 3 (1.6) | 5 (0.4) |
AE = adverse event, SAE = serious adverse event, TEAE = treatment-emergent AE.
TEAE was defined as an AE that began or worsened in severity after at least 1 dose of study drug had been administered.
4 (0.3%) patients reported SAEs during the study, with one patient reporting cholecystitis, one patient reporting salivary gland cyst.
| Type of AE . | Dapoxetine 30 mg only (N = 1,060) n (%) . | Dapoxetine 30→60 mg (N = 192) n (%) . | Total (N = 1,252) n (%) . |
|---|---|---|---|
| Number of patients with: | |||
| AEs | 186 (17.5) | 76 (39.6) | 262 (20.9) |
| SAEs∗ | 1 (0.1) | 3 (1.6) | 4 (0.3) |
| Drug-related AEs | 172 (16.2) | 71 (37.0) | 243 (19.4) |
| Drug-related SAEs | 0 | 0 | 0 |
| Number of patients with: | |||
| TEAEs | 167 (15.8) | 66 (34.4) | 233 (18.6) |
| Serious TEAEs | 0 | 0 | 0 |
| Drug-related TEAEs | 167 (15.8) | 66 (34.4) | 233 (18.6) |
| Drug-related serious TEAEs | 0 | 0 | 0 |
| Dizziness | 93 (8.8) | 38 (19.8) | 131 (10.5) |
| Nausea | 62 (5.8) | 24 (12.5) | 86 (6.9) |
| Headache | 19 (1.8) | 5 (2.6) | 24 (1.9) |
| Somnolence | 9 (0.8) | 4 (2.1) | 13 (1.0) |
| Thirst | 5 (0.5) | 3 (1.6) | 8 (0.6) |
| Erectile dysfunction | 2 (0.2) | 3 (1.6) | 5 (0.4) |
| Type of AE . | Dapoxetine 30 mg only (N = 1,060) n (%) . | Dapoxetine 30→60 mg (N = 192) n (%) . | Total (N = 1,252) n (%) . |
|---|---|---|---|
| Number of patients with: | |||
| AEs | 186 (17.5) | 76 (39.6) | 262 (20.9) |
| SAEs∗ | 1 (0.1) | 3 (1.6) | 4 (0.3) |
| Drug-related AEs | 172 (16.2) | 71 (37.0) | 243 (19.4) |
| Drug-related SAEs | 0 | 0 | 0 |
| Number of patients with: | |||
| TEAEs | 167 (15.8) | 66 (34.4) | 233 (18.6) |
| Serious TEAEs | 0 | 0 | 0 |
| Drug-related TEAEs | 167 (15.8) | 66 (34.4) | 233 (18.6) |
| Drug-related serious TEAEs | 0 | 0 | 0 |
| Dizziness | 93 (8.8) | 38 (19.8) | 131 (10.5) |
| Nausea | 62 (5.8) | 24 (12.5) | 86 (6.9) |
| Headache | 19 (1.8) | 5 (2.6) | 24 (1.9) |
| Somnolence | 9 (0.8) | 4 (2.1) | 13 (1.0) |
| Thirst | 5 (0.5) | 3 (1.6) | 8 (0.6) |
| Erectile dysfunction | 2 (0.2) | 3 (1.6) | 5 (0.4) |
AE = adverse event, SAE = serious adverse event, TEAE = treatment-emergent AE.
TEAE was defined as an AE that began or worsened in severity after at least 1 dose of study drug had been administered.
4 (0.3%) patients reported SAEs during the study, with one patient reporting cholecystitis, one patient reporting salivary gland cyst.
Effectiveness
At week 4, of the 1,052 patients who received dapoxetine 30 mg only and were included in the effectiveness analysis population, treatment with dapoxetine was effective in 928 patients and the effectiveness rate was 88.2% (95% CI: 86.11, 90.10). In the effectiveness analysis population, of the 192 patients whose dapoxetine was up-titrated from 30 mg to 60 mg at week 4, treatment with dapoxetine was effective in 107 patients at week 8 and the effectiveness rate was 55.7% (95% CI: 48.40, 62.88). Overall, 83.2% (1,035) responded to dapoxetine at dosages equal to or less than 60 mg. Dapoxetine improved all aspects of PEP (Figure 3 ).
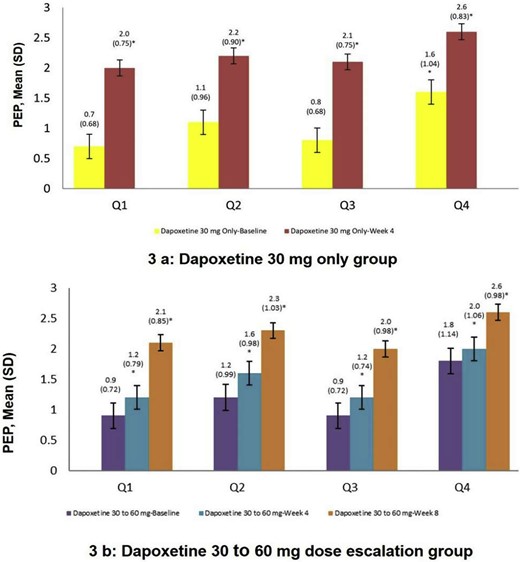
Comparsion of changes of Premature Ejaculation Profile (PEP) Questionnaire between before and after dapoxetine treatment. SD= standard deviation; P-value within each treatment group will be evaluated using a paired t-test. ∗: Week 4 vs baseline, P < .001. ∗∗: Week 8 vs baseline, P < .001. #: Week 8 vs week 4, P < .001. A baseline is defined as the value obtained at the consultation visit. Q1: Over the past 4 weeks, was your control over ejaculation during sexual intercourse? Q2: How distressed are you by how fast you ejaculate (come) during sexual (vaginal) intercourse?. Q3: Over the past 4 weeks, was your satisfaction with sexual intercourse?. Q4: To what extent does how fast you ejaculate (come) during sexual (vaginal) intercourse cause difficulty in your sexual relationship with your partner?
Discussion
The safety and effectiveness of dapoxetine on-demand administration were assessed in a large Chinese population. Treatment with dapoxetine 30 mg or 60 mg in this study did not unveil any new safety concerns in Chinese patients. Total TEAE incidence was 18.6%, only 10 discontinuations due to AEs, and no clinically meaningful laboratory or vital sign changes were found. Moreover, no syncope was reported. Serious AEs were considered not related to the study drug.
The primary objective of this trial regarding safety was met and the safety profile was consistent with the tolerability reported previously.18,19
Consistent with previous studies, dapoxetine on-demand improved ejaculation control and sexual satisfaction. Based on the PEP assessments, effectiveness was notable after 4 weeks with dapoxetine 30 mg treatment. Of 1,214 patients included in analysis 1,052 (87%), patients received dapoxetine 30 mg only, whereas only 192 (13%) patients had an insufficient response so that the dose was uptitrated from 30 mg to 60 mg and at 8 weeks the response was statistically superior to baseline as well as week 4. The effectiveness rate of dapoxetine 60 mg reached 55.7% in patients who did not respond to 30 mg. This is important to note as in real life situation, not all the patients would respond adequately at 30 mg and opportunity exist to increase the dosage to 60 mg. In addition, the overall effectiveness rate of 83.2% within the approved dosage limit of 60 mg or less confirms the clinical trial evidence. This increased dosage can be achieved while safety parameters are being still acceptable. Moreover, our results found that dapoxetine could significantly improve all aspects of PEP. The high compliance rate of around 85% is also an indicator of the tolerable safety profile. These results were consistent with previous studies.18
Inability to delay ejaculation and negative personal consequences are the important factors in PE definition.5 In this study, ejaculation control and sexual satisfaction were evaluated as PROs after dapoxetine treatment. Most Chinese patients (88.2%) reported dapoxetine on demand for 4 weeks significantly improved their ejaculation control and satisfaction.
Daily treatment with off-label SSRIs such as paroxetine, sertraline, fluoxetine, has been found effective in delaying ejaculation.15 However, both short- and long-term use of SSRIs causes treatment-emergent AEs and sexual dysfunction. Treatment-emergent sexual dysfunction related to antidepressants has been found in 25.8–80.3% of treated patients.29 Moreover, long-term use of SSRIs may affect the motility of spermatozoa.30,31 Treatment-related side effects of dapoxetine were uncommon and, as expected, dose-dependent. Although not evaluated in this study, earlier literature found no indication of an increased risk of suicidal ideation or suicide attempts and little indication of withdrawal symptoms with abrupt dapoxetine cessation.32 Dapoxetine is, in fact, a short-acting SSRI, with a pharmacokinetic profile suitable for on-demand treatment for PE.17 Moreover, results from 5 phase 3 trials (N = 6,081) demonstrated dapoxetine significantly improved all aspects of PE and was generally well tolerated.18 Our study confirms these data and demonstrates in Chinese men with PE the safety and effectiveness of dapoxetine on-demand.
This study has some limitations. First, the observation period was relatively short, therefore missing the long-term safety and effectiveness results. Second, as per our experimental design, only when patients did not respond to dapoxetine 30 mg, the dose has been adjusted to 60 mg missing a direct comparison of the safety and effectiveness of dapoxetine 30 mg versus 60 mg. Third, in this study for evaluation of effectiveness, an objective scale, such as stopwatch intravaginal ejaculatory latency time, was not used. Also during enrollment, no information was collected about lifelong PE or acquired PE; therefore, we could not calculate the potential difference in these 2 subgroups. However, the present study is one of the largest real-world studies on dapoxetine in Asian patients and adds to the strength of evidence of dapoxetine.
Conclusion
In Chinese patients with PE, on-demand dapoxetine 30 mg is a safe and effective treatment and dose can be safely increased to 60 mg when required.
Statement of authorship
J.P. and Z.Z. shared the study design. Z.Z., S.J., and E.A.J. played a critical role in the analysis of data and manuscript preparation. All other authors, including L.L., R.Z., D.H., J.L., N.L., L.L., Y.J., Y.L., Z.Z., and X.Z. participated in the acquisition of data and revising the intellectual content. All authors read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Funding This study and manuscript preparation was sponsored by A. Menarini Asia-Pacific Holdings Pte. Ltd which, however, did not influence the results independently collected by each center.
References
Author notes
Conflict of Interest: The authors declared no competing interests. E.A.J. has been a paid speaker and/or consultant for Bayer, Menarini, Otsuka, Pfizer, and Shionogi. S.J. is an employee of Menarini Asia Pacific.



