-
PDF
- Split View
-
Views
-
Cite
Cite
Corien M. Eeltink, Birgit I. Lissenberg-Witte, Luca Incrocci, Annemarie M.J. Braamse, Otto Visser, Josée Zijlstra, Irma M. Verdonck-de Leeuw, Sonja Zweegman, Self-Reported Sexual Function in Sexually Active Male Hodgkin Lymphoma Survivors, Sexual Medicine, Volume 8, Issue 3, September 2020, Pages 428–435, https://doi.org/10.1016/j.esxm.2020.04.005
Close - Share Icon Share
Abstract
Unambiguous data on sexual dysfunction after Hodgkin lymphoma (HL) treatment are scarce.
To form a baseline in this area, we compared patient-reported sexual function in sexually active male HL survivors in complete remission with a sexually active, age-matched, male Dutch sample population. Furthermore, we explored whether sociodemographic and clinical factors were associated with sexual dysfunction in HL survivors and investigated whether reporting to perceive sexual problems was indicative for sexual dysfunction.
This cross-sectional study included male patients with HL who were treated with chemotherapy and age-matched sexually active males.
Outcome measures included the internationally validated International Index of Erectile Function (IIEF) and self-reported sexual problems by adding 3 items to the study-specific questionnaire.
Erectile dysfunction (ED) occurred in 23.3% of the HL survivors vs in 23.0% of controls: respectively 13.3% and 12.3% had moderate to severe ED. However, more HL survivors positively answered the question whether they did perceive sexual problems than controls (20.0% vs 7.0%; P = .087). More patients treated with bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procabazine, and prednisone (BEACOPP) had sexual problems 33.3% vs 8.3% who were treated with doxorubicin, bleomycin, vinblastine, and dacarbazine (P = .057). Importantly, we found that the mean IIEF score for erectile function was 15.7 in HL survivors who reported to perceive sexual problems (moderate ED) vs 28.3 (normal) in those without perceiving sexual problems.
In general, sexual function of male HL survivors is comparable to that of matched normal controls. Perceiving sexual problems was associated with lower sexual function measured by the IIEF. None of the HL survivors who were treated with doxorubicin, bleomycin, vinblastine, and dacarbazine perceived sexual problems. However, one-third of HL survivors who were treated with BEACOPP did, including ED in one-third of the cases. This is an important consideration for daily clinical practice as BEACOPP is increasingly used as standard therapy in advanced-stage HL.
Introduction
During the past few decades, more effective therapy has led to better survival rates among patients with Hodgkin lymphoma (HL), and cures can presently be as high as 80–90%.1,2 Nevertheless, treatment-related toxicity is still a major concern, and while long-term follow-up has revealed much detailed information, unambiguous data on sexual dysfunction after HL treatment are scarce.
Overall, sexual dysfunction may consist of a loss of sexual desire, arousal, difficulties reaching orgasm, pain with erection or orgasm, sexual satisfaction, or discontinuation of sexual activity.3–11 Among men treated for cancer, the most common sexual problems are a loss of sexual desire and erectile problems.12 Sexual dysfunction can have a variety of causes such as testicular damage, especially due to alkylating treatment agents and radiotherapy.13,14 As a result, subnormal testosterone levels (hypogonadism) may lead to problems with sexual function.5–11 In addition, cancer and its treatment can have negative effects on physiological, as well as psychological and interpersonal factors, which subsequently may impact on sexual function and satisfaction.3–5,7,9–11
The fact that sexual dysfunction can be a problem in HL has been shown in a series of reports with 24 male HL survivors by Hannah et al, (1992)15 to 1,826 male HL survivors by Behringer et al (2013).16 Even so, the exact extent of the problem is unknown, and reported results vary widely from 20% to 63%.16–23 In addition, there are heterogeneous reports on these effects which compare patients to the general population, again with a large variation in results.16,22
A problem with any new avenue of retrospective research is the lack of stable reporting scenarios against which comparisons can be made. To start with, the aforementioned variation might simply be explained by differences in the definitions of sexual dysfunctions. These are often subjective ranging from reporting “decreased sexual enjoyment” to actual “erectile dysfunction.” In addition, various (non)standardized and (non)validated questionnaires have been used,3 and when comparing new findings, only a limited number of prior studies took the sexual dysfunction rate in the general male population into consideration.8,16,22,23 Critically, none of these studies used the International Index of Erectile Function (IIEF)24,25 questionnaire which has presently been adopted as the “gold standard” for efficacy assessment in clinical trials investigating erectile dysfunction and when measuring male sexual function in male cancer populations.4 In the largest series describing the outcome of 1,826 male HL survivors reported by the German Hodgkin Study Group, the European Organisation for Research and Treatment of Cancer-sexual function scale was used, based on 3 questions, and being validated in patients with testicular cancer only.16
There is little doubt that as patients live longer, sexual issues will become more important. To help form a base line for this research area, we compared self-reported sexual function in sexually active male HL survivors with that of a sample drawn from sexually active, age-matched, Dutch males. We used the standardized and validated IIEF questionnaire. To support early detection of sexual dysfunction in HL survivors in the future, we also investigated whether sociodemographic factors and treatment regimen were associated with sexual dysfunction. Finally, we investigated whether reporting to perceive sexual problems was indicative for sexual dysfunction.
Materials and methods
Study Design and Participants
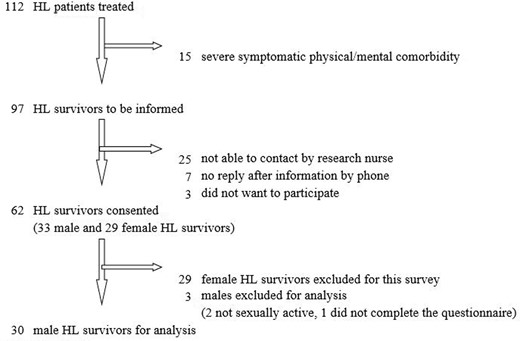
This cross-sectional survey study was conducted in the VU University Medical Center in 2008. From a cohort of 112 HL survivors, patients who were treated between September 2002 and December 2007 were identified. The recruitment of the sample is presented in Figure 1. Patients who were treated with either ABVD regimen—doxorubicin, bleomycin, vinblastine, and dacarbazine (baseline and/or escalated)—or BEACOPP regimen—bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procabazine, and prednisone, with or without adjuvant involved field—were also identified. Patients had to be in complete remission at the time of the study. Patients with severe physical or mental comorbidity, or who were not sexually active at the moment of the questionnaire, were excluded. Eligible patients were contacted and informed by telephone. After showing an interest to participate, the patient information letter, informed consent form, and questionnaire were sent by regular mail.
Control Population
In 2014, adult male members of a Dutch internet panel (www.panelclix.nl), representative of the general Dutch population, were asked if they might participate in a digital survey on sexual function for which a fee of 5 Euro per person was paid. Panelclix is an International Organization for Standardization (ISO)-certified European online recruitment agency. An online study–specific questionnaire was developed whereby information on age, marital status, and sexual activity was obtained. In addition, the IIEF questionnaire (discussed in the following sections) was used.
A total of 205 male participants completed the survey from which sexually inactive males were excluded (n = 13). This resulted in a database of 192 sexually active males. Each HL survivor (n = 30) was age-matched with 2 controls, randomly selected from the control population. 3 HL survivors could only be matched to one control, which resulted in a total of n = 57 controls.
Procedure
Sexual Function
Sexual function was evaluated by means of the IIEF. The IIEF is a validated, 15-item self-report questionnaire for the evaluation of male sexual function.25 The IIEF comprises 5 subscales, including erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. For grading erectile dysfunction, the cutoff scores as defined by Cappelleri et al24 have been used. For all other domains, no standard reference cutoffs have been identified; a higher score indicates better sexual function.24,25
Sexual Problems
In addition, perceived sexual problems were evaluated in the study-specific questionnaire by adding 3 items to it: (i) Do you perceive a sexual problem? (ii) If yes, please define the problem(s); (iii) Did the problem(s) improve, remain unchanged, resolve, or worsen since the treatment for HL began?
Sociodemographic and Clinical Characteristics
In addition, items on sociodemographic variables (age, committed relationship) were assessed in the study-specific questionnaire. Disease-specific items (date of HL diagnosis, stage of disease, treatment, time since treatment) were derived from medical records. Time in remission was calculated from date of complete remission until the date of questionnaire. Follow-up time (in months) was calculated from diagnosis until the date of questionnaire.
Ethical Consideration
The study was approved by the Ethics Committee of the VU University Medical Center, Amsterdam, the Netherlands. The study was conducted according to the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice Guidelines, and the EU directive for Good Clinical Practice (2001/20/EG).
Data Analysis
All statistical analyses were performed using SPSS version 22 (IBM Corp, Armonk, NY). Dichotomous variables are described by frequency and percentage. All subscales and the total score of the IIEF are described by mean and standard deviation, and all other continuous normally distributed variables by mean and standard deviation and continuous nonnormally distributed variables by median and range. Reporting the presence of a sexual problem and erectile dysfunction according to the IIEF and the scores of the subscales and total score of IIEF were compared between the HL survivors and the controls using the Fisher’s exact test for dichotomous outcomes and the Mann-Whitney U test for continuous subscales. Relations between clinical or sociodemographical factors and sexual function in HL survivors were assessed via the Fisher’s exact test (for dichotomous factors), independent samples t-test (for the continuous factor age), and Mann-Whitney test (for the IIEF subscales and other nonnormal continuous factors). P values < .05 were considered to be significant.
Results
Patient Sociodemographic and Clinical Characteristics
In total, 30 HL survivors were analyzed. Sociodemographic and clinical characteristics of male HL survivors are presented in Table 1. The median age of the HL survivors was 38 years (range: 22–63), and 90% were in a committed relationship. All survivors (n = 30) had been treated with combination chemotherapy, 12 survivors had been treated with 2–4 cycles ABVD, and 17 survivors with 4–8 cycles standard or dose-escalated BEACOPP. One survivor had been treated with a combined regimen of BEACOPP and ABVD. Adjuvant radiotherapy (involved field) was given to 15 patients, of whom 3 were treated with BEACOPP regimen. All 15 patients who did not receive radiotherapy received a BEACOPP regimen. 2 patients underwent pelvic irradiation, one in combination with ABVD and one in combination with BEACOPP. 2 patients had received autologous stem cell transplantation because of recurrent disease, both patients had a stage II disease, one received ABVD in combination with adjuvant radiotherapy, and the other patient received 8 cycles of BEACOPP in combination with adjuvant radiotherapy.
| Sample characteristics . | N = 30 . | |
|---|---|---|
| N . | % . | |
| Age at diagnosis (years) | ||
| Mean (SD) | 36 (10) | |
| Age at time of study (years) | ||
| Mean (SD) | 39 (10) | |
| Time in remission (months) | ||
| Median (range) | 38 (9–83) | |
| Follow-up time (months) | ||
| Median (range) | 47 (14–89) | |
| Relationship status | ||
| Committed relationship | 27 | 90.0 |
| No committed relationship | 3 | 10.0 |
| Stage of disease | ||
| I + II | 18 | 60.0 |
| III + IV | 12 | 40.0 |
| BEACOPP regimen (%) | 18 (60.0) | |
| 8 Cycles BEACOPP escalated | 8 | 44.4 |
| 8 Cycles BEACOPP | 2 | 11.1 |
| 6 Cycles BEACOPP escalated | 4 | 22.2 |
| 4 Cycles BEACOPP escalated + 4 cycles BEACOPP | 3 | 16.7 |
| 2 Cycles BEACOPP escalated + 2 cycles ABVD | 1 | 5.6 |
| ABVD regimen | 12 (40.0) | |
| 4 cycles ABVD | 7 | 58.3 |
| 2–4 cycles ABVD like | 5 | 41.7 |
| Involved field radiotherapy | ||
| No | 15 (50.0) | |
| BEACOPP regimen | 15 | 100.0 |
| ABVD regimen | 0 | 0.0 |
| Yes | 15 (50.0) | |
| BEACOPP regimen | 3 | 20.0 |
| ABVD regimen | 12 | 80.0 |
| Sample characteristics . | N = 30 . | |
|---|---|---|
| N . | % . | |
| Age at diagnosis (years) | ||
| Mean (SD) | 36 (10) | |
| Age at time of study (years) | ||
| Mean (SD) | 39 (10) | |
| Time in remission (months) | ||
| Median (range) | 38 (9–83) | |
| Follow-up time (months) | ||
| Median (range) | 47 (14–89) | |
| Relationship status | ||
| Committed relationship | 27 | 90.0 |
| No committed relationship | 3 | 10.0 |
| Stage of disease | ||
| I + II | 18 | 60.0 |
| III + IV | 12 | 40.0 |
| BEACOPP regimen (%) | 18 (60.0) | |
| 8 Cycles BEACOPP escalated | 8 | 44.4 |
| 8 Cycles BEACOPP | 2 | 11.1 |
| 6 Cycles BEACOPP escalated | 4 | 22.2 |
| 4 Cycles BEACOPP escalated + 4 cycles BEACOPP | 3 | 16.7 |
| 2 Cycles BEACOPP escalated + 2 cycles ABVD | 1 | 5.6 |
| ABVD regimen | 12 (40.0) | |
| 4 cycles ABVD | 7 | 58.3 |
| 2–4 cycles ABVD like | 5 | 41.7 |
| Involved field radiotherapy | ||
| No | 15 (50.0) | |
| BEACOPP regimen | 15 | 100.0 |
| ABVD regimen | 0 | 0.0 |
| Yes | 15 (50.0) | |
| BEACOPP regimen | 3 | 20.0 |
| ABVD regimen | 12 | 80.0 |
ABVD = doxorubicin, bleomycin, vinblastin, and dacarbazin; BEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procabazine, and prednisone; HL = Hodgkin lymphoma; SD = standard deviation.
| Sample characteristics . | N = 30 . | |
|---|---|---|
| N . | % . | |
| Age at diagnosis (years) | ||
| Mean (SD) | 36 (10) | |
| Age at time of study (years) | ||
| Mean (SD) | 39 (10) | |
| Time in remission (months) | ||
| Median (range) | 38 (9–83) | |
| Follow-up time (months) | ||
| Median (range) | 47 (14–89) | |
| Relationship status | ||
| Committed relationship | 27 | 90.0 |
| No committed relationship | 3 | 10.0 |
| Stage of disease | ||
| I + II | 18 | 60.0 |
| III + IV | 12 | 40.0 |
| BEACOPP regimen (%) | 18 (60.0) | |
| 8 Cycles BEACOPP escalated | 8 | 44.4 |
| 8 Cycles BEACOPP | 2 | 11.1 |
| 6 Cycles BEACOPP escalated | 4 | 22.2 |
| 4 Cycles BEACOPP escalated + 4 cycles BEACOPP | 3 | 16.7 |
| 2 Cycles BEACOPP escalated + 2 cycles ABVD | 1 | 5.6 |
| ABVD regimen | 12 (40.0) | |
| 4 cycles ABVD | 7 | 58.3 |
| 2–4 cycles ABVD like | 5 | 41.7 |
| Involved field radiotherapy | ||
| No | 15 (50.0) | |
| BEACOPP regimen | 15 | 100.0 |
| ABVD regimen | 0 | 0.0 |
| Yes | 15 (50.0) | |
| BEACOPP regimen | 3 | 20.0 |
| ABVD regimen | 12 | 80.0 |
| Sample characteristics . | N = 30 . | |
|---|---|---|
| N . | % . | |
| Age at diagnosis (years) | ||
| Mean (SD) | 36 (10) | |
| Age at time of study (years) | ||
| Mean (SD) | 39 (10) | |
| Time in remission (months) | ||
| Median (range) | 38 (9–83) | |
| Follow-up time (months) | ||
| Median (range) | 47 (14–89) | |
| Relationship status | ||
| Committed relationship | 27 | 90.0 |
| No committed relationship | 3 | 10.0 |
| Stage of disease | ||
| I + II | 18 | 60.0 |
| III + IV | 12 | 40.0 |
| BEACOPP regimen (%) | 18 (60.0) | |
| 8 Cycles BEACOPP escalated | 8 | 44.4 |
| 8 Cycles BEACOPP | 2 | 11.1 |
| 6 Cycles BEACOPP escalated | 4 | 22.2 |
| 4 Cycles BEACOPP escalated + 4 cycles BEACOPP | 3 | 16.7 |
| 2 Cycles BEACOPP escalated + 2 cycles ABVD | 1 | 5.6 |
| ABVD regimen | 12 (40.0) | |
| 4 cycles ABVD | 7 | 58.3 |
| 2–4 cycles ABVD like | 5 | 41.7 |
| Involved field radiotherapy | ||
| No | 15 (50.0) | |
| BEACOPP regimen | 15 | 100.0 |
| ABVD regimen | 0 | 0.0 |
| Yes | 15 (50.0) | |
| BEACOPP regimen | 3 | 20.0 |
| ABVD regimen | 12 | 80.0 |
ABVD = doxorubicin, bleomycin, vinblastin, and dacarbazin; BEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, oncovin, procabazine, and prednisone; HL = Hodgkin lymphoma; SD = standard deviation.
The median time of remission was 38 months (range: 9–83 months). The median follow-up time since treatment was 47 months (range: 14–89 months). In the medical records, no notes on provided sexual support were found.
Differences in Sexual Functioning
The IIEF questionnaire revealed no differences in sexual function between HL survivors and controls (Table 2). Also the degree of erectile dysfunction was comparable between HL survivors and controls: 23.3% of HL survivors reported mild to severe erectile dysfunction and 23.0% in controls (odds ratio 1.03) (Table 2).
Comparison of main outcome measures of HL survivors and age-matched controls
| Main outcome measures . | Score range . | HL survivors . | Age-matched controls . | |||
|---|---|---|---|---|---|---|
| N = 30 . | N = 57 . | P value . | ||||
| IIEF subscales | Mean | SD | Mean | SD | ||
| Erectile function | 1–30 | 25.8 | 7.3 | 26.1 | 6.1 | .60 |
| Orgasmic function | 0–10 | 9.2 | 2.0 | 9.4 | 2.0 | .42 |
| Sexual desire | 2–10 | 7.2 | 1.9 | 7.7 | 1.6 | .24 |
| Intercourse satisfaction | 0–15 | 10.2 | 4.2 | 10.3 | 4.4 | .73 |
| Overall satisfaction | 2–10 | 8.5 | 2.3 | 7.9 | 2.3 | .11 |
| IIEF total | 5–75 | 60.8 | 15.1 | 61.3 | 12.8 | .87 |
| Erectile function score | N | % | N | % | ||
| No erectile dysfunction | 26–30 | 23 | 76.7 | 44 | 77.0 | .68 |
| Mild erectile dysfunction | 22–25 | 1 | 3.3 | 2 | 3.5 | |
| Mild to moderate erectile dysfunction | 17–21 | 2 | 6.7 | 4 | 7.0 | |
| Moderate erectile dysfunction | 11–16 | 1 | 3.3 | 5 | 8.8 | |
| Severe erectile dysfunction | 1–10 | 3 | 10.0 | 2 | 3.5 | |
| Perceiving a sexual problem | .087 | |||||
| Yes | 6 | 20.0 | 4 | 7.0 | ||
| No | 24 | 80.0 | 53 | 93.0 | ||
| Main outcome measures . | Score range . | HL survivors . | Age-matched controls . | |||
|---|---|---|---|---|---|---|
| N = 30 . | N = 57 . | P value . | ||||
| IIEF subscales | Mean | SD | Mean | SD | ||
| Erectile function | 1–30 | 25.8 | 7.3 | 26.1 | 6.1 | .60 |
| Orgasmic function | 0–10 | 9.2 | 2.0 | 9.4 | 2.0 | .42 |
| Sexual desire | 2–10 | 7.2 | 1.9 | 7.7 | 1.6 | .24 |
| Intercourse satisfaction | 0–15 | 10.2 | 4.2 | 10.3 | 4.4 | .73 |
| Overall satisfaction | 2–10 | 8.5 | 2.3 | 7.9 | 2.3 | .11 |
| IIEF total | 5–75 | 60.8 | 15.1 | 61.3 | 12.8 | .87 |
| Erectile function score | N | % | N | % | ||
| No erectile dysfunction | 26–30 | 23 | 76.7 | 44 | 77.0 | .68 |
| Mild erectile dysfunction | 22–25 | 1 | 3.3 | 2 | 3.5 | |
| Mild to moderate erectile dysfunction | 17–21 | 2 | 6.7 | 4 | 7.0 | |
| Moderate erectile dysfunction | 11–16 | 1 | 3.3 | 5 | 8.8 | |
| Severe erectile dysfunction | 1–10 | 3 | 10.0 | 2 | 3.5 | |
| Perceiving a sexual problem | .087 | |||||
| Yes | 6 | 20.0 | 4 | 7.0 | ||
| No | 24 | 80.0 | 53 | 93.0 | ||
HL = Hodgkin lymphoma; IIEF = International Index of Erectile Function; SD = standard deviation.
Comparison of main outcome measures of HL survivors and age-matched controls
| Main outcome measures . | Score range . | HL survivors . | Age-matched controls . | |||
|---|---|---|---|---|---|---|
| N = 30 . | N = 57 . | P value . | ||||
| IIEF subscales | Mean | SD | Mean | SD | ||
| Erectile function | 1–30 | 25.8 | 7.3 | 26.1 | 6.1 | .60 |
| Orgasmic function | 0–10 | 9.2 | 2.0 | 9.4 | 2.0 | .42 |
| Sexual desire | 2–10 | 7.2 | 1.9 | 7.7 | 1.6 | .24 |
| Intercourse satisfaction | 0–15 | 10.2 | 4.2 | 10.3 | 4.4 | .73 |
| Overall satisfaction | 2–10 | 8.5 | 2.3 | 7.9 | 2.3 | .11 |
| IIEF total | 5–75 | 60.8 | 15.1 | 61.3 | 12.8 | .87 |
| Erectile function score | N | % | N | % | ||
| No erectile dysfunction | 26–30 | 23 | 76.7 | 44 | 77.0 | .68 |
| Mild erectile dysfunction | 22–25 | 1 | 3.3 | 2 | 3.5 | |
| Mild to moderate erectile dysfunction | 17–21 | 2 | 6.7 | 4 | 7.0 | |
| Moderate erectile dysfunction | 11–16 | 1 | 3.3 | 5 | 8.8 | |
| Severe erectile dysfunction | 1–10 | 3 | 10.0 | 2 | 3.5 | |
| Perceiving a sexual problem | .087 | |||||
| Yes | 6 | 20.0 | 4 | 7.0 | ||
| No | 24 | 80.0 | 53 | 93.0 | ||
| Main outcome measures . | Score range . | HL survivors . | Age-matched controls . | |||
|---|---|---|---|---|---|---|
| N = 30 . | N = 57 . | P value . | ||||
| IIEF subscales | Mean | SD | Mean | SD | ||
| Erectile function | 1–30 | 25.8 | 7.3 | 26.1 | 6.1 | .60 |
| Orgasmic function | 0–10 | 9.2 | 2.0 | 9.4 | 2.0 | .42 |
| Sexual desire | 2–10 | 7.2 | 1.9 | 7.7 | 1.6 | .24 |
| Intercourse satisfaction | 0–15 | 10.2 | 4.2 | 10.3 | 4.4 | .73 |
| Overall satisfaction | 2–10 | 8.5 | 2.3 | 7.9 | 2.3 | .11 |
| IIEF total | 5–75 | 60.8 | 15.1 | 61.3 | 12.8 | .87 |
| Erectile function score | N | % | N | % | ||
| No erectile dysfunction | 26–30 | 23 | 76.7 | 44 | 77.0 | .68 |
| Mild erectile dysfunction | 22–25 | 1 | 3.3 | 2 | 3.5 | |
| Mild to moderate erectile dysfunction | 17–21 | 2 | 6.7 | 4 | 7.0 | |
| Moderate erectile dysfunction | 11–16 | 1 | 3.3 | 5 | 8.8 | |
| Severe erectile dysfunction | 1–10 | 3 | 10.0 | 2 | 3.5 | |
| Perceiving a sexual problem | .087 | |||||
| Yes | 6 | 20.0 | 4 | 7.0 | ||
| No | 24 | 80.0 | 53 | 93.0 | ||
HL = Hodgkin lymphoma; IIEF = International Index of Erectile Function; SD = standard deviation.
Differences in Sexual Problems
HL survivors perceived more sexual problems than controls, although this was not statistically significant (20.0% vs 7.0%; P = .087) (Table 2). The following problems were reported by HL survivors: lack of sexual desire (n = 3), problems with getting aroused (n = 2), no firm erection (n = 2), problem getting too aroused (n = 1), erection not possible (n = 1), self perceived sexual unattractiveness (n = 1), and lack of condition (n = 1). Sexual problems were not present before the start of therapy but developed during or after treatment. None of the problems disappeared.
Differences in Sociodemographic and Clinical Characteristics
Table 3 presents the associations of sociodemographic and clinical factors with sexual function in HL survivors. Differences were observed between survivors treated by BEACOPP and those treated by ABVD, although these were not statistically significant. Overall satisfaction was found to be lower in survivors treated with BEACOPP (mean 7.8 ± 2.7 vs 9.5 ± 0.8 survivors treated with ABVD, P = .065). Accordingly, more HL survivors who were treated with a BEACOPP regimen perceived sexual problems than those who were treated with ABVD (6/18 [33.3%] vs 0/12 [0.0%], P = .057).
Associations of sociodemographic and clinical factors with sexual function in HL survivors
| Variable . | International Index of Erectile Function . | Erectile dysfunction . | Perceiving a sexual problem . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Erectile function . | Orgasmic function . | Sexual desire . | Intercourse satisfaction . | Overall satisfaction . | Total score IIEF . | Yes . | No . | Yes . | No . | |
| Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | N (%) . | N (%) . | N (%) . | N (%) . | |
| Relationship status | ||||||||||
| Committed relationship | 26.3 ± 6.6 | 9.6 ± 0.9 | 7.4 ± 1.8 | 10.6 ± 3.9 | 8.7 ± 2.0 | 62.6 ± 12.5 | 6 (22.2) | 21 (77.8) | 5 (18.5) | 22 (81.5) |
| No committed relationship | 21.0 ± 13.0 | 5.7 ± 5.1 | 5.3 ± 2.1 | 6.7 ± 5.9 | 6.0 ± 3.5 | 44.7 ± 29.4 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) |
| Stage of disease | ||||||||||
| I–II | 27.4 ± 5.2 | 9.4 ± 1.1 | 7.1 ± 1.7 | 10.8 ± 3.3 | 9.1 ± 1.5 | 63.8 ± 10.5 | 3 (16.7) | 15 (83.3) | 3 (14.3) | 15 (85.7) |
| III–IV | 23.3 ± 9.4 | 8.8 ± 2.9 | 7.3 ± 2.1 | 9.3 ± 5.3 | 7.6 ± 2.9 | 56.4 ± 20.0 | 4 (33.3) | 8 (66.7) | 3 (25.0) | 9 (75.0) |
| Chemotherapy | ||||||||||
| ABVD regimen | 28.3 ± 2.6 | 9.2 ± 1.3 | 7.7 ± 1.5 | 11.5 ± 2.1 | 9.5 ± 0.8∗ | 66.2 ± 5.6 | 1 (8.3) | 11 (91.7) | 0 (0.0)† | 12 (100.0) |
| BEACOPP regimen | 24.1 ± 8.9 | 9.2 ± 2.4 | 6.8 ± 2.0 | 9.4 ± 5.0 | 7.8 ± 2.7 | 57.3 ± 18.4 | 6 (33.3) | 12 (66.7) | 6 (33.3) | 12 (66.7) |
| Involved field radiotherapy | ||||||||||
| No | 24.3 ± 8.7 | 9.1 ± 2.6 | 6.9 ± 2.0 | 9.6 ± 4.8 | 7.8 ± 2.7 | 57.7 ± 17.9 | 5 (33.3) | 10 (66.7) | 4 (26.7) | 11 (73.3) |
| Yes | 27.3 ± 5.6 | 9.3 ± 1.2 | 7.4 ± 1.7 | 10.9 ± 3.6 | 9.1 ± 1.6 | 64.0 ± 11.5 | 2 (13.3) | 13 (86.7) | 2 (13.3) | 13 (86.7) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| Age at time of study (years) | 44.4 ± 13.8 | 38.0 ± 9.0 | 46 ± 13 | 38 ± 9 | ||||||
| Median (range) | Median (range) | Median (range) | Median (range) | |||||||
| Time since start treatment (months) | 29 (18–74) | 48 (14–89) | 51 (29–74) | 46 (14–89) | ||||||
| Time since remission (months) | 22 (9–60) | 44 (9–83) | 31 (22–60) | 41(9–83) | ||||||
| Variable . | International Index of Erectile Function . | Erectile dysfunction . | Perceiving a sexual problem . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Erectile function . | Orgasmic function . | Sexual desire . | Intercourse satisfaction . | Overall satisfaction . | Total score IIEF . | Yes . | No . | Yes . | No . | |
| Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | N (%) . | N (%) . | N (%) . | N (%) . | |
| Relationship status | ||||||||||
| Committed relationship | 26.3 ± 6.6 | 9.6 ± 0.9 | 7.4 ± 1.8 | 10.6 ± 3.9 | 8.7 ± 2.0 | 62.6 ± 12.5 | 6 (22.2) | 21 (77.8) | 5 (18.5) | 22 (81.5) |
| No committed relationship | 21.0 ± 13.0 | 5.7 ± 5.1 | 5.3 ± 2.1 | 6.7 ± 5.9 | 6.0 ± 3.5 | 44.7 ± 29.4 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) |
| Stage of disease | ||||||||||
| I–II | 27.4 ± 5.2 | 9.4 ± 1.1 | 7.1 ± 1.7 | 10.8 ± 3.3 | 9.1 ± 1.5 | 63.8 ± 10.5 | 3 (16.7) | 15 (83.3) | 3 (14.3) | 15 (85.7) |
| III–IV | 23.3 ± 9.4 | 8.8 ± 2.9 | 7.3 ± 2.1 | 9.3 ± 5.3 | 7.6 ± 2.9 | 56.4 ± 20.0 | 4 (33.3) | 8 (66.7) | 3 (25.0) | 9 (75.0) |
| Chemotherapy | ||||||||||
| ABVD regimen | 28.3 ± 2.6 | 9.2 ± 1.3 | 7.7 ± 1.5 | 11.5 ± 2.1 | 9.5 ± 0.8∗ | 66.2 ± 5.6 | 1 (8.3) | 11 (91.7) | 0 (0.0)† | 12 (100.0) |
| BEACOPP regimen | 24.1 ± 8.9 | 9.2 ± 2.4 | 6.8 ± 2.0 | 9.4 ± 5.0 | 7.8 ± 2.7 | 57.3 ± 18.4 | 6 (33.3) | 12 (66.7) | 6 (33.3) | 12 (66.7) |
| Involved field radiotherapy | ||||||||||
| No | 24.3 ± 8.7 | 9.1 ± 2.6 | 6.9 ± 2.0 | 9.6 ± 4.8 | 7.8 ± 2.7 | 57.7 ± 17.9 | 5 (33.3) | 10 (66.7) | 4 (26.7) | 11 (73.3) |
| Yes | 27.3 ± 5.6 | 9.3 ± 1.2 | 7.4 ± 1.7 | 10.9 ± 3.6 | 9.1 ± 1.6 | 64.0 ± 11.5 | 2 (13.3) | 13 (86.7) | 2 (13.3) | 13 (86.7) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| Age at time of study (years) | 44.4 ± 13.8 | 38.0 ± 9.0 | 46 ± 13 | 38 ± 9 | ||||||
| Median (range) | Median (range) | Median (range) | Median (range) | |||||||
| Time since start treatment (months) | 29 (18–74) | 48 (14–89) | 51 (29–74) | 46 (14–89) | ||||||
| Time since remission (months) | 22 (9–60) | 44 (9–83) | 31 (22–60) | 41(9–83) | ||||||
ABVD = adriamycin, bleomycin, vinblastin, and dacarbazin; BEACOPP = bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procabazine, and prednisone; HL = Hodgkin lymphoma; IIEF = International Index of Erectile Function; SD = standard deviation.
P = .065.
P = .057.
Associations of sociodemographic and clinical factors with sexual function in HL survivors
| Variable . | International Index of Erectile Function . | Erectile dysfunction . | Perceiving a sexual problem . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Erectile function . | Orgasmic function . | Sexual desire . | Intercourse satisfaction . | Overall satisfaction . | Total score IIEF . | Yes . | No . | Yes . | No . | |
| Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | N (%) . | N (%) . | N (%) . | N (%) . | |
| Relationship status | ||||||||||
| Committed relationship | 26.3 ± 6.6 | 9.6 ± 0.9 | 7.4 ± 1.8 | 10.6 ± 3.9 | 8.7 ± 2.0 | 62.6 ± 12.5 | 6 (22.2) | 21 (77.8) | 5 (18.5) | 22 (81.5) |
| No committed relationship | 21.0 ± 13.0 | 5.7 ± 5.1 | 5.3 ± 2.1 | 6.7 ± 5.9 | 6.0 ± 3.5 | 44.7 ± 29.4 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) |
| Stage of disease | ||||||||||
| I–II | 27.4 ± 5.2 | 9.4 ± 1.1 | 7.1 ± 1.7 | 10.8 ± 3.3 | 9.1 ± 1.5 | 63.8 ± 10.5 | 3 (16.7) | 15 (83.3) | 3 (14.3) | 15 (85.7) |
| III–IV | 23.3 ± 9.4 | 8.8 ± 2.9 | 7.3 ± 2.1 | 9.3 ± 5.3 | 7.6 ± 2.9 | 56.4 ± 20.0 | 4 (33.3) | 8 (66.7) | 3 (25.0) | 9 (75.0) |
| Chemotherapy | ||||||||||
| ABVD regimen | 28.3 ± 2.6 | 9.2 ± 1.3 | 7.7 ± 1.5 | 11.5 ± 2.1 | 9.5 ± 0.8∗ | 66.2 ± 5.6 | 1 (8.3) | 11 (91.7) | 0 (0.0)† | 12 (100.0) |
| BEACOPP regimen | 24.1 ± 8.9 | 9.2 ± 2.4 | 6.8 ± 2.0 | 9.4 ± 5.0 | 7.8 ± 2.7 | 57.3 ± 18.4 | 6 (33.3) | 12 (66.7) | 6 (33.3) | 12 (66.7) |
| Involved field radiotherapy | ||||||||||
| No | 24.3 ± 8.7 | 9.1 ± 2.6 | 6.9 ± 2.0 | 9.6 ± 4.8 | 7.8 ± 2.7 | 57.7 ± 17.9 | 5 (33.3) | 10 (66.7) | 4 (26.7) | 11 (73.3) |
| Yes | 27.3 ± 5.6 | 9.3 ± 1.2 | 7.4 ± 1.7 | 10.9 ± 3.6 | 9.1 ± 1.6 | 64.0 ± 11.5 | 2 (13.3) | 13 (86.7) | 2 (13.3) | 13 (86.7) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| Age at time of study (years) | 44.4 ± 13.8 | 38.0 ± 9.0 | 46 ± 13 | 38 ± 9 | ||||||
| Median (range) | Median (range) | Median (range) | Median (range) | |||||||
| Time since start treatment (months) | 29 (18–74) | 48 (14–89) | 51 (29–74) | 46 (14–89) | ||||||
| Time since remission (months) | 22 (9–60) | 44 (9–83) | 31 (22–60) | 41(9–83) | ||||||
| Variable . | International Index of Erectile Function . | Erectile dysfunction . | Perceiving a sexual problem . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Erectile function . | Orgasmic function . | Sexual desire . | Intercourse satisfaction . | Overall satisfaction . | Total score IIEF . | Yes . | No . | Yes . | No . | |
| Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | Mean ± SD . | N (%) . | N (%) . | N (%) . | N (%) . | |
| Relationship status | ||||||||||
| Committed relationship | 26.3 ± 6.6 | 9.6 ± 0.9 | 7.4 ± 1.8 | 10.6 ± 3.9 | 8.7 ± 2.0 | 62.6 ± 12.5 | 6 (22.2) | 21 (77.8) | 5 (18.5) | 22 (81.5) |
| No committed relationship | 21.0 ± 13.0 | 5.7 ± 5.1 | 5.3 ± 2.1 | 6.7 ± 5.9 | 6.0 ± 3.5 | 44.7 ± 29.4 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) |
| Stage of disease | ||||||||||
| I–II | 27.4 ± 5.2 | 9.4 ± 1.1 | 7.1 ± 1.7 | 10.8 ± 3.3 | 9.1 ± 1.5 | 63.8 ± 10.5 | 3 (16.7) | 15 (83.3) | 3 (14.3) | 15 (85.7) |
| III–IV | 23.3 ± 9.4 | 8.8 ± 2.9 | 7.3 ± 2.1 | 9.3 ± 5.3 | 7.6 ± 2.9 | 56.4 ± 20.0 | 4 (33.3) | 8 (66.7) | 3 (25.0) | 9 (75.0) |
| Chemotherapy | ||||||||||
| ABVD regimen | 28.3 ± 2.6 | 9.2 ± 1.3 | 7.7 ± 1.5 | 11.5 ± 2.1 | 9.5 ± 0.8∗ | 66.2 ± 5.6 | 1 (8.3) | 11 (91.7) | 0 (0.0)† | 12 (100.0) |
| BEACOPP regimen | 24.1 ± 8.9 | 9.2 ± 2.4 | 6.8 ± 2.0 | 9.4 ± 5.0 | 7.8 ± 2.7 | 57.3 ± 18.4 | 6 (33.3) | 12 (66.7) | 6 (33.3) | 12 (66.7) |
| Involved field radiotherapy | ||||||||||
| No | 24.3 ± 8.7 | 9.1 ± 2.6 | 6.9 ± 2.0 | 9.6 ± 4.8 | 7.8 ± 2.7 | 57.7 ± 17.9 | 5 (33.3) | 10 (66.7) | 4 (26.7) | 11 (73.3) |
| Yes | 27.3 ± 5.6 | 9.3 ± 1.2 | 7.4 ± 1.7 | 10.9 ± 3.6 | 9.1 ± 1.6 | 64.0 ± 11.5 | 2 (13.3) | 13 (86.7) | 2 (13.3) | 13 (86.7) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| Age at time of study (years) | 44.4 ± 13.8 | 38.0 ± 9.0 | 46 ± 13 | 38 ± 9 | ||||||
| Median (range) | Median (range) | Median (range) | Median (range) | |||||||
| Time since start treatment (months) | 29 (18–74) | 48 (14–89) | 51 (29–74) | 46 (14–89) | ||||||
| Time since remission (months) | 22 (9–60) | 44 (9–83) | 31 (22–60) | 41(9–83) | ||||||
ABVD = adriamycin, bleomycin, vinblastin, and dacarbazin; BEACOPP = bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procabazine, and prednisone; HL = Hodgkin lymphoma; IIEF = International Index of Erectile Function; SD = standard deviation.
P = .065.
P = .057.
Association Between Sexual Functioning According to IIEF vs Perceiving a Sexual Problem
Finally, we investigated whether reporting perceived sexual problems was indicative for sexual dysfunction as measured by the IIEF. All domains of the IIEF were found to be associated with perceiving sexual problems, except for orgasmic function (Table 4). In patients perceiving sexual problems, IIEF scores were lower, indicating less sexual function. In addition, in patients perceiving sexual problems, the mean IIEF score for erectile function was 15.7, indicative for moderate erectile dysfunction, whereas in patients without perceiving sexual problems, the mean IIEF score for erectile dysfunction was 28.3 being normal.
Comparison of main outcome measure between HL survivors with vs without a sexual problem
| . | . | HL survivors with a sexual problem . | HL survivors without a sexual problem . | . | ||
|---|---|---|---|---|---|---|
| IIEF subscales . | Score range . | N = 6 . | N = 24 . | P value . | ||
| . | . | Mean . | SD . | Mean . | SD . | |
| Erectile function∗ | 1–30 | 15.7 | 10.2 | 28.3 | 3.5 | .005∗ |
| Orgasmic function | 0–10 | 7.7 | 3.9 | 9.6 | 0.97 | .21 |
| Sexual desire | 2–10 | 5.7 | 2.2 | 7.5 | 1.6 | .05∗ |
| Intercourse satisfaction | 0–15 | 5.5 | 5.0 | 11.4 | 3.1 | .005∗ |
| Overall satisfaction | 2–10 | 5.5 | 3.2 | 9.2 | 1.2 | .003∗ |
| IIEF total | 5–75 | 40.0 | 20.9 | 66.0 | 7.3 | .001∗ |
| . | . | HL survivors with a sexual problem . | HL survivors without a sexual problem . | . | ||
|---|---|---|---|---|---|---|
| IIEF subscales . | Score range . | N = 6 . | N = 24 . | P value . | ||
| . | . | Mean . | SD . | Mean . | SD . | |
| Erectile function∗ | 1–30 | 15.7 | 10.2 | 28.3 | 3.5 | .005∗ |
| Orgasmic function | 0–10 | 7.7 | 3.9 | 9.6 | 0.97 | .21 |
| Sexual desire | 2–10 | 5.7 | 2.2 | 7.5 | 1.6 | .05∗ |
| Intercourse satisfaction | 0–15 | 5.5 | 5.0 | 11.4 | 3.1 | .005∗ |
| Overall satisfaction | 2–10 | 5.5 | 3.2 | 9.2 | 1.2 | .003∗ |
| IIEF total | 5–75 | 40.0 | 20.9 | 66.0 | 7.3 | .001∗ |
HL = Hodgkin lymphoma; IIEF = International Index of Erectile Function; SD = standard deviation.
P < .05.
Comparison of main outcome measure between HL survivors with vs without a sexual problem
| . | . | HL survivors with a sexual problem . | HL survivors without a sexual problem . | . | ||
|---|---|---|---|---|---|---|
| IIEF subscales . | Score range . | N = 6 . | N = 24 . | P value . | ||
| . | . | Mean . | SD . | Mean . | SD . | |
| Erectile function∗ | 1–30 | 15.7 | 10.2 | 28.3 | 3.5 | .005∗ |
| Orgasmic function | 0–10 | 7.7 | 3.9 | 9.6 | 0.97 | .21 |
| Sexual desire | 2–10 | 5.7 | 2.2 | 7.5 | 1.6 | .05∗ |
| Intercourse satisfaction | 0–15 | 5.5 | 5.0 | 11.4 | 3.1 | .005∗ |
| Overall satisfaction | 2–10 | 5.5 | 3.2 | 9.2 | 1.2 | .003∗ |
| IIEF total | 5–75 | 40.0 | 20.9 | 66.0 | 7.3 | .001∗ |
| . | . | HL survivors with a sexual problem . | HL survivors without a sexual problem . | . | ||
|---|---|---|---|---|---|---|
| IIEF subscales . | Score range . | N = 6 . | N = 24 . | P value . | ||
| . | . | Mean . | SD . | Mean . | SD . | |
| Erectile function∗ | 1–30 | 15.7 | 10.2 | 28.3 | 3.5 | .005∗ |
| Orgasmic function | 0–10 | 7.7 | 3.9 | 9.6 | 0.97 | .21 |
| Sexual desire | 2–10 | 5.7 | 2.2 | 7.5 | 1.6 | .05∗ |
| Intercourse satisfaction | 0–15 | 5.5 | 5.0 | 11.4 | 3.1 | .005∗ |
| Overall satisfaction | 2–10 | 5.5 | 3.2 | 9.2 | 1.2 | .003∗ |
| IIEF total | 5–75 | 40.0 | 20.9 | 66.0 | 7.3 | .001∗ |
HL = Hodgkin lymphoma; IIEF = International Index of Erectile Function; SD = standard deviation.
P < .05.
Discussion
This retrospective study was performed to form a baseline for research into sexual functioning among male HL survivors. Patients were compared to an age-matched male control population (Table 1) and, on average, had been treated with standard treatment regimens 4 years previous to this investigation. To the best of our knowledge, this is the first study to compare the IEFF scores with an age-matched male control population.
In general, sexual function of male HL survivors was found to be comparable to that of matched normal controls, with respect to all IIEF subscales comprising function, desire, and satisfaction. Furthermore, the degree of ED was comparable between HL survivors and controls: 23.3% in HL survivors and 23.0% in control—respectively 13.3% and 12.3% had moderate to severe erectile dysfunction. This has been reported previously and indicates that no additional care is needed in male HL survivors.16,22
However, the prevalence of sexual problems in our population of HL survivors was remarkably lower than that recorded in another which used the validated Global Sexual Satisfaction Index from the Derogatis Sexual Functioning Inventory.22 In this latter study, 54% of HL survivors (>7 years after treatment) reported decreased sexual activity, and 41% reported a decreased interest in sex. This may be due to the fact that our sample included a heterogeneously treated population, with 40% of early stage patients treated with ABVD and 60% of higher risk patients treated with BEACOPP. We found that although none of the HL survivors who were treated with ABVD perceived sexual problems, 33.3% of HL survivors who were treated with BEACOPP did. Behringer et al16 reported that only survivors with early stages of HL reached the same levels of sexual functioning after therapy compared with controls, while survivors with higher stages of HL were more likely to develop long-term sexual problems. This is probably explained by the fact that the BEACOPP schedule includes 2 alkylating agents, namely procarbazine and cyclophosphamide, while the ABVD regimen has only one, namely dacarbazine.
Alkylating agents can lead to damage of the gonads, a well-known cause of sexual dysfunction.6–11,13,14,16 Apart from these drugs, gonadal effects also depend on cumulative doses and combinations that have been applied.26 Given the type of sexual problems reported by HL survivors who received the BEACOPP regimen, in particular, the lack of desire and problems with arousal, hypogonadism probably plays a role.
Furthermore, we found that patients perceiving themselves the presence of sexual problems were associated with them having a lower sexual function measured by the IIEF. This is in line with our previous study on the sexual functioning of young female HL survivors.27
It appears that sexual dysfunction can easily be detected by early screening of sexual problems using just 3 questions. This would allow early specific intervention in the affected domain, as intervention strategies have been shown to minimize sexual dysfunction.5,28 We recommend monitoring these patients for perceiving a sexual problem, to prevent clinical symptoms.29
There are some limitations with the study to be acknowledged. First, many HL survivors have been excluded because of severe physical or mental comorbidity. In retrospect, it would have been better to have informed all patients instead of excluding on own interpretation. Second, our study design does not allow conclusions to be drawn on the evolution of sexual dysfunction over time, especially as early-onset sexual problems may be overlooked because of the 4-year median time between diagnosis and treatment. Moreover, it is possible that the previous treatment is not the sole influencer of the sexual dysfunction. Nevertheless, we feel the strength of our study is the identification and reporting of persistent sexual problems, which can now be addressed; after all, it has been previously reported that an improvement in sexual function is not to be expected for 2 years after hematopoietic stem cell transplantation.30 As late effects on gonadal function due to alkylating agents are present both after transplantation and after treatment for HL lymphoma, a similar pattern is to be expected in this patient population. Another possible criticism is that the control population consisted of paid members of an online panel, so bias might have been induced. However, we expect that men with sexual dysfunction are more willing to participate in a survey as opposed to men without problems, overrating the incidence in the normal population. In addition, we could only match for age, as other factors such as comorbidities, which affect sexual function, could not be corrected for. However, as our survivor population is young with a median age of 38, we suppose such influence is limited. Finally, owing to its introductory nature, this study concerns a relatively small number of participants. Even so, we found a (near) statistical significance. In our opinion, this work creates a baseline for further research and monitoring as we believe that increasing the number of patients will only strengthen our conclusions.
Conclusions
We found that sexual function of male HL survivors, as measured by the IIEF, is comparable to that of matched normal controls. However, the type of treatment seems important; while none of the HL survivors who were treated with ABVD perceived sexual problems, one-third of HL survivors who were treated with BEACOPP did, including erectile dysfunction in one-third of cases. Eliciting perceived sexual problems revealed by using just 3 questions was associated with lower sexual function measured by the IIEF. The use of screening for perceiving sexual problems using a simple tool to identify patients who might have sexual dysfunction should be further explored. The IIEF questionnaire to identify and quantify the nature and extent of sexual problems should be used in cases where patients report issues, permitting early intervention in affected domains and potentially improving quality of life. This is an important consideration for daily clinical practice as BEACOPP is increasingly used as standard therapy in advanced-stage HL.
Statement of authorship
Category 1
Conception and Design
Corien M. Eeltink; Birgit I. Lissenberg-Witte; Luca Incrocci; Irma M. Verdonck-de Leeuw; Sonja Zweegman
Acquisition of Data
Corien M. Eeltink
Analysis and Interpretation of Data
Corien M. Eeltink; Birgit I. Lissenberg-Witte; Luca Incrocci; Annemarie M.J. Braamse; Otto Visser; Josée Zijlstra; Irma M. Verdonck-de Leeuw; Sonja Zweegman
Category 2
Drafting the Article
Corien M. Eeltink; Birgit I. Lissenberg-Witte; Luca Incrocci; Annemarie M.J. Braamse; Otto Visser; Josée Zijlstra; Irma M. Verdonck-de Leeuw; Sonja Zweegman
Revising It for Intellectual Content
Corien M. Eeltink; Birgit I. Lissenberg-Witte; Luca Incrocci; Irma M. Verdonck-de Leeuw; Sonja Zweegman
Category 3
Final Approval of the Completed Article
Corien M. Eeltink; Birgit I. Lissenberg-Witte; Luca Incrocci; Annemarie M.J. Braamse; Otto Visser; Josée Zijlstra; Irma M. Verdonck-de Leeuw; Sonja Zweegman
FundingNone.
Acknowledgments
The authors thank their patients who participated in this study. They also thank Marleen Both for collecting the data and Dr Jack Franklin for the editorial support.
References
Author notes
Conflicts of Interest: The authors report no conflicts of interest.



