-
PDF
- Split View
-
Views
-
Cite
Cite
Smita Pattanaik, Pawan Kaundal, Ravimohan S. Mavuduru, Shrawan K. Singh, Arup K. Mandal, Endothelial Dysfunction in Patients With Erectile Dysfunction: A Double-Blind, Randomized-Control Trial Using Tadalafil, Sexual Medicine, Volume 7, Issue 1, March 2019, Pages 41–47, https://doi.org/10.1016/j.esxm.2018.11.008
Close - Share Icon Share
Abstract
To assess whether tadalafil improves endothelial dysfunction(EnD) in a placebo-controlled randomized-control trial.
Erectile dysfunction and EnD were assessed by the International Index of Erectile Function (IIEF-5) and flow-mediated dilation (FMD) of the brachial artery respectively, at baseline and 4 weeks by blinded observer. Patients with FMD of < 15% were randomized in 1:1 ratio to receive either placebo or tadalafil. Both placebo and tadalafil in similar-appearing capsules but coded separately, were dispensed by a blinded co-investigator. Compliance and drug-related events were recorded. The randomization codes were then decoded and appropriate statistical tests applied.
89 patients were randomized and 82 completed the study. Both groups were comparable. Posttreatment, there were significant improvements in IIEF-5 score (pre- vs posttreatment; tadalafil: 11.432 vs 15.937, P < .001 and placebo 11.232 vs 14.935, P < .00) and FMD% pre- vs posttreatment; tadalafil: 11.222 vs 13.827, P < .001 and placebo: 11.617 vs 14.027, P < .001). Intergroup comparison did not show any significant difference in IIEF scores (mean change in tadalafil vs placebo group: 3.719 vs 4.433, P = .223) and FMD% (mean change tadalafil vs placebo group: 2.426 and 2.829, P = .528). The adverse events were significantly more in the tadalafil group (tadalafil vs placebo 14 adverse reactions [ADR] vs 5 ADR, P < .001).
The response of low-dose tadalafil on IIEF and FMD is largely similar to placebo; however, the utility of FMD% in young patients and placebo effect needs to be studied further.
Introduction
Erectile dysfunction (ED) is defined as the consistent inability to attain and maintain erection of the penis to permit satisfactory sexual intercourse.1 ED has myriad causes but the final common pathway in the majority of subjects with organic ED is endothelial dysfunction (EnD). EnD is physiological dysfunction of normal biochemical processes of the endothelial cells lining the blood vessels of the corpora cavernosa leading to decreased endothelial nitric oxide release and impaired vasodilatation.2 EnD is an important early event of atherosclerotic vascular disease and it has been shown that EnD correlates well with incidence as well as severity of coronary artery disease (CAD).3 Several non-invasive techniques have been devised to assess endothelial dysfunction (EnD). Ultrasonographic assessment of flow-mediated vasodilatation (FMD) of brachial artery, is a reliable, validated, and frequently used non-invasive tool for assessment of endothelial function.4 It has also been recommended as a parameter for evaluation of interventions.5 Impaired brachial artery FMD has been found to be the single best predictor of long-term cardiovascular adverse events in young men with no apparent cardiovascular risk factors.3,6 Several studies have suggested that ED could be the forerunner of cardiovascular disease and impaired brachial artery FMD could be the first manifestation of onset of ED.7–9
Phosphodiesterase inhibitors (PDEI) are used for the symptomatic treatment of ED and administered “as and when required basis.” There have been several studies suggesting that continuous use of phosphodiesterase type 5 inhibitors may actually improve EnD in subjects with ED using varied methods.10–13 Based on evidence, it has been hypothesized that chronic use of PDEI may be beneficial for ED subjects.10 2 studies have demonstrated that EnD as measured by brachial artery FMD improved significantly with daily use of tadalafil over 4–6 weeks14,15 and conversely there are studies with contrasting findings suggesting that the effect of tadalafil on markers of EnD is modest.16–18 Considering the limitations of the earlier reported studies, this study was conducted to evaluate the effect of tadalafil on brachial artery FMD.
Methods
Study Design
This was a prospective randomized double-blind placebo-controlled trial conducted in the outpatient setting. Ethical approval was obtained from the Institutional Ethics Committee before beginning. The study was conducted in accordance with the principles of the Declaration of Helsinki and the Indian Council of Medical Research guidelines on biomedical research.
Patient Selection
Subjects between the ages of 18 and 60 years, presenting to Men’s Health Clinic with complaints of ED, were screened. A written informed consent was obtained from all the participants. The routine tests at our center included thyroid function test, tests for gonadal function, fasting blood glucose, and fasting lipid profile. Based on history, physical examination, and routine investigation participants with psychogenic and hormonal causes were excluded. Those with impaired glucose tolerance test, diabetes mellitus, hypertension, and deranged lipid profile were also excluded. Participants having history of intake of tadalafil or similar drugs (other PDEI) in the past were also excluded from the study. Younger subjects (< age 40 years) were first evaluated by a psychiatrist for psychogenic cause of ED. Only after clearance by the psychiatrist were the subjects included in the study.
Study Conduct and Follow-Up
After obtaining written informed consent, detailed history and focused physical examinations were performed. The erectile function was assessed by the International Index of Erectile Function (IIEF-5) questionnaire and the brachial FMD was evaluated by high-resolution ultrasonography at baseline. Participants were randomized in 1:1 ratio to receive either tadalafil 10 mg or placebo once daily by a computer-generated pseudo-random number list. Both placebo and tadalafil were similar-looking tablets and were dispensed in similar-appearing bottles with dosage for 4 weeks. A co-investigator who was not involved in the process of selection of the subjects or evaluation of the end points dispensed the study drug. The participants were encouraged to perform sexual acts at least once a week.
The primary outcome in this study was the effect of tadalafil on brachial artery FMD. The secondary outcomes were effect on erectile function and drug-related adverse events. All participants were assessed for compliance and any drug-related adverse events. The patient-reported compliance to drug intake was relied upon. Each patient was given a compliance assessment card and was instructed to mark the days on which the drug was taken. The empty drug bottles were also checked for compliance and intake of ≥ 80% was considered adequate. They were asked to return or contact by telephone at 2 weeks for any issues related to the drug. The final evaluation of erectile function and brachial FMD was done at the study closure (4 weeks). Both the subjects and the investigator evaluating IIEF-5 and FMD remained blinded to the treatment throughout the study period. The randomization codes were decoded only after completion the study (ie, after the last enrolled patient completed 4 weeks of treatment).
Method of FMD Assessment
FMD of the brachial artery was performed by 1 investigator throughout the course of study who was masked to the study intervention. It was estimated using high resolution ultrasound 7 MHz. Participants fasted for at least 8–12 hours before the study and all vasoactive medications were withheld for at least 4 half-lives. They were instructed to abstain from alcohol, caffeine, high-fat foods, and vitamin C or smoking for at least 4–6 hours before the study. The participant was positioned supine and instructed to lie quietly for at least 10 minutes before scan. The right brachial artery was studied in full and imaged 10–15 cm above the antecubital fossa. During image acquisition, anatomic landmarks such as veins and fascial planes were noted to help maintain the same image of the artery throughout the study. The diameter of the brachial artery was measured from longitudinal images in which the lumen-intima interface was visualized on the near (anterior) and far (posterior) walls. Thereafter, arterial occlusion was created by inflation of the cuff to 50 mm Hg above systolic pressure for 5 minutes followed by deflation. The diameter of vessel lumen 1 minute after cuff deflation was used to calculate FMD. FMD was expressed as change in poststimulus diameter as a percentage of the baseline diameter. The higher the FMD% change, the better the EnD.
Statistical Analysis
Brachial artery FMD has been reported to be a highly variable parameter. This study intended to detect the difference between the difference of pre- and posttreatment values of tadalafil and placebo groups. Assuming a true difference in means of post- and preFMD between the tadalafil and the placebo group of 3 units, and a pooled SD of 3 units, the study required a sample size of 39 per group to achieve a power of 90% and at a level of significance of 5%, for declaring that the test drug is superior to the active control drug at 5 units margin of superiority. The data obtained were expressed as mean ± SD or percentages. Student’s t-test and chi-square test were applied as applicable. Statistical significance was defined as P < .05.
Results
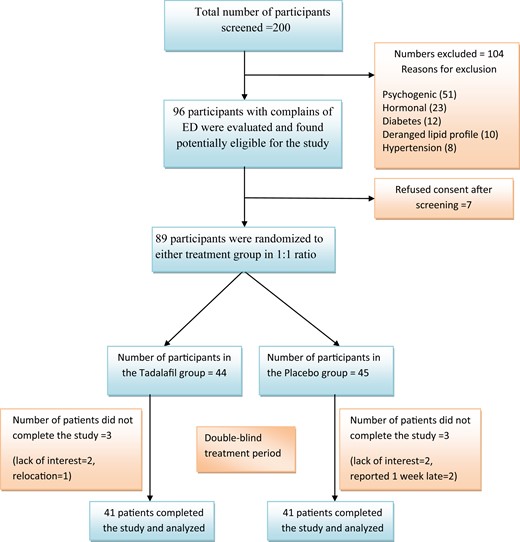
200 subjects with ED were screened during the study period, of which 111 were excluded owing to psychogenic causes (51), hormonal causes (23), impaired glucose tolerance test and diabetes (12), deranged lipid profile (10), hypertension (8), and refusal to participate (7). The flow of patients during the study is shown in Figure 1. A total of 89 participants were randomized to receive tadalafil (n = 44) or placebo (n = 45) for 4 weeks’ duration. The posttreatment data could not be obtained for 3 participants in the tadalafil group and 4 in the placebo group. Hence, 41 participants completed the study in each group.
Demographic Profile
The majority of the participants were young men with a mean age of 31.1 ± 8.9 and 33.3 ± 7.5 years in tadalafil and placebo group, respectively. The baseline FMD and IIEF-5 scores were comparable at baseline. The mean sexual acts per week were 4.8 and 4.9 per week in tadalafil and placebo group, respectively, during the study period. The demographic profile of both groups at baseline is presented in Table 1.
| . | Tadalafil group (n = 41) . | Placebo group (n = 41) . | P value . |
|---|---|---|---|
| Age (y) | 31.3 ± 8.9 | 33.1 ± 7.5 | .36 |
| BMI (kg/m2) | 22.8 ± 3.0 | 23.4 ± 2.19 | .32 |
| Smoking (Yes/No) | 10/41 | 6/41 | .26 |
| Fasting blood sugar (mg/dL) | 95.9 ±14.1 | 92.9 ± 10.2 | .25 |
| Fasting serum TC | 191.5 ± 37.1 | 188.2 ± 46.5 | .72 |
| Serum TG | 169.9 ± 117.4 | 165.1 ± 107.8 | .87 |
| Serum LDL | 121.2 ± 29.5 | 121.9 ± 38.3 | .91 |
| Serum HDL | 44.4 ± 7.6 | 44.6 ± 9.2 | .51 |
| Serum testosterone | 17.1 ± 5.8 | 16.8 ± 5.2 | .86 |
| Duration of ED (y) | 1.8 ± 1.7 | 1.9 ± 1.7 | |
| IIEF score at baseline | 11.4 ± 3 .2 | 11.2 ± 3.1 | .75 |
| ED severity based on IIEF-5 score | |||
| Severe | 5/41 | 5/41 | .73 |
| Moderate | 14/41 | 17/41 | |
| Mild to moderate | 19/41 | 18/41 | |
| Mild | 3/41 | 1/41 | |
| Brachial artery FMD% | 11.0 ± 2.1 | 11.6 ± 1.7 | .17 |
| . | Tadalafil group (n = 41) . | Placebo group (n = 41) . | P value . |
|---|---|---|---|
| Age (y) | 31.3 ± 8.9 | 33.1 ± 7.5 | .36 |
| BMI (kg/m2) | 22.8 ± 3.0 | 23.4 ± 2.19 | .32 |
| Smoking (Yes/No) | 10/41 | 6/41 | .26 |
| Fasting blood sugar (mg/dL) | 95.9 ±14.1 | 92.9 ± 10.2 | .25 |
| Fasting serum TC | 191.5 ± 37.1 | 188.2 ± 46.5 | .72 |
| Serum TG | 169.9 ± 117.4 | 165.1 ± 107.8 | .87 |
| Serum LDL | 121.2 ± 29.5 | 121.9 ± 38.3 | .91 |
| Serum HDL | 44.4 ± 7.6 | 44.6 ± 9.2 | .51 |
| Serum testosterone | 17.1 ± 5.8 | 16.8 ± 5.2 | .86 |
| Duration of ED (y) | 1.8 ± 1.7 | 1.9 ± 1.7 | |
| IIEF score at baseline | 11.4 ± 3 .2 | 11.2 ± 3.1 | .75 |
| ED severity based on IIEF-5 score | |||
| Severe | 5/41 | 5/41 | .73 |
| Moderate | 14/41 | 17/41 | |
| Mild to moderate | 19/41 | 18/41 | |
| Mild | 3/41 | 1/41 | |
| Brachial artery FMD% | 11.0 ± 2.1 | 11.6 ± 1.7 | .17 |
BMI = body mass index; ED = erectile dysfunction; FMD% = percentage change in the flow-mediated dilation of brachial artery; HDL = high-density lipoprotein; IIEF-5 (International Index of Erectile Function) score; 5–7 = severe; 8–11 = moderate; 12–16 = mild to moderate; 17–21 = mild ED; LDL = low-density lipoprotein; TC = total cholesterol; TG = triglycerides.
| . | Tadalafil group (n = 41) . | Placebo group (n = 41) . | P value . |
|---|---|---|---|
| Age (y) | 31.3 ± 8.9 | 33.1 ± 7.5 | .36 |
| BMI (kg/m2) | 22.8 ± 3.0 | 23.4 ± 2.19 | .32 |
| Smoking (Yes/No) | 10/41 | 6/41 | .26 |
| Fasting blood sugar (mg/dL) | 95.9 ±14.1 | 92.9 ± 10.2 | .25 |
| Fasting serum TC | 191.5 ± 37.1 | 188.2 ± 46.5 | .72 |
| Serum TG | 169.9 ± 117.4 | 165.1 ± 107.8 | .87 |
| Serum LDL | 121.2 ± 29.5 | 121.9 ± 38.3 | .91 |
| Serum HDL | 44.4 ± 7.6 | 44.6 ± 9.2 | .51 |
| Serum testosterone | 17.1 ± 5.8 | 16.8 ± 5.2 | .86 |
| Duration of ED (y) | 1.8 ± 1.7 | 1.9 ± 1.7 | |
| IIEF score at baseline | 11.4 ± 3 .2 | 11.2 ± 3.1 | .75 |
| ED severity based on IIEF-5 score | |||
| Severe | 5/41 | 5/41 | .73 |
| Moderate | 14/41 | 17/41 | |
| Mild to moderate | 19/41 | 18/41 | |
| Mild | 3/41 | 1/41 | |
| Brachial artery FMD% | 11.0 ± 2.1 | 11.6 ± 1.7 | .17 |
| . | Tadalafil group (n = 41) . | Placebo group (n = 41) . | P value . |
|---|---|---|---|
| Age (y) | 31.3 ± 8.9 | 33.1 ± 7.5 | .36 |
| BMI (kg/m2) | 22.8 ± 3.0 | 23.4 ± 2.19 | .32 |
| Smoking (Yes/No) | 10/41 | 6/41 | .26 |
| Fasting blood sugar (mg/dL) | 95.9 ±14.1 | 92.9 ± 10.2 | .25 |
| Fasting serum TC | 191.5 ± 37.1 | 188.2 ± 46.5 | .72 |
| Serum TG | 169.9 ± 117.4 | 165.1 ± 107.8 | .87 |
| Serum LDL | 121.2 ± 29.5 | 121.9 ± 38.3 | .91 |
| Serum HDL | 44.4 ± 7.6 | 44.6 ± 9.2 | .51 |
| Serum testosterone | 17.1 ± 5.8 | 16.8 ± 5.2 | .86 |
| Duration of ED (y) | 1.8 ± 1.7 | 1.9 ± 1.7 | |
| IIEF score at baseline | 11.4 ± 3 .2 | 11.2 ± 3.1 | .75 |
| ED severity based on IIEF-5 score | |||
| Severe | 5/41 | 5/41 | .73 |
| Moderate | 14/41 | 17/41 | |
| Mild to moderate | 19/41 | 18/41 | |
| Mild | 3/41 | 1/41 | |
| Brachial artery FMD% | 11.0 ± 2.1 | 11.6 ± 1.7 | .17 |
BMI = body mass index; ED = erectile dysfunction; FMD% = percentage change in the flow-mediated dilation of brachial artery; HDL = high-density lipoprotein; IIEF-5 (International Index of Erectile Function) score; 5–7 = severe; 8–11 = moderate; 12–16 = mild to moderate; 17–21 = mild ED; LDL = low-density lipoprotein; TC = total cholesterol; TG = triglycerides.
Effect of Treatment of Brachial Artery FMD
The mean pre- and posttreatment brachial artery FMD in the tadalafil group were 11.0 ± 2.1 and 13.8 ± 2.7%. There was a significant difference (P = .00) between the pre- and posttreatment values. Similarly, there was also a significant difference between the pre- and posttreatment FMD in the placebo group (11.6 ± 1.7 vs 14.0 ± 2.7, P = .00). There was no statistical difference between tadalafil and placebo arm with respect to brachial artery FMD at 4 weeks (P = .53) and mean change in the FMD with treatment were 2.8 ± 2.8 and 2.4 ± 2.6 in the tadalafil and placebo group, respectively (Table 2).
| Treatment group . | Pretreatment . | Posttreatment . | Change from baseline . | P value (comparison between the groups) . |
|---|---|---|---|---|
| Brachial FMD% . | ||||
| Tadalafil (n = 41) | 11.2 ± 2.1 | 13.8 ± 2.7 | 2.8 ± 2.8 | .53 |
| Placebo (n = 41) | 11.6 ± 1.7 | 14.0 ± 2.7 | 2.4 ± 2.6 | |
| IIEF-5 score | ||||
| Tadalafil (n = 41) | 11.4 ± 3 .2 | 15.9 ± 3.7 | 4.5 ± 3.2 | .22 |
| Placebo (n = 41) | 11.2 ± 3.1 | 14.9 ± 3.5 | 3.7 ± 1.9 | |
| Treatment group . | Pretreatment . | Posttreatment . | Change from baseline . | P value (comparison between the groups) . |
|---|---|---|---|---|
| Brachial FMD% . | ||||
| Tadalafil (n = 41) | 11.2 ± 2.1 | 13.8 ± 2.7 | 2.8 ± 2.8 | .53 |
| Placebo (n = 41) | 11.6 ± 1.7 | 14.0 ± 2.7 | 2.4 ± 2.6 | |
| IIEF-5 score | ||||
| Tadalafil (n = 41) | 11.4 ± 3 .2 | 15.9 ± 3.7 | 4.5 ± 3.2 | .22 |
| Placebo (n = 41) | 11.2 ± 3.1 | 14.9 ± 3.5 | 3.7 ± 1.9 | |
FMD% = percentage change in the flow-mediated dilation of brachial artery; IIEF = International Index of Erectile Function.
| Treatment group . | Pretreatment . | Posttreatment . | Change from baseline . | P value (comparison between the groups) . |
|---|---|---|---|---|
| Brachial FMD% . | ||||
| Tadalafil (n = 41) | 11.2 ± 2.1 | 13.8 ± 2.7 | 2.8 ± 2.8 | .53 |
| Placebo (n = 41) | 11.6 ± 1.7 | 14.0 ± 2.7 | 2.4 ± 2.6 | |
| IIEF-5 score | ||||
| Tadalafil (n = 41) | 11.4 ± 3 .2 | 15.9 ± 3.7 | 4.5 ± 3.2 | .22 |
| Placebo (n = 41) | 11.2 ± 3.1 | 14.9 ± 3.5 | 3.7 ± 1.9 | |
| Treatment group . | Pretreatment . | Posttreatment . | Change from baseline . | P value (comparison between the groups) . |
|---|---|---|---|---|
| Brachial FMD% . | ||||
| Tadalafil (n = 41) | 11.2 ± 2.1 | 13.8 ± 2.7 | 2.8 ± 2.8 | .53 |
| Placebo (n = 41) | 11.6 ± 1.7 | 14.0 ± 2.7 | 2.4 ± 2.6 | |
| IIEF-5 score | ||||
| Tadalafil (n = 41) | 11.4 ± 3 .2 | 15.9 ± 3.7 | 4.5 ± 3.2 | .22 |
| Placebo (n = 41) | 11.2 ± 3.1 | 14.9 ± 3.5 | 3.7 ± 1.9 | |
FMD% = percentage change in the flow-mediated dilation of brachial artery; IIEF = International Index of Erectile Function.
Effect of Treatment in Erectile Function
Tadalafil 10 mg per day treatment significantly improved the mean posttreatment IIEF-5 score compared with the mean pretreatment score (15.9 ± 3.7 vs 11.4 ± 3.7, P = .00). The posttreatment IIEF-5 score also showed significant improvement than the mean pretreatment IIEF-5 score (14.9 ± 3.5 vs 11.2 ± 3.3, P = .001) in the placebo group. However, the extent of improvements in scores was similar between the groups and there was no difference obtained between tadalafil and placebo (Table 2).
IIEF-5 Responders
Considering the minimally important clinical differences (MICD) for response as 4-point improvement in the IIEF-5 scores, those participants who had a posttreatment increase in IIEF-5 score of > 4 were defined as “responders.” In the tadalafil group, 27 subjects were responders and 14 were non-responders. Interestingly, in placebo group, 24 subjects were found to be responders and only 17 were non-responders. Therefore, there was no difference between the proportion of responders and non-responders between tadalafil and placebo groups (P = .32) (Table 3).
Comparing IIEF-5 responders and non-responders within and between the groups
| Treatment group . | Response . | Number of participants . | Change in FMD% (mean ± SD) . | Within-group comparison (P value) . | Between-group comparison (P value) . |
|---|---|---|---|---|---|
| Tadalafil (n = 41) | Yes | 27 (65.8%) | 3.7 ± 2.2 | Responder vs non-responder in tadalafil group, P = .003 | Responders in tadalafil vs placebo group, P = .31 |
| No | 14 (34.2%) | 1.0 ± 3.1 | |||
| Placebo (n = 41) | Yes | 24 (58.5%) | 3.8 ± 2.4 | Responder vs non-responder in tadalafil group, P = .004 | Non-responders in tadalafil vs placebo group, P = .31 |
| No | 17 (41.5%) | 1.4 ± 2.3 |
| Treatment group . | Response . | Number of participants . | Change in FMD% (mean ± SD) . | Within-group comparison (P value) . | Between-group comparison (P value) . |
|---|---|---|---|---|---|
| Tadalafil (n = 41) | Yes | 27 (65.8%) | 3.7 ± 2.2 | Responder vs non-responder in tadalafil group, P = .003 | Responders in tadalafil vs placebo group, P = .31 |
| No | 14 (34.2%) | 1.0 ± 3.1 | |||
| Placebo (n = 41) | Yes | 24 (58.5%) | 3.8 ± 2.4 | Responder vs non-responder in tadalafil group, P = .004 | Non-responders in tadalafil vs placebo group, P = .31 |
| No | 17 (41.5%) | 1.4 ± 2.3 |
FMD% = change in flow-mediated dilatation; IIEF-5 score = International Index of Erectile Function questionnaire score.
Comparing IIEF-5 responders and non-responders within and between the groups
| Treatment group . | Response . | Number of participants . | Change in FMD% (mean ± SD) . | Within-group comparison (P value) . | Between-group comparison (P value) . |
|---|---|---|---|---|---|
| Tadalafil (n = 41) | Yes | 27 (65.8%) | 3.7 ± 2.2 | Responder vs non-responder in tadalafil group, P = .003 | Responders in tadalafil vs placebo group, P = .31 |
| No | 14 (34.2%) | 1.0 ± 3.1 | |||
| Placebo (n = 41) | Yes | 24 (58.5%) | 3.8 ± 2.4 | Responder vs non-responder in tadalafil group, P = .004 | Non-responders in tadalafil vs placebo group, P = .31 |
| No | 17 (41.5%) | 1.4 ± 2.3 |
| Treatment group . | Response . | Number of participants . | Change in FMD% (mean ± SD) . | Within-group comparison (P value) . | Between-group comparison (P value) . |
|---|---|---|---|---|---|
| Tadalafil (n = 41) | Yes | 27 (65.8%) | 3.7 ± 2.2 | Responder vs non-responder in tadalafil group, P = .003 | Responders in tadalafil vs placebo group, P = .31 |
| No | 14 (34.2%) | 1.0 ± 3.1 | |||
| Placebo (n = 41) | Yes | 24 (58.5%) | 3.8 ± 2.4 | Responder vs non-responder in tadalafil group, P = .004 | Non-responders in tadalafil vs placebo group, P = .31 |
| No | 17 (41.5%) | 1.4 ± 2.3 |
FMD% = change in flow-mediated dilatation; IIEF-5 score = International Index of Erectile Function questionnaire score.
Change in FMD in IIEF-5 Responders
We looked at the FMD% responses in the participants in both the groups per their IIEF-5 response status. The mean change in brachial artery FMD of IIEF-5 responders was significantly better after treatment than those of IIEF-5 non-responders. For the tadalafil group, the FMD changes were 3.7 ± 2.2 in responders compared with 1.0 ± 3.1 in non-responders, P = .003. Similarly, in the placebo group, the FMD changes were 3.8 ± 2.4 vs 1.4 ± 2.3 for responders and non-responders respectively, P = .004. FMD responses were similar for the responders (P = .31) and non-responders (P = .54) comparing the tadalafil and placebo groups.
FMD Response and ED Severity
The severity of ED and the mean baseline FMD in each category in both the treatment groups were similar and there was no difference between tadalafil and placebo groups (Tables 1 and 4). The change in FMD between the tadalafil and placebo group for each ED severity category was compared. There was significant difference observed between the drug and placebo group for severe ED (P = .02), whereas the categories viz. mild, mild to moderate, and moderate, the change in FMD was not significant (P = .26, P = .91, and P = .92) (Table 4).
Adverse Events
All the reported adverse effects (AE) were mild in nature and the total number of AE reported in the tadalafil group (14) were more than in the placebo group (5). Headache was the most frequently noted side effect seen in 13 subjects (10 participants in tadalafil and 3 in the placebo group). 3 had dyspeptic symptoms (2 in tadalafil and 1 in placebo group). 2 participants complained of loin pain and both belonged to tadalafil group. The occurrence of AE was significant in the tadalafil group compared with the placebo group (P = .034). However, none of the participants discontinued the medication caused by the AE. Comparing both the groups, the occurrence of AE had no bearing with the IIEF-5 response.
Discussion
EnD, the final common pathway for manifestation of ED, has been a fertile area of research in the recent years. However, the evidence so far has been conflicting.10,11,13–16 Studies have also suggested that low-dose daily tadalafil is safe and efficacious, thereby providing a steady-state level of drug for its beneficial effect on ED; also, such a measure could actually improve EnD.12 We used a robust study design comparing the effect of administration of tadalafil and placebo for their effect on EnD using brachial artery FMD. Our study found that there is no difference between tadalafil and placebo. Rosano et al14 in 2005 published a study that suggested that chronic treatment with tadalafil (20 mg every alternate day for 4 weeks) improved the brachial artery FMD by about 5% compared with no improvement in the placebo group. This study was conducted on 32 men who had an increased cardiovascular risk (with >2 risk factors for CAD, resulting in a 10-year cardiovascular risk >20%), only 50% of them had complained of ED and the blinding of treatment assignment and FMD evaluation was not described. We used a randomized double-blind design with optimal sample size to investigate whether tadalafil was superior to placebo. However, the participants in our study were young men with no cardiovascular risk but only complained of ED. Although our inclusion criteria were broad for age up to 60 years, the majority of our participants were young. This could be because of the reluctance in the older men in our setting to seek medical help for ED owing to cultural beliefs and practices or men in the older age group were excluded because of the presence of cardiovascular risk factors. Nevertheless, the baseline FMD was similar in both the groups and we did not find tadalafil to improve the brachial FMD significantly compared with placebo. Although our study falls in line with the other studies by Pecilline et al16 that evaluated the markers of endothelial repair and the integrated analysis by Prost et al18 suggesting no effect of tadalafil on EnD, both the studies did not use FMD for assessments.
The participant population in our study is an important group for study with emerging reports of subclinical EnD owing to low-grade endothelial inflammation presenting as ED in young Asian men with no apparent cardiovascular risk.19 Moreover, it has also been shown that brachial artery FMD is significantly impaired in young men with ED and no other clinical cardiovascular disease compared with men without ED.20
A significant placebo response was observed in our study and the improvement in erectile function was similar in both tadalafil and placebo groups. The number of responders to the drug was significantly higher in the tadalafil treatment group correlating to the pharmacologic profile of the drug, and the response to the placebo was also considerably high. Placebo response is not unusual for treatment of ED and has been well documented.21 The placebo response in our study could be explained by the “Howthorne effect,” which is the participant expectation that one had a 50% likelihood of getting assigned to active drug tadalafil in the study, which resulted in improvement in erectile function. Moreover, it has also been shown that the odds of placebo response in ED is >7 times higher in men <45 years of age.22 Because our study population was comprised of young men, the robust response to placebo is well explained in this context. In the study by Ajuro et al,21 the magnitude of placebo response ranged from about 31% to 39%, but in our study it was found to be about 78%.
It was interesting to note that FMD improvement was significantly higher in the IIEF-5 responders compared with non-responders in either of the treatment groups. Although the variability of this parameter in the population is large, the reproducibility of FMD has been found to be good provided the standard operating procedures are employed.23 The interobserver variability in our study was negligible because 1 investigator who was blinded to the treatment assignment did the measurements. Therefore, the effect observed in our study is a true effect. It is possible that subjects with improved erections are physically more active and may have had better improvements in FMD. We did not assess the physical activity status of participants; hence, it might be a limitation in true interpretation of this observation. In addition, MCID of IIEF-524 has been defined as any 4-point improvement; however, the similar MCID for FMD is not defined. This further limits the interpretation of results. Nevertheless, ours is the first study reporting the effect of placebo on FMD.
The mean change in brachial artery FMD in participants with severe ED from baseline was significantly higher in the tadalafil group compared with the placebo group and change in IIEF-5 scores in this group also had a trend toward significant difference although the baseline FMD and IIEF-5 score in both the groups were similar (Table 4). The changes in IIEF-5 score and FMD were not significant in any other ED severity group. This finding implies that EnD is higher in severe grades of ED and that there is a possible true role of tadalafil in improving EnD over placebo. In mild grades of ED, the EnD may not be severe enough to benefit from treatment of active drug; hence, there is a similar response in both active drug and the placebo. However, this study was not specifically designed to answer the effect on FMD in severe ED, and this hypothesis needs further validation.
| ED severity . | Group . | N . | Baseline IIEF-5 . | Change in IIEF-5 . | P value . | Baseline FMD% . | Change in FMD% . | P value . |
|---|---|---|---|---|---|---|---|---|
| Severe | Tadalafil | 5 | 6.2 ± 1.2 | 6.4 ± 3.1 | .06 | 9.7 ± 1.8 | 4.6 ± 1.9 | .02 |
| Placebo | 5 | 6.6 ± 1.6 | 3.2 ±1.5 | 11.3 ± 1.6 | 1.6 ± 1.4 | |||
| Moderate | Tadalafil | 14 | 9.6 ± 3.1 | 4.5 ± 2.6 | .74 | 10.6 ± 1.9 | 3.3 ± 3.6 | .92 |
| Placebo | 17 | 9.5 ± 2.1 | 4.2 ± 2.1 | 11.5 ± 1.6 | 3.4 ± 3.3 | |||
| Mild to moderate | Tadalafil | 19 | 13.3 ± 2.1 | 4.5 ± 3.4 | .24 | 11.9 ± 2.3 | 1.9 ± 2.8 | .91 |
| Placebo | 18 | 13.7 ± 1.5 | 3.4 ± 1.9 | 11.5 ± 1.6 | 1.8 ± 2.0 | |||
| Mild | Tadalafil | 3 | 17.3 ± 2.3 | 0.7 ± 3.5 | .62 | 9.7 ± 1.3 | 2.8 ± 1.8 | .26 |
| Placebo | 1 | 13.9 | 3.0 | 20.0 | 0.4 |
| ED severity . | Group . | N . | Baseline IIEF-5 . | Change in IIEF-5 . | P value . | Baseline FMD% . | Change in FMD% . | P value . |
|---|---|---|---|---|---|---|---|---|
| Severe | Tadalafil | 5 | 6.2 ± 1.2 | 6.4 ± 3.1 | .06 | 9.7 ± 1.8 | 4.6 ± 1.9 | .02 |
| Placebo | 5 | 6.6 ± 1.6 | 3.2 ±1.5 | 11.3 ± 1.6 | 1.6 ± 1.4 | |||
| Moderate | Tadalafil | 14 | 9.6 ± 3.1 | 4.5 ± 2.6 | .74 | 10.6 ± 1.9 | 3.3 ± 3.6 | .92 |
| Placebo | 17 | 9.5 ± 2.1 | 4.2 ± 2.1 | 11.5 ± 1.6 | 3.4 ± 3.3 | |||
| Mild to moderate | Tadalafil | 19 | 13.3 ± 2.1 | 4.5 ± 3.4 | .24 | 11.9 ± 2.3 | 1.9 ± 2.8 | .91 |
| Placebo | 18 | 13.7 ± 1.5 | 3.4 ± 1.9 | 11.5 ± 1.6 | 1.8 ± 2.0 | |||
| Mild | Tadalafil | 3 | 17.3 ± 2.3 | 0.7 ± 3.5 | .62 | 9.7 ± 1.3 | 2.8 ± 1.8 | .26 |
| Placebo | 1 | 13.9 | 3.0 | 20.0 | 0.4 |
ED = erectile dysfunction; FMD% = percent change in flow-mediated dilatation expressed as mean ± SD; IIEF 5 score = International Index of Erectile Function questionnaire score; 5–7 = severe; 8–11 = moderate; 12–16 = mild to moderate; 17–21 = mild ED.
| ED severity . | Group . | N . | Baseline IIEF-5 . | Change in IIEF-5 . | P value . | Baseline FMD% . | Change in FMD% . | P value . |
|---|---|---|---|---|---|---|---|---|
| Severe | Tadalafil | 5 | 6.2 ± 1.2 | 6.4 ± 3.1 | .06 | 9.7 ± 1.8 | 4.6 ± 1.9 | .02 |
| Placebo | 5 | 6.6 ± 1.6 | 3.2 ±1.5 | 11.3 ± 1.6 | 1.6 ± 1.4 | |||
| Moderate | Tadalafil | 14 | 9.6 ± 3.1 | 4.5 ± 2.6 | .74 | 10.6 ± 1.9 | 3.3 ± 3.6 | .92 |
| Placebo | 17 | 9.5 ± 2.1 | 4.2 ± 2.1 | 11.5 ± 1.6 | 3.4 ± 3.3 | |||
| Mild to moderate | Tadalafil | 19 | 13.3 ± 2.1 | 4.5 ± 3.4 | .24 | 11.9 ± 2.3 | 1.9 ± 2.8 | .91 |
| Placebo | 18 | 13.7 ± 1.5 | 3.4 ± 1.9 | 11.5 ± 1.6 | 1.8 ± 2.0 | |||
| Mild | Tadalafil | 3 | 17.3 ± 2.3 | 0.7 ± 3.5 | .62 | 9.7 ± 1.3 | 2.8 ± 1.8 | .26 |
| Placebo | 1 | 13.9 | 3.0 | 20.0 | 0.4 |
| ED severity . | Group . | N . | Baseline IIEF-5 . | Change in IIEF-5 . | P value . | Baseline FMD% . | Change in FMD% . | P value . |
|---|---|---|---|---|---|---|---|---|
| Severe | Tadalafil | 5 | 6.2 ± 1.2 | 6.4 ± 3.1 | .06 | 9.7 ± 1.8 | 4.6 ± 1.9 | .02 |
| Placebo | 5 | 6.6 ± 1.6 | 3.2 ±1.5 | 11.3 ± 1.6 | 1.6 ± 1.4 | |||
| Moderate | Tadalafil | 14 | 9.6 ± 3.1 | 4.5 ± 2.6 | .74 | 10.6 ± 1.9 | 3.3 ± 3.6 | .92 |
| Placebo | 17 | 9.5 ± 2.1 | 4.2 ± 2.1 | 11.5 ± 1.6 | 3.4 ± 3.3 | |||
| Mild to moderate | Tadalafil | 19 | 13.3 ± 2.1 | 4.5 ± 3.4 | .24 | 11.9 ± 2.3 | 1.9 ± 2.8 | .91 |
| Placebo | 18 | 13.7 ± 1.5 | 3.4 ± 1.9 | 11.5 ± 1.6 | 1.8 ± 2.0 | |||
| Mild | Tadalafil | 3 | 17.3 ± 2.3 | 0.7 ± 3.5 | .62 | 9.7 ± 1.3 | 2.8 ± 1.8 | .26 |
| Placebo | 1 | 13.9 | 3.0 | 20.0 | 0.4 |
ED = erectile dysfunction; FMD% = percent change in flow-mediated dilatation expressed as mean ± SD; IIEF 5 score = International Index of Erectile Function questionnaire score; 5–7 = severe; 8–11 = moderate; 12–16 = mild to moderate; 17–21 = mild ED.
There were mild side effects in the tadalafil group and more than in the placebo group. Although there was a significantly higher number of treatment-emergent side effects in the tadalafil group, largely both medications were well tolerated without any drop-outs owing to side effects.
Limitations
First, predominant inclusion of young individuals with ED.
Higher baseline FMD response in the participants even before treatment. The reported FMD in a normal man is 8.9 ± 3.1% (range 0.6–17.2) as reported by Adams et al25 and 7.8 ± 3.1 (range 1.6 to 14.0) as reported by Dallia et al.26 In ED patients, the FMD was reported to be lower by an average of 2.0; however, there is a good overlap of values among normal and ED subjects between the studies. In our study, the FMD was found to be 11 ± 2.1 and 11.6 ± 1.7 in both, which overlaps within the ranges reported by the earlier studies.
Unexplained and placebo response to FMD.
Conclusion
We conclude that the response of low-dose, once-daily tadalafil (10 mg per day) over a short-term period (4 weeks) on IIEF and FMD is largely similar to placebo in younger subjects without any comorbidities, except those with severe ED. However, to draw meaningful conclusions, further studies are warranted.
Funding
None.
Statement of Authorship
Category 1
Conception and Design
Pawan Kaundal; Ravimohan S. Mavuduru; Smita Pattanik
Acquisition of Data
Pawan Kaundal; Ravimohan S. Mavuduru; Smita Pattanik
Analysis and Interpretation of Data
Pawan Kaundal; Ravimohan S. Mavuduru; Smita Pattanik
Category 2
Drafting the Article
Smita Pattanik; Ravimohan S. Mavuduru
Revising It for Intellectual Content
Shrawan K. Singh; Arup K. Mandal; Smita Pattanik; Ravimohan S. Mavuduru
Category 3
Final Approval of the Completed Article
Smita Pattanaik; Pawan Kaundal; Ravimohan S. Mavuduru; Shrawan K. Singh; Arup K. Mandal
References
Author notes
Conflict of Interest: The authors report no conflicts of interest.



