-
PDF
- Split View
-
Views
-
Cite
Cite
Annie C Lajoie, Anne-Louise Lafontaine, R John Kimoff, Andrea Benedetti, Ann R Robinson, Marie Létourneau, Joelle Crane, Amanda Scanga, Francine Noel, Marta Kaminska, Cognition and obstructive sleep apnea in Parkinson’s disease: randomized controlled trial of positive airway pressure, Sleep, 2025;, zsaf038, https://doi.org/10.1093/sleep/zsaf038
Close - Share Icon Share
Abstract
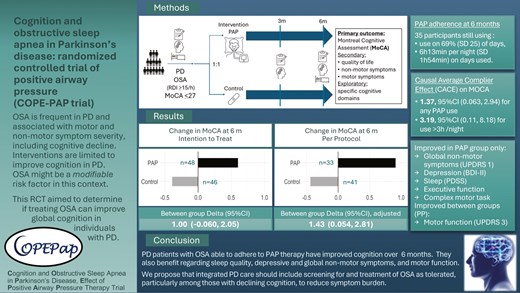
This randomized controlled trial assessed the effects of positive airway pressure (PAP) treatment of obstructive sleep apnea (OSA) on cognition in patients with Parkinson’s disease (PD).
Individuals with PD with Montreal Cognitive Assessment (MoCA) ≤ 27 and OSA were randomized to PAP or nasal dilator strips (placebo) for 6 months. The primary outcome was the change in MoCA from baseline to 6 months compared by t-test between groups by intention to treat (ITT). Sensitivity and per protocol (PP) analyses were performed, adjusting for potential confounders. Secondary outcomes included patient-reported and motor outcomes. Exploratory outcomes comprised detailed neurocognitive tests.
We randomized 94 participants (31% female) with a mean age of 67.3 (standard deviation 10.5) years, body mass index of 28.1 (4.7) kg/m2, and MoCA of 22.7 (3.5). The change in MoCA in the PAP group (n = 48) was 0.60, 95% CI [−0.08, 1.29] and in the control group (n = 46) −0.39, 95% CI [−1.21, 0.43]; between-group difference 1.00, 95% CI [−0.06, 2.05] (ITT). Adjusted ITT analyses showed improved MoCA by 1.44, 95% CI [0.09, 2.79], in treated versus control groups. In PP analyses, the adjusted between-group difference was 1.43, 95% CI [0.054, 2.81] between PAP (n = 33) versus control (n = 41) groups. Nonmotor symptoms, depression and sleep quality scores, and performance on certain executive and psychomotor tasks improved with PAP. PP analyses also showed significant improvement in motor function in PAP compared to control groups.
Evaluation for OSA in PD patients with reduced cognition should be considered as OSA treatment may improve cognitive function, and possibly patient-reported and motor outcomes.
Registered as “Cognition and Obstructive Sleep Apnea in Parkinson’s Disease, Effect of Positive Airway Pressure Therapy (COPE-PAP)” at ClinicalTrials.gov. ID: NCT02209363. https://clinicaltrials.gov/study/NCT02209363?term=kaminska&rank=4.
- body mass index procedure
- parkinson disease
- airway pressure
- obstructive sleep apnea
- cognition
- depressive disorders
- mental processes
- sleep disorders
- unified parkinson's disease rating scale
- cognitive ability
- per protocol analysis
- intention to treat
- neurocognitive testing
- primary outcome measure
- sleep quality
- montreal cognitive assessment
This is the largest and longest randomized controlled trial (RCT) of treatment for obstructive sleep apnea (OSA) in a neurodegenerative condition, namely Parkinson’s disease (PD). PD is the fastest growing neurodegenerative disorder of aging, while sleep and sleep disorders have come into focus as important determinants of brain health. OSA is common in the general population, including older adults, and in PD. Observational data are accumulating that treating OSA can improve cognitive outcomes in aging, and in PD. However, this is the first RCT of positive airway pressure (PAP) therapy over a 6-month period, which shows this therapy is feasible in PD, and suggests benefit with respect to cognition and other outcomes. This has important implications for further research and clinical care of individuals with PD.
Introduction
Obstructive sleep apnea (OSA) is a treatable, highly prevalent sleep disorder in aging individuals and is characterized by recurrent upper airway obstruction with intermittent hypoxemia, sleep fragmentation, and hemodynamic changes. OSA is associated with cognitive impairment in the general population and with cognitive decline in aging [1, 2].
Parkinson’s disease (PD) is the second most frequent neurodegenerative disorder and the most rapidly increasing in prevalence [3]. In addition to the classic motor symptoms, nonmotor symptoms (NMS), including cognitive dysfunction and sleep disorders, greatly impair the quality of life (QoL) of PD patients [4]. Cognitive dysfunction is found in 20–40% of patients with early PD [5]. PD-related dementia ultimately affects up to 80% of patients, leading to a major personal, societal, and health-care burden [6]. Sleep disorders are associated with worsened health-related QoL in PD [7]. OSA affects 20–60% of PD patients and evidence suggests it has deleterious effects on NMS in PD, including cognitive function [8]. Previous work from our group found lower global cognition in PD patients with OSA [9].
Positive airway pressure (PAP) therapy is the recommended first-line treatment for symptomatic OSA. In the general population, response to PAP therapy has been variable and incomplete regarding cognitive function [1]. In older individuals with more severe OSA, improvement in cognition and neuroimaging abnormalities have been demonstrated in a randomized controlled trial (RCT) of PAP therapy [10]. PAP has also been studied in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) with some encouraging results in observational studies [11]. In a prospective observational study in PD patients, our group observed significant improvement in cognitive function and other NMS after 12 months of PAP treatment [12]. The effect on cognitive function was more marked in the subgroup with reduced baseline cognition. RCTs of PAP in neurodegenerative conditions to date have been of short duration and limited sample sizes [13, 14]. PAP therapy nonetheless has been shown to successfully correct OSA, improve sleep quality, and reduce excessive daytime sleepiness in PD patients [15]. Cognitive function, however, did not improve in the single short-term RCT in PD after 3 or 6 weeks of PAP therapy [16].
The primary objective of this study was to assess the effects of 6 months of PAP therapy compared to placebo on global cognition assessed using the MoCA score in PD patients who have OSA and evidence of MCI. The secondary objectives are to assess the effect of 6 months of PAP therapy on MoCA scores in a per protocol (efficacy) analysis and on QoL and other PD NMS. In addition, we explored the effect of PAP on specific domains of neurocognitive function.
Materials and Methods
Trial design
This was a single-blind, parallel group RCT of auto-PAP (APAP, treatment) versus nasal dilator strips (NDS, control) used for 6 months. All testing was performed at sites of a single center—the McGill University Health Centre (MUHC, Glen site, and Montreal Neurological Institute) in Montreal, Canada. The study was approved by the Institutional Research Ethics Board (14-187 GEN). The study has been registered (ClinicalTrials.gov ID: NCT02209363) and the complete protocol has been previously published [17].
Participants
Participants were recruited primarily from the McGill Movement Disorder Clinic, as well as from a general neurology clinic at an affiliated hospital, through the MUHC Sleep Clinic and through the Quebec Parkinson Network. Inclusion criteria were: diagnosis of PD [18], clinical impression of MCI with a Montreal Cognitive Assessment (MoCA) score ≤ 27, OSA with a respiratory disturbance index (RDI) ≥ 15/hour, and adequate knowledge of English or French. Exclusion criteria included active OSA treatment and major comorbidities [17].
Procedures
Participants underwent standard overnight polysomnography (PSG, Nihon Kohden). Standard full PSG channels were recorded, including six EEG channels (C3, C4, F3, F4, O1, and O2), bilateral tibialis anterior EMG and digital video. Scoring was done manually using standard American Academy of Sleep Medicine (AASM) clinical criteria [19]using the recommended 1A hypopnea definition by a certified technologist, with expert physician review. Subjects with a total sleep time <2 hours were convened for a second study to improve the diagnostic accuracy of OSA.
On a separate day, participants underwent an assessment which included questionnaires, the Movement Disorder Society revision of the Unified PD Rating Scale (MDS-UPDRS) and full neurocognitive testing.
Interventions
The active intervention consisted of PAP therapy, which was initiated in auto-PAP mode with subsequent adjustments based on clinical judgment. Patients randomized to APAP therapy were referred to our partner PAP provider who provided specific teaching, follow-up, and troubleshooting of technical issues via standardized protocols, in cooperation with the study staff. About 1 week after the initiation visit with the PAP provider, subjects were contacted by the study sleep nurse for troubleshooting if needed. For patients who remained unable to use APAP, a home visit could be performed by the sleep nurse. A respiratory therapist from the PAP provider company also remained available and in contact with the participants and study staff as needed to optimize therapy. Reports were obtained remotely from a modem attached to the PAP device and reviewed by an expert sleep physician after 1, 3, and 6 months to assess treatment efficacy and adherence, at the time of clinical follow-up.
NDS was used as a placebo. Subjects randomized to NDS were given a labeled supply of strips and participated in a teaching session on their proper use with a research nurse. Participants were called at 1 week to evaluate any issues with NDS. Subjects were also seen by the sleep physician and research nurse at 1, 3, and 6 months, mimicking visits for the APAP group.
Outcomes
The primary outcome measure was the mean change in the MoCA test score from baseline to 6 months. Alternative versions (mocacognition.com) were used at each time point to minimize potential learning effects. Secondary outcomes included changes in QoL and NMS burden. We used the PDQ-39, a validated PD-specific QoL measure. The MDS-UPDRS part 1 subscale (Non-Motor Aspects of Experiences of Daily Living) was the global measure of PD NMS. A higher score implies greater symptoms. Additional outcomes included mood (Beck Depression Inventory II, BDI-II), sleep quality (PD Sleep Scale, PDSS), and daytime sleepiness (Epworth Sleepiness Scale). Exploratory outcome measures consisted of specific domains of cognitive function. Patients underwent a detailed neurocognitive assessment of five domains of cognitive function: attention/working memory, executive function, language, memory, and visuospatial function. Our battery was chosen based on the MDS Task Force recommendations (Supplementary Material). These assessments at baseline and at 6-month follow-up were conducted in-person with the neuropsychologist at the Montreal Neurological Hospital. In exceptional circumstances (primarily related to the COVID-19 pandemic), certain 3 months assessments could be conducted remotely.
Sample size
In our pilot observational work, the change in MoCA score with 6 months of PAP treatment was 2.5 (standard deviation [SD] 2.4), whereas the change in those untreated for OSA (with or without OSA) was 0.14 (SD 2.6), yielding a between-group difference of 2.36. Our sample size was calculated using a conservative estimate of 2.2, SD for change of 2.7, and assuming intention to treat. Using 1:1 randomization, we would need 25 subjects per group (α = 0.05, power 80%). To account for 20% noncompliance and 5% loss to follow-up, the adjusted sample size was 90, or 45 per group.
Randomization and masking
Randomization was obtained through the database software (Dacima Software Inc.) after inclusion/exclusion data and data for stratification were entered. Blocked randomization (permuted blocks, size 4 and 6) was performed, stratified by severity of cognitive dysfunction (MoCA < 24 vs. 24–26 vs. 27) to avoid imbalance between groups. Treatment allocation was communicated by the software directly to the unblinded research nurse, who arranged treatment initiation.
Complete subject blinding was not possible in this study, but subjects were not aware that NDS is a placebo, as they were told that two treatments for OSA were being compared. Subjects were asked not to disclose their treatment to the outcome assessor. Outcome assessments (MoCA, MDS-UPDRS, and neurocognitive testing) were performed by a single trained and blinded assessor. The assessor answered a blinding survey. Questionnaires were self-reported by participants.
Statistical methods
The primary outcome (change in MoCA score from baseline to 6 months) was compared between groups using a t-test under intention to treat (ITT); subjects randomized but lost to follow-up were included, with the last measurement carried forward. Similar analyses were done for other outcomes. Measurements were also carried backward in the case of prior missing data for secondary and exploratory outcomes. If there was no follow-up data, baseline missing items were imputed as follows: for MDS-UPDRS according to [20], PDSS items from the mean of other items, for PDQ from the mean of other items of the subscore. Other questionnaires were either complete or entirely missing. Supplementary Table S2 describes the number of questionnaires with imputed data. We estimated the Causal Average Complier Effect for the primary outcome as per [21], with confidence intervals estimated using bootstrapping with R = 1000.
In sensitivity analyses for the primary outcome, we adjusted for potential confounders, determined a priori (age, sex, body mass index [BMI], depression scores, and psychoactive medication change during the course of the trial) and from unbalanced baseline characteristics (hypertension), via linear regression. Finally, a subgroup analysis of patients without psychoactive medication change throughout the study was performed.
In per protocol (efficacy) analyses, we compared outcome measures from baseline to 6 months in subjects who were still using PAP therapy at 6 months (any use) and underwent outcome assessment at 6 months, and control participants who underwent the 6-month assessment, adjusting for confounders as above. Exploratory analyses were conducted to determine the effect of baseline OSA severity and related variables on change in MoCA in the PAP-treated group, per protocol group (Supplementary Material). Analyses were done using R version 4.0.3 (R Foundation).
Role of the funding source
The funder had no role in study design, in the collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Results
Participants
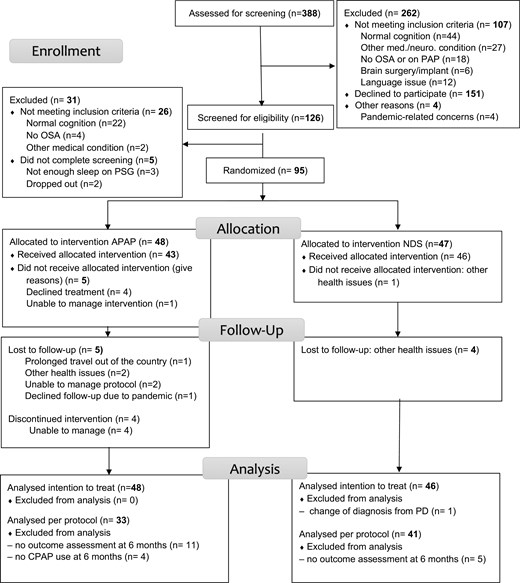
A total of 48 participants were randomized to the treatment arm and 47 to the control arm (CONSORT diagram in Figure 1). All were analyzed in ITT except one participant in the control arm whose diagnosis was changed from PD. Per protocol, analyses were performed on 33 participants in the treatment arm who still used PAP therapy at 6 months and who completed the assessment at that timepoint, and 41 in the control group. Baseline characteristics are shown in Table 1 (and Supplementary Table S1) and were generally similar between study groups, though the control group had a greater proportion of females, and had slightly more hypertension.
| Group . | PAP N = 48 . | PAP per protocol N = 33 . | Control N = 46 . | Control per protocol N = 41 . |
|---|---|---|---|---|
| Age (years) | 66.0 (10.4) | 64.0 (11.1) | 68.8 (10.4) | 69.1 (10.2) |
| Sex male, n (%) | 37 (77) | 23 (70) | 28 (61) | 25 (61) |
| BMI (kg/m2) | 27.4 (4.7) | 27.8 (5.5) | 28.8 (4.6) | 28.9 (4.7) |
| Level of education | ||||
| No high school diploma, n (%) | 5 (10.4) | 4 (12.1) | 3 (6.5) | 2 (4.9) |
| High school diploma, n (%) | 9 (18.8) | 6 (18.2) | 9 (19.6) | 6 (14.6) |
| Technical degree, n (%) | 8 (16.7) | 4 (12.1) | 7 (15.2) | 6 (14.6) |
| Bachelor’s degree, n (%) | 13 (27.1) | 9 (27.3) | 18 (39.1) | 18 (43.9) |
| Post-graduate degree, n (%) | 13 (27.1) | 10 (30.3) | 9 (19.6) | 9 (22) |
| Living with partner, n (%) | 35 (72.9) | 24 (72.7) | 26 (56.5) | 22 (53.7) |
| Currently smoking, n (%) | 4 (8.3) | 2 (6.1) | 4 (8.7) | 4 (9.8) |
| Smoking history (pack-years) | 8 (15.9) | 7.3 (16.9) | 8.9 (17.1) | 9.4 (18) |
| Alcohol consumption (drinks/week) | 2.6 (4.7) | 2.3 (3.9) | 3.2 (6.2) | 3.4 (6.5) |
| Age at PD diagnosis (years) | 59.5 (9.9) | 58.3 (10.3) | 62.0 (12.9) | 62.3 (13.1) |
| LED (mg) | 673.1 (393.2) | 666.3 (373.2) | 702.1 (428.5) | 690.8 (448.4) |
| RBD definite*, n (%) | 8 (17) | 5 (15) | 7 (15) | 7 (17) |
| RBD probable†, n (%) | 22 (46) | 14 (42) | 28 (57) | 24 (59) |
| Comorbidities, n (%) | ||||
| Hypertension | 6 (13) | 4 (12) | 11 (24) | 10 (24) |
| Diabetes | 6 (13) | 4 (12) | 5 (11) | 4 (10) |
| Dyslipidemia | 6 (13) | 6 (18) | 8 (17) | 7 (17) |
| Coronary artery disease | 1 (2) | 0 (0) | 4 (9) | 4 (10) |
| Atrial fibrillation | 3 (6) | 0 (0) | 2 (4) | 2 (5) |
| Chronic kidney disease | 0 (0) | 0 (0) | 3 (7) | 3 (7) |
| COPD | 1 (2) | 1 (3) | 0 (0) | 0 (0) |
| Hypothyroidism | 2 (4) | 2 (6) | 0 (0) | 0 (0) |
| Polysomnography results | ||||
| TST (minute) | 319.0 (67.4) | 319.2 (67.1) | 305.7 (66.5) | 308.2 (66.2) |
| Sleep efficiency (%) | 71.8 (15.1) | 72.0 (16.0) | 69.1 (14.3) | 69.4 (13.9) |
| % N1 stage | 27.2 (15.3) | 25.8 (14.1) | 28.7 (14.3) | 28.3 (13.4) |
| % N2 stage | 50.5 (14.0) | 49.4 (13.6) | 53.2 (13.5) | 54.0 (12.6) |
| % N3 stage | 12.2 (13.6) | 13.8 (15.0) | 9.4 (11.3) | 10.0 (11.7) |
| % R stage | 10.1 (7.5) | 11.0 (7.9) | 10.2 (7.5) | 9.5 (6.8) |
| WASO (minute) | 113.9 (73.9) | 112.6 (79.9) | 120.3 (68.3) | 119.9 (65.5) |
| Total arousal index | 56.6 (20.3) | 54.0 (17.4) | 59.1 (25.7) | 57.8 (22.4) |
| Respiratory arousal index | 48.2 (21.8) | 46.7 (18.8) | 49.7 (23.0) | 49.1 (20.9) |
| Spontaneous arousal index | 10.8 (7.2) | 9.9 (6.4) | 11.4 (7.1) | 10.8 (7.0) |
| PLM arousal index | 0.5 (1.3) | 0.6 (1.5) | 1.1 (4.4) | 0.7 (3.6) |
| PLM sleep index | 8.4 (17.4) | 8.3 (19.2) | 3.2 (8.1) | 2.8 (7.4) |
| AHI | 35.1 (20.3) | 34.4 (18.6) | 35.4 (24.8) | 35.8 (24.5) |
| RDI | 47.4 (20.1) | 46.7 (18.2) | 48.6 (20.1) | 48.8 (20.2) |
| ODI | 8.9 (7.4) | 9.5 (8.4) | 12.7 (14.9) | 13.2 (15.4) |
| T90% | 0.3 (0.7) | 0.2 (0.5) | 2.1 (4.1) | 2.3 (4.3) |
| Group . | PAP N = 48 . | PAP per protocol N = 33 . | Control N = 46 . | Control per protocol N = 41 . |
|---|---|---|---|---|
| Age (years) | 66.0 (10.4) | 64.0 (11.1) | 68.8 (10.4) | 69.1 (10.2) |
| Sex male, n (%) | 37 (77) | 23 (70) | 28 (61) | 25 (61) |
| BMI (kg/m2) | 27.4 (4.7) | 27.8 (5.5) | 28.8 (4.6) | 28.9 (4.7) |
| Level of education | ||||
| No high school diploma, n (%) | 5 (10.4) | 4 (12.1) | 3 (6.5) | 2 (4.9) |
| High school diploma, n (%) | 9 (18.8) | 6 (18.2) | 9 (19.6) | 6 (14.6) |
| Technical degree, n (%) | 8 (16.7) | 4 (12.1) | 7 (15.2) | 6 (14.6) |
| Bachelor’s degree, n (%) | 13 (27.1) | 9 (27.3) | 18 (39.1) | 18 (43.9) |
| Post-graduate degree, n (%) | 13 (27.1) | 10 (30.3) | 9 (19.6) | 9 (22) |
| Living with partner, n (%) | 35 (72.9) | 24 (72.7) | 26 (56.5) | 22 (53.7) |
| Currently smoking, n (%) | 4 (8.3) | 2 (6.1) | 4 (8.7) | 4 (9.8) |
| Smoking history (pack-years) | 8 (15.9) | 7.3 (16.9) | 8.9 (17.1) | 9.4 (18) |
| Alcohol consumption (drinks/week) | 2.6 (4.7) | 2.3 (3.9) | 3.2 (6.2) | 3.4 (6.5) |
| Age at PD diagnosis (years) | 59.5 (9.9) | 58.3 (10.3) | 62.0 (12.9) | 62.3 (13.1) |
| LED (mg) | 673.1 (393.2) | 666.3 (373.2) | 702.1 (428.5) | 690.8 (448.4) |
| RBD definite*, n (%) | 8 (17) | 5 (15) | 7 (15) | 7 (17) |
| RBD probable†, n (%) | 22 (46) | 14 (42) | 28 (57) | 24 (59) |
| Comorbidities, n (%) | ||||
| Hypertension | 6 (13) | 4 (12) | 11 (24) | 10 (24) |
| Diabetes | 6 (13) | 4 (12) | 5 (11) | 4 (10) |
| Dyslipidemia | 6 (13) | 6 (18) | 8 (17) | 7 (17) |
| Coronary artery disease | 1 (2) | 0 (0) | 4 (9) | 4 (10) |
| Atrial fibrillation | 3 (6) | 0 (0) | 2 (4) | 2 (5) |
| Chronic kidney disease | 0 (0) | 0 (0) | 3 (7) | 3 (7) |
| COPD | 1 (2) | 1 (3) | 0 (0) | 0 (0) |
| Hypothyroidism | 2 (4) | 2 (6) | 0 (0) | 0 (0) |
| Polysomnography results | ||||
| TST (minute) | 319.0 (67.4) | 319.2 (67.1) | 305.7 (66.5) | 308.2 (66.2) |
| Sleep efficiency (%) | 71.8 (15.1) | 72.0 (16.0) | 69.1 (14.3) | 69.4 (13.9) |
| % N1 stage | 27.2 (15.3) | 25.8 (14.1) | 28.7 (14.3) | 28.3 (13.4) |
| % N2 stage | 50.5 (14.0) | 49.4 (13.6) | 53.2 (13.5) | 54.0 (12.6) |
| % N3 stage | 12.2 (13.6) | 13.8 (15.0) | 9.4 (11.3) | 10.0 (11.7) |
| % R stage | 10.1 (7.5) | 11.0 (7.9) | 10.2 (7.5) | 9.5 (6.8) |
| WASO (minute) | 113.9 (73.9) | 112.6 (79.9) | 120.3 (68.3) | 119.9 (65.5) |
| Total arousal index | 56.6 (20.3) | 54.0 (17.4) | 59.1 (25.7) | 57.8 (22.4) |
| Respiratory arousal index | 48.2 (21.8) | 46.7 (18.8) | 49.7 (23.0) | 49.1 (20.9) |
| Spontaneous arousal index | 10.8 (7.2) | 9.9 (6.4) | 11.4 (7.1) | 10.8 (7.0) |
| PLM arousal index | 0.5 (1.3) | 0.6 (1.5) | 1.1 (4.4) | 0.7 (3.6) |
| PLM sleep index | 8.4 (17.4) | 8.3 (19.2) | 3.2 (8.1) | 2.8 (7.4) |
| AHI | 35.1 (20.3) | 34.4 (18.6) | 35.4 (24.8) | 35.8 (24.5) |
| RDI | 47.4 (20.1) | 46.7 (18.2) | 48.6 (20.1) | 48.8 (20.2) |
| ODI | 8.9 (7.4) | 9.5 (8.4) | 12.7 (14.9) | 13.2 (15.4) |
| T90% | 0.3 (0.7) | 0.2 (0.5) | 2.1 (4.1) | 2.3 (4.3) |
*RBD: questionnaire positive and PSG evidence.
†Not including definite.
Data as mean (SD) unless otherwise specified. Abbreviations: TST, total sleep time; WASO, wake after sleep onset; PLM, periodic leg movements; AHI, apnea-hypopnea index; RDI, respiratory disturbance index; ODI, oxygen desaturation index; T90%, time with oxygen saturation below 90%; LED, levodopa equivalent daily dose.
| Group . | PAP N = 48 . | PAP per protocol N = 33 . | Control N = 46 . | Control per protocol N = 41 . |
|---|---|---|---|---|
| Age (years) | 66.0 (10.4) | 64.0 (11.1) | 68.8 (10.4) | 69.1 (10.2) |
| Sex male, n (%) | 37 (77) | 23 (70) | 28 (61) | 25 (61) |
| BMI (kg/m2) | 27.4 (4.7) | 27.8 (5.5) | 28.8 (4.6) | 28.9 (4.7) |
| Level of education | ||||
| No high school diploma, n (%) | 5 (10.4) | 4 (12.1) | 3 (6.5) | 2 (4.9) |
| High school diploma, n (%) | 9 (18.8) | 6 (18.2) | 9 (19.6) | 6 (14.6) |
| Technical degree, n (%) | 8 (16.7) | 4 (12.1) | 7 (15.2) | 6 (14.6) |
| Bachelor’s degree, n (%) | 13 (27.1) | 9 (27.3) | 18 (39.1) | 18 (43.9) |
| Post-graduate degree, n (%) | 13 (27.1) | 10 (30.3) | 9 (19.6) | 9 (22) |
| Living with partner, n (%) | 35 (72.9) | 24 (72.7) | 26 (56.5) | 22 (53.7) |
| Currently smoking, n (%) | 4 (8.3) | 2 (6.1) | 4 (8.7) | 4 (9.8) |
| Smoking history (pack-years) | 8 (15.9) | 7.3 (16.9) | 8.9 (17.1) | 9.4 (18) |
| Alcohol consumption (drinks/week) | 2.6 (4.7) | 2.3 (3.9) | 3.2 (6.2) | 3.4 (6.5) |
| Age at PD diagnosis (years) | 59.5 (9.9) | 58.3 (10.3) | 62.0 (12.9) | 62.3 (13.1) |
| LED (mg) | 673.1 (393.2) | 666.3 (373.2) | 702.1 (428.5) | 690.8 (448.4) |
| RBD definite*, n (%) | 8 (17) | 5 (15) | 7 (15) | 7 (17) |
| RBD probable†, n (%) | 22 (46) | 14 (42) | 28 (57) | 24 (59) |
| Comorbidities, n (%) | ||||
| Hypertension | 6 (13) | 4 (12) | 11 (24) | 10 (24) |
| Diabetes | 6 (13) | 4 (12) | 5 (11) | 4 (10) |
| Dyslipidemia | 6 (13) | 6 (18) | 8 (17) | 7 (17) |
| Coronary artery disease | 1 (2) | 0 (0) | 4 (9) | 4 (10) |
| Atrial fibrillation | 3 (6) | 0 (0) | 2 (4) | 2 (5) |
| Chronic kidney disease | 0 (0) | 0 (0) | 3 (7) | 3 (7) |
| COPD | 1 (2) | 1 (3) | 0 (0) | 0 (0) |
| Hypothyroidism | 2 (4) | 2 (6) | 0 (0) | 0 (0) |
| Polysomnography results | ||||
| TST (minute) | 319.0 (67.4) | 319.2 (67.1) | 305.7 (66.5) | 308.2 (66.2) |
| Sleep efficiency (%) | 71.8 (15.1) | 72.0 (16.0) | 69.1 (14.3) | 69.4 (13.9) |
| % N1 stage | 27.2 (15.3) | 25.8 (14.1) | 28.7 (14.3) | 28.3 (13.4) |
| % N2 stage | 50.5 (14.0) | 49.4 (13.6) | 53.2 (13.5) | 54.0 (12.6) |
| % N3 stage | 12.2 (13.6) | 13.8 (15.0) | 9.4 (11.3) | 10.0 (11.7) |
| % R stage | 10.1 (7.5) | 11.0 (7.9) | 10.2 (7.5) | 9.5 (6.8) |
| WASO (minute) | 113.9 (73.9) | 112.6 (79.9) | 120.3 (68.3) | 119.9 (65.5) |
| Total arousal index | 56.6 (20.3) | 54.0 (17.4) | 59.1 (25.7) | 57.8 (22.4) |
| Respiratory arousal index | 48.2 (21.8) | 46.7 (18.8) | 49.7 (23.0) | 49.1 (20.9) |
| Spontaneous arousal index | 10.8 (7.2) | 9.9 (6.4) | 11.4 (7.1) | 10.8 (7.0) |
| PLM arousal index | 0.5 (1.3) | 0.6 (1.5) | 1.1 (4.4) | 0.7 (3.6) |
| PLM sleep index | 8.4 (17.4) | 8.3 (19.2) | 3.2 (8.1) | 2.8 (7.4) |
| AHI | 35.1 (20.3) | 34.4 (18.6) | 35.4 (24.8) | 35.8 (24.5) |
| RDI | 47.4 (20.1) | 46.7 (18.2) | 48.6 (20.1) | 48.8 (20.2) |
| ODI | 8.9 (7.4) | 9.5 (8.4) | 12.7 (14.9) | 13.2 (15.4) |
| T90% | 0.3 (0.7) | 0.2 (0.5) | 2.1 (4.1) | 2.3 (4.3) |
| Group . | PAP N = 48 . | PAP per protocol N = 33 . | Control N = 46 . | Control per protocol N = 41 . |
|---|---|---|---|---|
| Age (years) | 66.0 (10.4) | 64.0 (11.1) | 68.8 (10.4) | 69.1 (10.2) |
| Sex male, n (%) | 37 (77) | 23 (70) | 28 (61) | 25 (61) |
| BMI (kg/m2) | 27.4 (4.7) | 27.8 (5.5) | 28.8 (4.6) | 28.9 (4.7) |
| Level of education | ||||
| No high school diploma, n (%) | 5 (10.4) | 4 (12.1) | 3 (6.5) | 2 (4.9) |
| High school diploma, n (%) | 9 (18.8) | 6 (18.2) | 9 (19.6) | 6 (14.6) |
| Technical degree, n (%) | 8 (16.7) | 4 (12.1) | 7 (15.2) | 6 (14.6) |
| Bachelor’s degree, n (%) | 13 (27.1) | 9 (27.3) | 18 (39.1) | 18 (43.9) |
| Post-graduate degree, n (%) | 13 (27.1) | 10 (30.3) | 9 (19.6) | 9 (22) |
| Living with partner, n (%) | 35 (72.9) | 24 (72.7) | 26 (56.5) | 22 (53.7) |
| Currently smoking, n (%) | 4 (8.3) | 2 (6.1) | 4 (8.7) | 4 (9.8) |
| Smoking history (pack-years) | 8 (15.9) | 7.3 (16.9) | 8.9 (17.1) | 9.4 (18) |
| Alcohol consumption (drinks/week) | 2.6 (4.7) | 2.3 (3.9) | 3.2 (6.2) | 3.4 (6.5) |
| Age at PD diagnosis (years) | 59.5 (9.9) | 58.3 (10.3) | 62.0 (12.9) | 62.3 (13.1) |
| LED (mg) | 673.1 (393.2) | 666.3 (373.2) | 702.1 (428.5) | 690.8 (448.4) |
| RBD definite*, n (%) | 8 (17) | 5 (15) | 7 (15) | 7 (17) |
| RBD probable†, n (%) | 22 (46) | 14 (42) | 28 (57) | 24 (59) |
| Comorbidities, n (%) | ||||
| Hypertension | 6 (13) | 4 (12) | 11 (24) | 10 (24) |
| Diabetes | 6 (13) | 4 (12) | 5 (11) | 4 (10) |
| Dyslipidemia | 6 (13) | 6 (18) | 8 (17) | 7 (17) |
| Coronary artery disease | 1 (2) | 0 (0) | 4 (9) | 4 (10) |
| Atrial fibrillation | 3 (6) | 0 (0) | 2 (4) | 2 (5) |
| Chronic kidney disease | 0 (0) | 0 (0) | 3 (7) | 3 (7) |
| COPD | 1 (2) | 1 (3) | 0 (0) | 0 (0) |
| Hypothyroidism | 2 (4) | 2 (6) | 0 (0) | 0 (0) |
| Polysomnography results | ||||
| TST (minute) | 319.0 (67.4) | 319.2 (67.1) | 305.7 (66.5) | 308.2 (66.2) |
| Sleep efficiency (%) | 71.8 (15.1) | 72.0 (16.0) | 69.1 (14.3) | 69.4 (13.9) |
| % N1 stage | 27.2 (15.3) | 25.8 (14.1) | 28.7 (14.3) | 28.3 (13.4) |
| % N2 stage | 50.5 (14.0) | 49.4 (13.6) | 53.2 (13.5) | 54.0 (12.6) |
| % N3 stage | 12.2 (13.6) | 13.8 (15.0) | 9.4 (11.3) | 10.0 (11.7) |
| % R stage | 10.1 (7.5) | 11.0 (7.9) | 10.2 (7.5) | 9.5 (6.8) |
| WASO (minute) | 113.9 (73.9) | 112.6 (79.9) | 120.3 (68.3) | 119.9 (65.5) |
| Total arousal index | 56.6 (20.3) | 54.0 (17.4) | 59.1 (25.7) | 57.8 (22.4) |
| Respiratory arousal index | 48.2 (21.8) | 46.7 (18.8) | 49.7 (23.0) | 49.1 (20.9) |
| Spontaneous arousal index | 10.8 (7.2) | 9.9 (6.4) | 11.4 (7.1) | 10.8 (7.0) |
| PLM arousal index | 0.5 (1.3) | 0.6 (1.5) | 1.1 (4.4) | 0.7 (3.6) |
| PLM sleep index | 8.4 (17.4) | 8.3 (19.2) | 3.2 (8.1) | 2.8 (7.4) |
| AHI | 35.1 (20.3) | 34.4 (18.6) | 35.4 (24.8) | 35.8 (24.5) |
| RDI | 47.4 (20.1) | 46.7 (18.2) | 48.6 (20.1) | 48.8 (20.2) |
| ODI | 8.9 (7.4) | 9.5 (8.4) | 12.7 (14.9) | 13.2 (15.4) |
| T90% | 0.3 (0.7) | 0.2 (0.5) | 2.1 (4.1) | 2.3 (4.3) |
*RBD: questionnaire positive and PSG evidence.
†Not including definite.
Data as mean (SD) unless otherwise specified. Abbreviations: TST, total sleep time; WASO, wake after sleep onset; PLM, periodic leg movements; AHI, apnea-hypopnea index; RDI, respiratory disturbance index; ODI, oxygen desaturation index; T90%, time with oxygen saturation below 90%; LED, levodopa equivalent daily dose.
Change in MoCA score
Table 2 shows the outcome scores at each timepoint. In ITT analyses (Table 3), the MoCA score improved at 6 months in the treatment arm by 0.60 (SD 2.37) and declined by 0.39 (SD 2.79) with no statistically significant difference within or between groups (1.00, 95% CI [−0.060, 2.05], Figure 2).
| PAP | Control | |||||
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| MoCA | 22.6 (3.8) N = 48 | 22.8 (3.7) N = 37 | 24.0 (3.5) N = 37 | 22.7 (3.1) N = 46 | 22.7 (3.6) N = 41 | 22.1 (3.8) N = 41 |
| PDQ-39 | 19.6 (12.3) N = 46 | 16.2 (10.1) N = 35 | 16.8 (10.6) N = 36 | 20.3 (11.5) N = 46 | 21.6 (13.2) N = 38 | 21.2 (12.5) N = 40 |
| UPDRS total | 59.6 (24.3) N = 47 | 53.7 (23.7) N = 35 | 52.2 (20.8) N = 35 | 61.1 (20.4) N = 46 | 60.9 (21.9) N = 39 | 59.8 (22.1) N = 37 |
| UPDRS 1 | 13.3 (5.2) N = 47 | 11.1 (5.9) N = 35 | 10.9 (5.6) N = 36 | 13.5 (5.4) N = 46 | 12.2 (6.5) N = 39 | 12.5 (6.7) N = 40 |
| UPDRS 2 | 11.9 (8.2) N = 47 | 10.3 (6.1) N = 35 | 11.1 (6.5) n = 36 | 13.0 (7.2) N = 46 | 13.0 (8.4) N = 39 | 12.8 (8.3) n = 37 |
| UPDRS 3 | 30.6 (13.8) N = 48 | 27.8 (13.4) N = 35 | 27.6 (12.8) N = 36 | 30.1 (11.2) N = 46 | 31.2 (10.8) n = 39 | 30.8 (10.7) n = 40 |
| UPDRS 4 | 4.1 (3.2) N = 48 | 4.5 (3.9) N = 35 | 4.0 (4.0) N = 36 | 4.5 (3.8) N = 46 | 4.6 (3.7) N = 39 | 3.8 (3.7) n = 40 |
| ESS | 9.8 (4.6) N = 48 | 9.2 (4.3) N = 34 | 8.9 (5.3) N = 36 | 10.3 (4.3) N = 46 | 8.0 (4.7) N = 37 | 9.1 (4.7) n = 39 |
| BDI-II | 11.7 (7.5) N = 48 | 9.8 (7.5) N = 37 | 8.9 (6.5) N = 37 | 13.2 (8.3) N = 46 | 11.5 (7.9 N = 41 | 11.3 (8.4) N = 41 |
| PDSS | 85.7 (21.4) N = 48 | 97.5 (27.0) N = 35 | 97.1 (26.7) N = 36 | 83.8 (24.3) N = 46 | 95.3 (22.7) N = 36 | 89.1 (23.6) N = 39 |
| PAP | Control | |||||
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| MoCA | 22.6 (3.8) N = 48 | 22.8 (3.7) N = 37 | 24.0 (3.5) N = 37 | 22.7 (3.1) N = 46 | 22.7 (3.6) N = 41 | 22.1 (3.8) N = 41 |
| PDQ-39 | 19.6 (12.3) N = 46 | 16.2 (10.1) N = 35 | 16.8 (10.6) N = 36 | 20.3 (11.5) N = 46 | 21.6 (13.2) N = 38 | 21.2 (12.5) N = 40 |
| UPDRS total | 59.6 (24.3) N = 47 | 53.7 (23.7) N = 35 | 52.2 (20.8) N = 35 | 61.1 (20.4) N = 46 | 60.9 (21.9) N = 39 | 59.8 (22.1) N = 37 |
| UPDRS 1 | 13.3 (5.2) N = 47 | 11.1 (5.9) N = 35 | 10.9 (5.6) N = 36 | 13.5 (5.4) N = 46 | 12.2 (6.5) N = 39 | 12.5 (6.7) N = 40 |
| UPDRS 2 | 11.9 (8.2) N = 47 | 10.3 (6.1) N = 35 | 11.1 (6.5) n = 36 | 13.0 (7.2) N = 46 | 13.0 (8.4) N = 39 | 12.8 (8.3) n = 37 |
| UPDRS 3 | 30.6 (13.8) N = 48 | 27.8 (13.4) N = 35 | 27.6 (12.8) N = 36 | 30.1 (11.2) N = 46 | 31.2 (10.8) n = 39 | 30.8 (10.7) n = 40 |
| UPDRS 4 | 4.1 (3.2) N = 48 | 4.5 (3.9) N = 35 | 4.0 (4.0) N = 36 | 4.5 (3.8) N = 46 | 4.6 (3.7) N = 39 | 3.8 (3.7) n = 40 |
| ESS | 9.8 (4.6) N = 48 | 9.2 (4.3) N = 34 | 8.9 (5.3) N = 36 | 10.3 (4.3) N = 46 | 8.0 (4.7) N = 37 | 9.1 (4.7) n = 39 |
| BDI-II | 11.7 (7.5) N = 48 | 9.8 (7.5) N = 37 | 8.9 (6.5) N = 37 | 13.2 (8.3) N = 46 | 11.5 (7.9 N = 41 | 11.3 (8.4) N = 41 |
| PDSS | 85.7 (21.4) N = 48 | 97.5 (27.0) N = 35 | 97.1 (26.7) N = 36 | 83.8 (24.3) N = 46 | 95.3 (22.7) N = 36 | 89.1 (23.6) N = 39 |
Missing data for individual items on questionnaires were imputed as per the methods; missing entire questionnaires were not imputed for this table. Results as mean and standard deviation. Abbreviations: MoCA, Montreal Cognitive Assessment; PDQ-39, Parkinson’s Disease Quality of Life Questionnaire—30 questions; UPDRS, Movement Disorder Society Revision of the Unified Parkinson’s Disease Rating Scale; ESS, Epworth Sleepiness Scale; BDI-II, Beck Depression Inventory II; PDSS, Parkinson’s Disease Sleep Scale.
| PAP | Control | |||||
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| MoCA | 22.6 (3.8) N = 48 | 22.8 (3.7) N = 37 | 24.0 (3.5) N = 37 | 22.7 (3.1) N = 46 | 22.7 (3.6) N = 41 | 22.1 (3.8) N = 41 |
| PDQ-39 | 19.6 (12.3) N = 46 | 16.2 (10.1) N = 35 | 16.8 (10.6) N = 36 | 20.3 (11.5) N = 46 | 21.6 (13.2) N = 38 | 21.2 (12.5) N = 40 |
| UPDRS total | 59.6 (24.3) N = 47 | 53.7 (23.7) N = 35 | 52.2 (20.8) N = 35 | 61.1 (20.4) N = 46 | 60.9 (21.9) N = 39 | 59.8 (22.1) N = 37 |
| UPDRS 1 | 13.3 (5.2) N = 47 | 11.1 (5.9) N = 35 | 10.9 (5.6) N = 36 | 13.5 (5.4) N = 46 | 12.2 (6.5) N = 39 | 12.5 (6.7) N = 40 |
| UPDRS 2 | 11.9 (8.2) N = 47 | 10.3 (6.1) N = 35 | 11.1 (6.5) n = 36 | 13.0 (7.2) N = 46 | 13.0 (8.4) N = 39 | 12.8 (8.3) n = 37 |
| UPDRS 3 | 30.6 (13.8) N = 48 | 27.8 (13.4) N = 35 | 27.6 (12.8) N = 36 | 30.1 (11.2) N = 46 | 31.2 (10.8) n = 39 | 30.8 (10.7) n = 40 |
| UPDRS 4 | 4.1 (3.2) N = 48 | 4.5 (3.9) N = 35 | 4.0 (4.0) N = 36 | 4.5 (3.8) N = 46 | 4.6 (3.7) N = 39 | 3.8 (3.7) n = 40 |
| ESS | 9.8 (4.6) N = 48 | 9.2 (4.3) N = 34 | 8.9 (5.3) N = 36 | 10.3 (4.3) N = 46 | 8.0 (4.7) N = 37 | 9.1 (4.7) n = 39 |
| BDI-II | 11.7 (7.5) N = 48 | 9.8 (7.5) N = 37 | 8.9 (6.5) N = 37 | 13.2 (8.3) N = 46 | 11.5 (7.9 N = 41 | 11.3 (8.4) N = 41 |
| PDSS | 85.7 (21.4) N = 48 | 97.5 (27.0) N = 35 | 97.1 (26.7) N = 36 | 83.8 (24.3) N = 46 | 95.3 (22.7) N = 36 | 89.1 (23.6) N = 39 |
| PAP | Control | |||||
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| MoCA | 22.6 (3.8) N = 48 | 22.8 (3.7) N = 37 | 24.0 (3.5) N = 37 | 22.7 (3.1) N = 46 | 22.7 (3.6) N = 41 | 22.1 (3.8) N = 41 |
| PDQ-39 | 19.6 (12.3) N = 46 | 16.2 (10.1) N = 35 | 16.8 (10.6) N = 36 | 20.3 (11.5) N = 46 | 21.6 (13.2) N = 38 | 21.2 (12.5) N = 40 |
| UPDRS total | 59.6 (24.3) N = 47 | 53.7 (23.7) N = 35 | 52.2 (20.8) N = 35 | 61.1 (20.4) N = 46 | 60.9 (21.9) N = 39 | 59.8 (22.1) N = 37 |
| UPDRS 1 | 13.3 (5.2) N = 47 | 11.1 (5.9) N = 35 | 10.9 (5.6) N = 36 | 13.5 (5.4) N = 46 | 12.2 (6.5) N = 39 | 12.5 (6.7) N = 40 |
| UPDRS 2 | 11.9 (8.2) N = 47 | 10.3 (6.1) N = 35 | 11.1 (6.5) n = 36 | 13.0 (7.2) N = 46 | 13.0 (8.4) N = 39 | 12.8 (8.3) n = 37 |
| UPDRS 3 | 30.6 (13.8) N = 48 | 27.8 (13.4) N = 35 | 27.6 (12.8) N = 36 | 30.1 (11.2) N = 46 | 31.2 (10.8) n = 39 | 30.8 (10.7) n = 40 |
| UPDRS 4 | 4.1 (3.2) N = 48 | 4.5 (3.9) N = 35 | 4.0 (4.0) N = 36 | 4.5 (3.8) N = 46 | 4.6 (3.7) N = 39 | 3.8 (3.7) n = 40 |
| ESS | 9.8 (4.6) N = 48 | 9.2 (4.3) N = 34 | 8.9 (5.3) N = 36 | 10.3 (4.3) N = 46 | 8.0 (4.7) N = 37 | 9.1 (4.7) n = 39 |
| BDI-II | 11.7 (7.5) N = 48 | 9.8 (7.5) N = 37 | 8.9 (6.5) N = 37 | 13.2 (8.3) N = 46 | 11.5 (7.9 N = 41 | 11.3 (8.4) N = 41 |
| PDSS | 85.7 (21.4) N = 48 | 97.5 (27.0) N = 35 | 97.1 (26.7) N = 36 | 83.8 (24.3) N = 46 | 95.3 (22.7) N = 36 | 89.1 (23.6) N = 39 |
Missing data for individual items on questionnaires were imputed as per the methods; missing entire questionnaires were not imputed for this table. Results as mean and standard deviation. Abbreviations: MoCA, Montreal Cognitive Assessment; PDQ-39, Parkinson’s Disease Quality of Life Questionnaire—30 questions; UPDRS, Movement Disorder Society Revision of the Unified Parkinson’s Disease Rating Scale; ESS, Epworth Sleepiness Scale; BDI-II, Beck Depression Inventory II; PDSS, Parkinson’s Disease Sleep Scale.
Change in Outcome Measures by Intention to Treat (from Baseline to 6 Months)
| PAP | Control | Between groups | |||
| 6 months mean (SD) | Delta (SD) 95% CI | 6 month mean (SD) | Delta (SD) 95% CI | Delta (95% CI) | |
| MoCA | 23.21 (4.02) | 0.60 (2.37) (−0.083, 1.29) | 22.30 (3.82) | −0.39 (2.79) (−1.21, 0.43) | 1.00 (−0.060, 2.05) |
| PDQ-39 | 18.4 (13.2) | −1.11 (5.65) (−2.79, 0.57) | 16.1 (10.3) | 0.85 (7.83) (−1.48, 3.17) | −1.96 (−4.79, 0.87) |
| UPDRS total | 57.36 (26.23) | −2.53 (13.79) (−6.58, 1.52) | 61.30 (24.02) | 0.22 (13.17) (−3.69, 4.13) | −2.75 (−8.30, 2.80) |
| UPDRS 1 | 11.57 (5.80) | −1.68 (3.44) (−2.69, −0.67)* | 12.5 (6.69) | −0.96 (4.98) (−2.43, 0.52) | −0.72 (−2.49, 1.04) |
| UPDRS 2 | 12.51 (8.32) | 0.64 (4.87) (−0.79, 2.07) | 13.43 (8.84) | 0.46 (5.05) (−1.04, 1.95) | 0.18 (−1.86, 2.23) |
| UPDRS 3 | 29.48 (14.11) | −1.29 (8.24) (−3.68, 1.01) | 31.41 (11.84) | 1.30 (7.94) (−1.05, 3.66) | −2.59 (−5.91, 0.72) |
| UPDRS 4 | 3.96 (4.02) | −0.167 (3.32) (−1.13, 0.780) | 3.96 (3.73) | −0.587 (2.79) (−1.41, 0.24) | 0.42 (−0.83, 1.67) |
| ESS | 8.88 (4.88) | −0.88 (4.55) (−2.19, 0.45) | 8.93 (4.62) | −1.35 (4.03) (−2.54, −0.15)* | 0.47 (−1.29, 2.23) |
| BDI-II | 9.29 (6.45) | −2.31 (4.90) (−3.74, −0.89)* | 11.83 (8.32) | −1.41 (6.85) (−3.45, 0.62) | −0.90 (−3.35, 1.55) |
| PDSS | 95.68 (24.41) | 9.72 (21.14) (3.59, 15.86)* | 87.57 (23.27) | 3.64 (23.30) (−3.01, 10.28) | 6.08 (−2.83, 10.01) |
| PAP | Control | Between groups | |||
| 6 months mean (SD) | Delta (SD) 95% CI | 6 month mean (SD) | Delta (SD) 95% CI | Delta (95% CI) | |
| MoCA | 23.21 (4.02) | 0.60 (2.37) (−0.083, 1.29) | 22.30 (3.82) | −0.39 (2.79) (−1.21, 0.43) | 1.00 (−0.060, 2.05) |
| PDQ-39 | 18.4 (13.2) | −1.11 (5.65) (−2.79, 0.57) | 16.1 (10.3) | 0.85 (7.83) (−1.48, 3.17) | −1.96 (−4.79, 0.87) |
| UPDRS total | 57.36 (26.23) | −2.53 (13.79) (−6.58, 1.52) | 61.30 (24.02) | 0.22 (13.17) (−3.69, 4.13) | −2.75 (−8.30, 2.80) |
| UPDRS 1 | 11.57 (5.80) | −1.68 (3.44) (−2.69, −0.67)* | 12.5 (6.69) | −0.96 (4.98) (−2.43, 0.52) | −0.72 (−2.49, 1.04) |
| UPDRS 2 | 12.51 (8.32) | 0.64 (4.87) (−0.79, 2.07) | 13.43 (8.84) | 0.46 (5.05) (−1.04, 1.95) | 0.18 (−1.86, 2.23) |
| UPDRS 3 | 29.48 (14.11) | −1.29 (8.24) (−3.68, 1.01) | 31.41 (11.84) | 1.30 (7.94) (−1.05, 3.66) | −2.59 (−5.91, 0.72) |
| UPDRS 4 | 3.96 (4.02) | −0.167 (3.32) (−1.13, 0.780) | 3.96 (3.73) | −0.587 (2.79) (−1.41, 0.24) | 0.42 (−0.83, 1.67) |
| ESS | 8.88 (4.88) | −0.88 (4.55) (−2.19, 0.45) | 8.93 (4.62) | −1.35 (4.03) (−2.54, −0.15)* | 0.47 (−1.29, 2.23) |
| BDI-II | 9.29 (6.45) | −2.31 (4.90) (−3.74, −0.89)* | 11.83 (8.32) | −1.41 (6.85) (−3.45, 0.62) | −0.90 (−3.35, 1.55) |
| PDSS | 95.68 (24.41) | 9.72 (21.14) (3.59, 15.86)* | 87.57 (23.27) | 3.64 (23.30) (−3.01, 10.28) | 6.08 (−2.83, 10.01) |
*p < 0.05.
Abbreviations: MoCA, Montreal Cognitive Assessment; PDQ-39, Parkinson’s Disease Quality of Life Questionnaire—30 questions; UPDRS, Movement Disorder Society Revision of the Unified Parkinson’s Disease Rating Scale; ESS, Epworth Sleepiness Scale; BDI-II, Beck Depression Inventory II; PDSS, Parkinson’s Disease Sleep Scale.
Change in Outcome Measures by Intention to Treat (from Baseline to 6 Months)
| PAP | Control | Between groups | |||
| 6 months mean (SD) | Delta (SD) 95% CI | 6 month mean (SD) | Delta (SD) 95% CI | Delta (95% CI) | |
| MoCA | 23.21 (4.02) | 0.60 (2.37) (−0.083, 1.29) | 22.30 (3.82) | −0.39 (2.79) (−1.21, 0.43) | 1.00 (−0.060, 2.05) |
| PDQ-39 | 18.4 (13.2) | −1.11 (5.65) (−2.79, 0.57) | 16.1 (10.3) | 0.85 (7.83) (−1.48, 3.17) | −1.96 (−4.79, 0.87) |
| UPDRS total | 57.36 (26.23) | −2.53 (13.79) (−6.58, 1.52) | 61.30 (24.02) | 0.22 (13.17) (−3.69, 4.13) | −2.75 (−8.30, 2.80) |
| UPDRS 1 | 11.57 (5.80) | −1.68 (3.44) (−2.69, −0.67)* | 12.5 (6.69) | −0.96 (4.98) (−2.43, 0.52) | −0.72 (−2.49, 1.04) |
| UPDRS 2 | 12.51 (8.32) | 0.64 (4.87) (−0.79, 2.07) | 13.43 (8.84) | 0.46 (5.05) (−1.04, 1.95) | 0.18 (−1.86, 2.23) |
| UPDRS 3 | 29.48 (14.11) | −1.29 (8.24) (−3.68, 1.01) | 31.41 (11.84) | 1.30 (7.94) (−1.05, 3.66) | −2.59 (−5.91, 0.72) |
| UPDRS 4 | 3.96 (4.02) | −0.167 (3.32) (−1.13, 0.780) | 3.96 (3.73) | −0.587 (2.79) (−1.41, 0.24) | 0.42 (−0.83, 1.67) |
| ESS | 8.88 (4.88) | −0.88 (4.55) (−2.19, 0.45) | 8.93 (4.62) | −1.35 (4.03) (−2.54, −0.15)* | 0.47 (−1.29, 2.23) |
| BDI-II | 9.29 (6.45) | −2.31 (4.90) (−3.74, −0.89)* | 11.83 (8.32) | −1.41 (6.85) (−3.45, 0.62) | −0.90 (−3.35, 1.55) |
| PDSS | 95.68 (24.41) | 9.72 (21.14) (3.59, 15.86)* | 87.57 (23.27) | 3.64 (23.30) (−3.01, 10.28) | 6.08 (−2.83, 10.01) |
| PAP | Control | Between groups | |||
| 6 months mean (SD) | Delta (SD) 95% CI | 6 month mean (SD) | Delta (SD) 95% CI | Delta (95% CI) | |
| MoCA | 23.21 (4.02) | 0.60 (2.37) (−0.083, 1.29) | 22.30 (3.82) | −0.39 (2.79) (−1.21, 0.43) | 1.00 (−0.060, 2.05) |
| PDQ-39 | 18.4 (13.2) | −1.11 (5.65) (−2.79, 0.57) | 16.1 (10.3) | 0.85 (7.83) (−1.48, 3.17) | −1.96 (−4.79, 0.87) |
| UPDRS total | 57.36 (26.23) | −2.53 (13.79) (−6.58, 1.52) | 61.30 (24.02) | 0.22 (13.17) (−3.69, 4.13) | −2.75 (−8.30, 2.80) |
| UPDRS 1 | 11.57 (5.80) | −1.68 (3.44) (−2.69, −0.67)* | 12.5 (6.69) | −0.96 (4.98) (−2.43, 0.52) | −0.72 (−2.49, 1.04) |
| UPDRS 2 | 12.51 (8.32) | 0.64 (4.87) (−0.79, 2.07) | 13.43 (8.84) | 0.46 (5.05) (−1.04, 1.95) | 0.18 (−1.86, 2.23) |
| UPDRS 3 | 29.48 (14.11) | −1.29 (8.24) (−3.68, 1.01) | 31.41 (11.84) | 1.30 (7.94) (−1.05, 3.66) | −2.59 (−5.91, 0.72) |
| UPDRS 4 | 3.96 (4.02) | −0.167 (3.32) (−1.13, 0.780) | 3.96 (3.73) | −0.587 (2.79) (−1.41, 0.24) | 0.42 (−0.83, 1.67) |
| ESS | 8.88 (4.88) | −0.88 (4.55) (−2.19, 0.45) | 8.93 (4.62) | −1.35 (4.03) (−2.54, −0.15)* | 0.47 (−1.29, 2.23) |
| BDI-II | 9.29 (6.45) | −2.31 (4.90) (−3.74, −0.89)* | 11.83 (8.32) | −1.41 (6.85) (−3.45, 0.62) | −0.90 (−3.35, 1.55) |
| PDSS | 95.68 (24.41) | 9.72 (21.14) (3.59, 15.86)* | 87.57 (23.27) | 3.64 (23.30) (−3.01, 10.28) | 6.08 (−2.83, 10.01) |
*p < 0.05.
Abbreviations: MoCA, Montreal Cognitive Assessment; PDQ-39, Parkinson’s Disease Quality of Life Questionnaire—30 questions; UPDRS, Movement Disorder Society Revision of the Unified Parkinson’s Disease Rating Scale; ESS, Epworth Sleepiness Scale; BDI-II, Beck Depression Inventory II; PDSS, Parkinson’s Disease Sleep Scale.

Change in MoCA scores at 6 months. ITT: intention to treat, p = 0.065 between groups. PP: per protocol, p = 0.038 between groups.
Sensitivity analyses were performed using linear regression. Under ITT, adjusting for age, sex, and BMI, the difference in change in MoCA at 6 months between groups was 1.28, 95% CI (0.18, 2.37), showing an improvement in the PAP group compared with the control group. Additionally adjusting for baseline depression scores, hypertension, and change in psychoactive medication during the study period, the difference in change in MoCA between groups was 1.44, 95% CI (0.09, 2.79). In a subset of 59 participants who had no change in medication over 6 months (25 in the PAP group, 34 control participants), adjusting for age, sex, BMI, baseline depression scores, and hypertension, the MoCA improved in the PAP group compared with the control group by 1.46 points, 95% CI (0.01, 2.90).
In per protocol analyses (Table 4), the MoCA score improved at 6 months by 0.91 (SD 2.54) in the treatment arm and declined by 0.44 (SD 2.93) with a significant difference between groups (1.35, 95% CI [0.078, 2.62], Figure 2). Analyses adjusted for age, sex, BMI, baseline depression scores, hypertension, and change in psychoactive medication showed improvement in MoCA by 1.43, 95% CI (0.054, 2.81).
| PAP (n = 33) | Control (n = 41) | Between groups | ||||
| 6 months mean (SD) | Delta (SD) (95% CI) | 6 months mean (SD) | Delta (SD) (95% CI) | Delta (95% CI) unadjusted | Delta (95% CI) adjusted | |
| MoCA | 24.30 (3.35) | 0.91 (2.54) (0.008, 1.81)* | 22.12 (3.85) | −0.44 (2.93) (−1.36, 0.49) | 1.35 (0.078, 2.62) * | 1.43 (0.054, 2.81)* |
| PDQ-39 | 16.13 (10.26) | −1.64 (6.54) (−3.96, 0.68) | 21.16 (12.32) | 1.03 (8.27) (−1.58, 3.64) | −2.67 (−6.11 0.76) | −2.66 (−6.55, 1.23) |
| UPDRS total | 51.33 (20.86) | −3.70 (14.76) (−8.93, 1.54) | 60.05 (22.36) | 0.39 (13.93) (−4.01, 4.79) | −4.09 (−10.81, 2.63) | −5.83 (−12.9, 1.27) |
| UPDRS 1 | 10.73 (5.78) | −2.09 (3.84) (−3.45, −0.73)* | 12.27 (6.80) | −1.10 (5.26) (−2.76, 0.53) | −0.99 (−3.10, 1.12) | −0.97 (−3.38, 1.45) |
| UPDRS 2 | 10.79 (6.50) | 0.76 (5.50) (−1.19, 2.71) | 13.39 (8.76) | 0.512 (5.35) (−1.18, 2.20) | 0.25 (−2.29, 2.78) | 0.25 (−2.43, 2.93) |
| UPDRS 3 | 25.88 (11.48) | −1.85 (7.94) (−4.66, 0.97) | 30.66 (10.63) | 1.54 (8.38) (−5.91, 0.72) | −3.39 (−7.18, 0.41) | −5.07 (−9.09, −1.06)* |
| UPDRS 4 | 3.94 (4.02) | −0.515 (3.27) (−1.67, 0.64) | 3.73 (3.73) | −0.56 (2.90) (−1.48, 0.35) | −0.045 (−1.41, 1.50) | −0.041 (−1.60, 1.52) |
| ESS | 8.93 (4.63) | −1.21 (4.99) (−3.01, 0.83) | 8.93 (4.68) | −1.58 (4.19) (−2.91, −2.63)* | 0.34 (−1.78, 2.47) | −0.10 (−2.36, 2.17) |
| BDI-II | 8.42 (6.30) | −3.12 (5.42) (−5.04, −1.20)* | 11.24 (8.38) | −1.68 (7.20) (−3.95, 0.59) | −1.44 (−4.36, 1.49) | −1.82 (−4.76, 1.12) |
| PDSS | 98.34 (26.92) | 13.18 (23.31) (4.91, 21.44)* | 88.44 (23.55) | 5.30 (22.08) (−1.67, 12.17) | 7.88 (−2.75, 18.51) | 7.25 (−4.32, 18.8) |
| PAP (n = 33) | Control (n = 41) | Between groups | ||||
| 6 months mean (SD) | Delta (SD) (95% CI) | 6 months mean (SD) | Delta (SD) (95% CI) | Delta (95% CI) unadjusted | Delta (95% CI) adjusted | |
| MoCA | 24.30 (3.35) | 0.91 (2.54) (0.008, 1.81)* | 22.12 (3.85) | −0.44 (2.93) (−1.36, 0.49) | 1.35 (0.078, 2.62) * | 1.43 (0.054, 2.81)* |
| PDQ-39 | 16.13 (10.26) | −1.64 (6.54) (−3.96, 0.68) | 21.16 (12.32) | 1.03 (8.27) (−1.58, 3.64) | −2.67 (−6.11 0.76) | −2.66 (−6.55, 1.23) |
| UPDRS total | 51.33 (20.86) | −3.70 (14.76) (−8.93, 1.54) | 60.05 (22.36) | 0.39 (13.93) (−4.01, 4.79) | −4.09 (−10.81, 2.63) | −5.83 (−12.9, 1.27) |
| UPDRS 1 | 10.73 (5.78) | −2.09 (3.84) (−3.45, −0.73)* | 12.27 (6.80) | −1.10 (5.26) (−2.76, 0.53) | −0.99 (−3.10, 1.12) | −0.97 (−3.38, 1.45) |
| UPDRS 2 | 10.79 (6.50) | 0.76 (5.50) (−1.19, 2.71) | 13.39 (8.76) | 0.512 (5.35) (−1.18, 2.20) | 0.25 (−2.29, 2.78) | 0.25 (−2.43, 2.93) |
| UPDRS 3 | 25.88 (11.48) | −1.85 (7.94) (−4.66, 0.97) | 30.66 (10.63) | 1.54 (8.38) (−5.91, 0.72) | −3.39 (−7.18, 0.41) | −5.07 (−9.09, −1.06)* |
| UPDRS 4 | 3.94 (4.02) | −0.515 (3.27) (−1.67, 0.64) | 3.73 (3.73) | −0.56 (2.90) (−1.48, 0.35) | −0.045 (−1.41, 1.50) | −0.041 (−1.60, 1.52) |
| ESS | 8.93 (4.63) | −1.21 (4.99) (−3.01, 0.83) | 8.93 (4.68) | −1.58 (4.19) (−2.91, −2.63)* | 0.34 (−1.78, 2.47) | −0.10 (−2.36, 2.17) |
| BDI-II | 8.42 (6.30) | −3.12 (5.42) (−5.04, −1.20)* | 11.24 (8.38) | −1.68 (7.20) (−3.95, 0.59) | −1.44 (−4.36, 1.49) | −1.82 (−4.76, 1.12) |
| PDSS | 98.34 (26.92) | 13.18 (23.31) (4.91, 21.44)* | 88.44 (23.55) | 5.30 (22.08) (−1.67, 12.17) | 7.88 (−2.75, 18.51) | 7.25 (−4.32, 18.8) |
*p < 0.05.
Analyses adjusted for age, sex, BMI, hypertension, psychoactive medication change, and baseline depression score. Abbreviations: MoCA, Montreal Cognitive Assessment; PDQ-39, Parkinson’s Disease Quality of Life Questionnaire—30 questions; UPDRS, Movement Disorder Society Revision of the Unified Parkinson’s Disease Rating Scale; ESS, Epworth Sleepiness Scale; BDI-II: Beck Depression Inventory II; PDSS, Parkinson’s Disease Sleep Scale.
| PAP (n = 33) | Control (n = 41) | Between groups | ||||
| 6 months mean (SD) | Delta (SD) (95% CI) | 6 months mean (SD) | Delta (SD) (95% CI) | Delta (95% CI) unadjusted | Delta (95% CI) adjusted | |
| MoCA | 24.30 (3.35) | 0.91 (2.54) (0.008, 1.81)* | 22.12 (3.85) | −0.44 (2.93) (−1.36, 0.49) | 1.35 (0.078, 2.62) * | 1.43 (0.054, 2.81)* |
| PDQ-39 | 16.13 (10.26) | −1.64 (6.54) (−3.96, 0.68) | 21.16 (12.32) | 1.03 (8.27) (−1.58, 3.64) | −2.67 (−6.11 0.76) | −2.66 (−6.55, 1.23) |
| UPDRS total | 51.33 (20.86) | −3.70 (14.76) (−8.93, 1.54) | 60.05 (22.36) | 0.39 (13.93) (−4.01, 4.79) | −4.09 (−10.81, 2.63) | −5.83 (−12.9, 1.27) |
| UPDRS 1 | 10.73 (5.78) | −2.09 (3.84) (−3.45, −0.73)* | 12.27 (6.80) | −1.10 (5.26) (−2.76, 0.53) | −0.99 (−3.10, 1.12) | −0.97 (−3.38, 1.45) |
| UPDRS 2 | 10.79 (6.50) | 0.76 (5.50) (−1.19, 2.71) | 13.39 (8.76) | 0.512 (5.35) (−1.18, 2.20) | 0.25 (−2.29, 2.78) | 0.25 (−2.43, 2.93) |
| UPDRS 3 | 25.88 (11.48) | −1.85 (7.94) (−4.66, 0.97) | 30.66 (10.63) | 1.54 (8.38) (−5.91, 0.72) | −3.39 (−7.18, 0.41) | −5.07 (−9.09, −1.06)* |
| UPDRS 4 | 3.94 (4.02) | −0.515 (3.27) (−1.67, 0.64) | 3.73 (3.73) | −0.56 (2.90) (−1.48, 0.35) | −0.045 (−1.41, 1.50) | −0.041 (−1.60, 1.52) |
| ESS | 8.93 (4.63) | −1.21 (4.99) (−3.01, 0.83) | 8.93 (4.68) | −1.58 (4.19) (−2.91, −2.63)* | 0.34 (−1.78, 2.47) | −0.10 (−2.36, 2.17) |
| BDI-II | 8.42 (6.30) | −3.12 (5.42) (−5.04, −1.20)* | 11.24 (8.38) | −1.68 (7.20) (−3.95, 0.59) | −1.44 (−4.36, 1.49) | −1.82 (−4.76, 1.12) |
| PDSS | 98.34 (26.92) | 13.18 (23.31) (4.91, 21.44)* | 88.44 (23.55) | 5.30 (22.08) (−1.67, 12.17) | 7.88 (−2.75, 18.51) | 7.25 (−4.32, 18.8) |
| PAP (n = 33) | Control (n = 41) | Between groups | ||||
| 6 months mean (SD) | Delta (SD) (95% CI) | 6 months mean (SD) | Delta (SD) (95% CI) | Delta (95% CI) unadjusted | Delta (95% CI) adjusted | |
| MoCA | 24.30 (3.35) | 0.91 (2.54) (0.008, 1.81)* | 22.12 (3.85) | −0.44 (2.93) (−1.36, 0.49) | 1.35 (0.078, 2.62) * | 1.43 (0.054, 2.81)* |
| PDQ-39 | 16.13 (10.26) | −1.64 (6.54) (−3.96, 0.68) | 21.16 (12.32) | 1.03 (8.27) (−1.58, 3.64) | −2.67 (−6.11 0.76) | −2.66 (−6.55, 1.23) |
| UPDRS total | 51.33 (20.86) | −3.70 (14.76) (−8.93, 1.54) | 60.05 (22.36) | 0.39 (13.93) (−4.01, 4.79) | −4.09 (−10.81, 2.63) | −5.83 (−12.9, 1.27) |
| UPDRS 1 | 10.73 (5.78) | −2.09 (3.84) (−3.45, −0.73)* | 12.27 (6.80) | −1.10 (5.26) (−2.76, 0.53) | −0.99 (−3.10, 1.12) | −0.97 (−3.38, 1.45) |
| UPDRS 2 | 10.79 (6.50) | 0.76 (5.50) (−1.19, 2.71) | 13.39 (8.76) | 0.512 (5.35) (−1.18, 2.20) | 0.25 (−2.29, 2.78) | 0.25 (−2.43, 2.93) |
| UPDRS 3 | 25.88 (11.48) | −1.85 (7.94) (−4.66, 0.97) | 30.66 (10.63) | 1.54 (8.38) (−5.91, 0.72) | −3.39 (−7.18, 0.41) | −5.07 (−9.09, −1.06)* |
| UPDRS 4 | 3.94 (4.02) | −0.515 (3.27) (−1.67, 0.64) | 3.73 (3.73) | −0.56 (2.90) (−1.48, 0.35) | −0.045 (−1.41, 1.50) | −0.041 (−1.60, 1.52) |
| ESS | 8.93 (4.63) | −1.21 (4.99) (−3.01, 0.83) | 8.93 (4.68) | −1.58 (4.19) (−2.91, −2.63)* | 0.34 (−1.78, 2.47) | −0.10 (−2.36, 2.17) |
| BDI-II | 8.42 (6.30) | −3.12 (5.42) (−5.04, −1.20)* | 11.24 (8.38) | −1.68 (7.20) (−3.95, 0.59) | −1.44 (−4.36, 1.49) | −1.82 (−4.76, 1.12) |
| PDSS | 98.34 (26.92) | 13.18 (23.31) (4.91, 21.44)* | 88.44 (23.55) | 5.30 (22.08) (−1.67, 12.17) | 7.88 (−2.75, 18.51) | 7.25 (−4.32, 18.8) |
*p < 0.05.
Analyses adjusted for age, sex, BMI, hypertension, psychoactive medication change, and baseline depression score. Abbreviations: MoCA, Montreal Cognitive Assessment; PDQ-39, Parkinson’s Disease Quality of Life Questionnaire—30 questions; UPDRS, Movement Disorder Society Revision of the Unified Parkinson’s Disease Rating Scale; ESS, Epworth Sleepiness Scale; BDI-II: Beck Depression Inventory II; PDSS, Parkinson’s Disease Sleep Scale.
The Complier Average Causal Effect (CACE) on MoCA was 1.37, 95% CI (0.063, 2.94), based on a compliance rate of 0.729 for any PAP use at 6 months (35 of 48 randomized, including two adherent participants without outcome data at 6 months, in whom prior available data were carried forward). Using compliance defined as using >3 hours per night on all nights, resulting in a compliance rate of 31% (15 of 48), the CACE would be 3.19, 95% CI (0.11, 8.18).
When the per-protocol PAP group was divided into categories of OSA severity, the mean MoCA change from baseline to 6 months increased with increasing AHI categories, albeit without statistical significance for trend (Supplementary Table S3). MoCA change showed a linear association with the AHI in REM sleep (Beta 0.0414, 95% CI [0.0022, 0.0805], p = 0.039) but not with overall AHI, RDI, and AHI in non-REM or hypoxemia measures (Supplementary Figure S1).
Secondary outcomes
Results for secondary outcomes are shown in Tables 3 and 4. There was significant improvement, in both ITT and per-protocol analyses, in MDS-UPDRS part 1, BDI-II, and PDSS scores in the PAP group but not in the control group. Differences in change between groups were not statistically significant, except for a significant improvement in MDS-UPDRS part 3 (motor) in PAP versus control groups in adjusted per protocol analyses.
Exploratory outcomes
Neurocognitive assessment results are shown in Supplementary Tables S4 and S5. Participants in the APAP group had improvement in several executive and psychomotor tests in both ITT and per protocol analyses: inhibition/switching task, Tower of London number of moves, correct score, execution time and total time (per protocol only), and complex motor tasks (left-hand tapping, bi-manual in-phase, and out-of-phase tapping). The control group showed improvement in letter sequencing (per protocol only), number/letter switching, two language tasks, and right-hand tapping (ITT only). No statistically significant between-group differences were found.
Treatment adherence
At 6 months, 35 participants were still using PAP with a mean use of 3 hours 6 minutes per night (SD 1 hour 58 minutes) on all days and 6 hours 13 minutes per night (SD 1 hour 54 minutes) on days used, with use on 69.0% (SD 25.2) of days (Supplementary Figure S2). The median residual AHI was 2.9/hour. Due to the COVID-19 pandemic and other reasons, three of those participants did not complete the final outcome assessment, resulting in a per-protocol group of 33 participants. Among those, the mean nightly PAP use was 3 hour 14 minutes (SD 1 hour 57 minutes) on all days and 4 hour 19 minutes (SD 1 hour 53 minutes) on days used, with use on 70.9% (SD 24.1) of days. The median residual AHI was 2.9/hour.
Blinding
The outcome assessor answered the question “Which group do you believe this patient belongs to?” at follow-up assessment visits, with possible answers “CPAP,” “NDS,” or “I don’t know.” At 6-month follow-up, the assessor correctly guessed the treatment in 57% of participants in the PAP group and 0% of the NDS group (one believed to be in APAP group, remainder “I don’t know”). Overall, the assessor remained blinded in 73% of 6-month assessments.
Adverse Events
Two participants randomized to APAP had a stroke: one prior to initiating treatment who was withdrawn from the study, and one after the 3-month visit who was withdrawn from further outcomes assessment (but continued PAP use). One participant in the APAP group was diagnosed with melanoma of the face which required resection, leading the patient to drop out of the study. One participant in the control group had an acute coronary event requiring angioplasty and stent insertion but continued in the study.
Discussion
OSA has been associated with lower MoCA scores in the general population and in PD [9, 22, 23]. The MoCA has higher sensitivity for MCI in OSA compared with the Mini-Mental State Examination [24]. Our group previously found improvements in MoCA with PAP treatment in an observational study of PD patients over 12 months [12]. In this RCT, we evaluated the effect of PAP therapy on change in MoCA score over 6 months in PD patients with mildly reduced cognition. Although the primary analysis does not, strictly speaking, show a statistically significant result, the 95% CI for the primary outcome (−0.06, 2.05) is in majority above the null and suggests a benefit, with a point estimate of 1 point on the MOCA score in the PAP group versus control, in a conservative ITT analysis. Moreover, per protocol analyses show a significant MoCA improvement with PAP therapy, compared with baseline and compared with the control group, with a difference between treated and control groups of 1.43 (95% CI: 0.054, 2.81). Our conservative estimate for CACE, which estimates the impact of an intervention among treatment compliers in randomized trials [21], was 1.32. This was calculated based on the proportion of participants with any use of PAP at 6 months. The CACE for nightly use >3 hours per night was 3.18. This high value seems clinically unrealistic, but it indicates that the benefit could be considerable with consistent PAP use. However, less than half of individuals were able to use the device to this level consistently (Supplementary Figure S2). We also performed sensitivity analyses, using ITT adjusting for baseline variables that were considered potential confounders, which demonstrated a significant improvement in MoCA with PAP compared with placebo. Additionally, medication change over 6 months in an evolving disorder with multiple nonmotor symptoms is difficult to avoid and was not specified as an exclusion criterion to reduce recruitment impediments and maximize the generalizability of results. As psychoactive medications may affect cognition, we performed sensitivity analyses in a subset without changes in such medication during the study, adjusting for confounders, that also showed significant improvement in MoCA in the PAP group versus controls.
The MCID for MoCA, which is generally considered a screening tool, is not known. However, Chen et al. found a decline of 0.31 annually in PD patients cognitively normal at baseline and 1.63 in cognitively impaired [25]. The MoCA is a sensitive predictor of cognitive decline in PD [26]. In a 2-year longitudinal study, a 1-point lower baseline MoCA score was associated with a 34% greater risk of progression to mild cognitive impairment or dementia [27]. Mild cognitive impairment, in turn, is associated with greater progression of motor dysfunction [28]. Hence the magnitude of improvement with PAP we describe over approximately 6 months appears to be clinically meaningful. Notably, MoCA improvement could represent (a) performance recovery due to correction of OSA and its immediate adverse consequences such as sleep disruption, but with no effect on the longer-term progression of cognitive dysfunction, or (b) disease modification, that is, a change in the course of long-term cognitive decline and of the neurodegenerative process with the treatment of OSA. Furthermore, longer-term studies will be required to clarify this point.
In non-PD individuals, treatment of OSA has yielded variable effects on cognitive function [1], which may have depended on patient population, OSA severity, tasks tested, and duration of treatment. In elderly individuals with severe OSA, PAP therapy did show benefit in several cognitive domains after 3 months [10]. Sustained treatment of OSA, but not a first night of CPAP, also benefited motor learning [29]. Similarly, a study in non-PD individuals with severe OSA found partial improvement in white matter abnormalities on MRI at 3 months but almost complete reversal at 12 months with cognitive improvement paralleling these findings [30]. Individuals with PD may be particularly vulnerable to adverse consequences of OSA on the brain. A previous RCT of PAP in PD found improved objective sleep quality and sleepiness [15], but no significant improvement in a composite cognitive outcome measure after 3 or 6 weeks of treatment [16]. It is likely that treatment duration was too short in that study. PD patients may need a longer adaptation period to PAP therapy and changes in cognition may require several months of treatment, as suggested by the current study and our previous 12-month observational study [12].
Our data suggest that individuals with more severe OSA benefit most from PAP therapy, but the benefit could not be excluded for any severity of OSA comprised in this study given our sample size. Interestingly, we found that MoCA improvement was related to REM AHI but not non-REM AHI. OSA specifically in REM sleep has been associated with adverse systemic outcomes [31], and with greater cognitive impairment in older individuals at risk for dementia [32]. Further research is needed to clarify the impact of REM and non-REM-related OSA on cognition in PD.
Detailed neurocognitive testing suggests improvement with PAP primarily in executive function, and in complex motor tasks. Executive dysfunction is the most frequent type of cognitive impairment in PD [31], and also in OSA, along with attention and working memory [2, 32]. Our findings suggest OSA treatment could translate into improved daily functioning. The control group showed improvement in several tasks that could be random, or related to a learning effect, with similar improvement (e.g. Boston Naming Test) in the APAP group, though not statistically significant, possibly due to fewer individuals completing the assessment in this group.
We also found improvements, in both ITT and per protocol analyses, in global nonmotor symptoms scores, depression scores, and sleep quality in the PAP group only. Improvements in the treatment group are noteworthy and consistent with previous observational data with respect to nonmotor symptoms and sleep quality. Baseline scores indicate minimal depression overall in our group, making any significant improvement that much more notable. Studies targeting patients more symptomatic in these domains would be useful to clarify these effects. Improved sleepiness (ESS) in the control group could be a chance finding, or regression to the mean, given slightly higher baseline ESS values in controls and similar values in both groups at follow up; the MCID for ESS being 2 points, the improvement in controls is not considered clinically significant. The MDS-UPDRS part 3 (motor) showed a divergence between groups, which was statistically significant in the per protocol analysis, with improvement in the PAP group and deterioration in controls. The MCID for improvement has been reported at −3.25 and for deterioration 4.63 [33], suggesting our adjusted point estimate for between-group differences of more than 5 to be clinically important. In our previous observational study, PAP treatment of OSA resulted in a stabilization of motor function over 1 year [34]. Additional research is warranted to further explore the effect of OSA and its treatment on PD motor impairment.
Regarding QoL (PDQ-39), there was an improvement in the PAP group and deterioration in the control group, though without statistical significance. This also indicates no deterioration related to PAP therapy which could be considered a burden in this patient population.
Through this trial, we found that PAP therapy is feasible in a large proportion of PD patients with OSA over a 6-month period with adherence similar to other PAP trials in the general population [35, 36]. In our protocol, participants received considerable support and encouragement from the PAP provider respiratory therapist as well as the study sleep nurse and sleep physician, which may have helped with the initiation and maintenance of PAP therapy throughout the study. Nevertheless, several participants were unable to manage PAP therapy. Other studies have found low adherence in PD [37], likely in relation to their specific study populations and possibly other factors like treatment support. Conversely, a short-term trial found excellent adherence [16], though longer-term adherence was unknown in that study. Given the cognitive benefit of PAP that our results substantiate, with relatively modest adherence, we suggest that programs to support PAP therapy among those with OSA and cognitive decline should become a part of PD care. While patients with advanced PD may no longer be able to use this form of treatment, adoption of PAP when indicated early on in the course of PD may prove most useful and help delay decline.
Limitations of the study include a higher than anticipated noncompletion rate, resulting in part from the COVID-19 pandemic. The relatively demanding protocol resulted in some missing data. The magnitude of the change in MoCA with PAP was lower than expected based on observational data, such that the study was somewhat underpowered. Participants could not be blinded, but NDS strips have a known placebo effect [38]. We did not believe it would be ethically justifiable or scientifically valid due to potential adverse effects on sleep to use sham PAP as a placebo in this already impaired population. Additionally, though the assessor was to be blinded according to protocol, several patients disclosed their assigned treatment, resulting in unblinding of the assessor in a proportion of cases but it is unlikely that this affected the results significantly. Our participants were recruited primarily from a Movement Disorder clinic and from a general neurology clinic in a major urban center, and most had a relatively high level of education. It is possible that they were not completely representative of the entire community of PD patients.
Conclusion
This is the largest and longest duration RCT to date of PAP treatment of OSA in a neurodegenerative condition. Our results suggest those PD patients who are able to adhere to this therapy over a 6-month period benefit with respect to cognition, primarily in executive and psychomotor function, and also sleep quality, depression symptoms, and nonmotor symptoms globally, as well as motor function. We propose that integrated PD care should include screening for and treatment of OSA, to reduce the burden of these symptoms among patients.
Acknowledgments
We would like to thank the McGill Movement Disorder Clinic staff and neurologists, particularly Drs. Michael Sidel and Daniel Rabinovitch, for their enthusiastic support of the study and help with recruitment. We thank VitalAire staff for their flexibility and their professional support of our participants for PAP therapy.
Funding
Canadian Institutes for Health Research (MOP 136806); Philips (in kind); Vitalaire (in kind).
Disclosure Statements
Financial disclosure: Annie C. Lajoie and Anne-Louise Lafontaine: none. R. John Kimoff: research operating grants from: Canadian Institutes of Health Research, The Multiple Sclerosis Society of Canada, and Fonds de Recherche du Quebec—Santé; research operating funds from Signifier Medical, Bresotec Inc.; in-kind research equipment support: PAP devices from ResMed; VitalAire Inc.—speaker fee. Andrea Benedetti, Ann R. Robinson, Marie Létourneau, Joelle Crane, Amanda Scanga, and Francine Noel: none. Marta Kaminska: research grants or in-kind support from Vitalaire, Philips, Canadian Institutes of Health Research, Weston Brain Institute, Temple University, and Fonds de recherche du Quebec—santé; speaker honoraria from eMedevents, Canadian Society of Respiratory Therapists, Ordre professionnel des inhalothérapeutes du Québec, and the Quebec Sleep Network; Advisory Board for Biron soins du sommeil and Jazz. Non-financial disclosure: Annie C. Lajoie and Anne-Louise Lafontaine: none. R. John Kimoff: advisory board: Powell-Mansfield Inc, Eisai Inc. Andrea Benedetti, Ann R. Robinson, Marie Létourneau, Joelle Crane, Amanda Scanga, and Francine Noel: none. Marta Kaminska: Chair of Canadian Thoracic Society Home Mechanical Ventilation Assembly.
Author contributions
Annie C. Lajoie (Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing—original draft), Anne-Louise Lafontaine (Conceptualization, Resources, Writing—review & editing), R. John Kimoff (Conceptualization, Resources, Writing—review & editing), Andrea Benedetti (Conceptualization, Formal analysis, Methodology, Validation, Writing—review & editing), Ann R. Robinson (Investigation, Methodology, Writing—review & editing), Marie Létourneau (Investigation, Methodology, Writing—review & editing), Joelle Crane (Investigation, Writing—review & editing), Amanda Scanga (Data curation, Formal analysis, Investigation, Visualization), Francine Noel (Investigation), and Marta Kaminska (Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing)
Data Availability
The data underlying this article cannot be shared publicly as this was not approved by the institutional Research Ethics Board.




Comments