-
PDF
- Split View
-
Views
-
Cite
Cite
Deepti Tiwari, Pushpa Kewlani, Laxman Singh, Sandeep Rawat, Indra D Bhatt, Rakesh C Sundriyal, Veena Pande, A review on natural bioactive compounds of Taraxacum officinale Weber: a potential anticancer plant, RPS Pharmacy and Pharmacology Reports, Volume 3, Issue 2, April 2024, rqae009, https://doi.org/10.1093/rpsppr/rqae009
Close - Share Icon Share
Abstract
This review analyzed available literature on traditional/ethnomedicinal knowledge, phytochemical composition, anticancer activity reported in vitro and in vivo studies, and the toxicological activity of Taraxacum officinale. The aim is to provide an in-depth analysis of existing research on the anticancer potential of T. officinale.
The data was extracted using four search engines, Google Scholar, Web of Science, Scopus, and Pubmed, and systematically analyzed to identify effective plant-based substances for cancer treatment. The different parts of the plant are the source of different bioactive compounds that exhibit several pharmacological activities like antidiabetic, hepatoprotective, antimicrobial, anticancer, analgesic, etc. Traditionally, it is used to treat various ailments such as migraines, cardiac complaints, jaundice, fever, liver and kidney disorders, and hepatitis. Different biologically active compounds isolated from T. officinale are widely investigated against various pharmacological activities, including cancer.
The available evidence on the bioactive potential of Taraxacum officinale provides direction for identifying and developing herbal agents to prevent different types of cancers in the future. However, there is a need to examine the clinical validation of pure compounds for drug development.
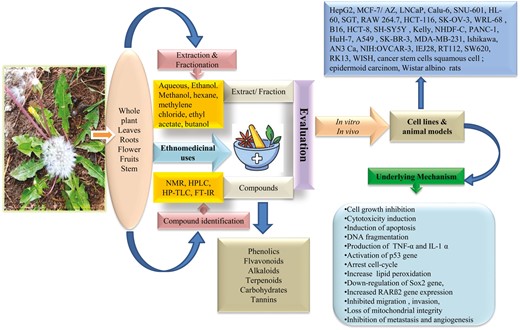
Introduction
Cancer is one of the most deadly diseases after cardiovascular disease, characterized by the uncontrolled malignant growth of body cells. According to a recent report, cancer accounted for 9.6 million deaths in 2018 worldwide [1]. Over the last few decades, discoveries of plant-driven medicine have become a route of novel molecule identification for cancer treatment. The National Cancer Institute (NCI), USA, began to screen plant extracts from higher plants with antitumor activity in 1957. After that, around 35 000 plant species were investigated as anticancer drugs [2]. Currently, over 3000 medicinal plants have been reported to possess potential anticancer compounds worldwide [3]. Over 60% of anticancer drugs are discovered in plants and animals. Plant-derived natural compounds are considered the most important source, accounting for approximately 75% of new drug discoveries for cancer [4]. Scientists are working worldwide on isolating, identifying, and characterizing purified compounds from plant origin to provide a strong scientific basis for plant-based drugs for cancer treatment [5]. For example, Podophyllotoxin isolated from Podophyllum hexandrum, vinblastin and vincristine from Catharanthus roseus, curcumin from Curcuma longa, taxol from Taxus species, homoharringtonine from Cephalotaxus harringtonii and Cephalotaxus fortunei, genistein from Genista tinctoria, gossypol from Gossypium hirsutum, and lodopyridone from Saccharomonospora species are among few important leads [6–12]. The plant-derived natural products are reported to selectively kill cancer cells without affecting the growth of normal cells due to the chemical diversity, inherent biological activity, structural complexity, and low side effects [13, 14], and by regulating extrinsic, intrinsic, and autophagic pathways [14]. Various plant-based compounds are considered potent anticancer agents due to their specific interference with drug targets, such as topoisomerase I and II inhibitors, mitosis, protein kinase, DNA synthesis, and DNA interactive agents [15]. Modern approaches such as virtual screening, molecular modelling, natural product library, hyphenated analytical techniques, database mining, and improved in vitro and in vivo bioassay models are being used for novel drug discovery from natural products [16, 17].
Taraxacum officinale Weber, commonly known as dandelion, is a popular plant in herbal medicine belonging to the family Asteraceae. The name Taraxacum is derived from two Greek words, ‘taraxos’ (disorder) and ‘akos’ (remedy or curative), and the term officinale describes the medicinal properties of the plant [18, 19]. It is a perennial herb with a milky latex, growing up to 40–60 cm tall in a rosette form against the ground [19]. The leaves are lobed or toothed, 5–20 cm long, and dark green in colour. The roots are thick and well developed, 15–45 cm long and 1.5–2.5 cm in diameter. The flowering stalks (5–40 cm) arise from the middle of rosette leaves, which carry a solitary and terminal inflorescence. The flowers are semi-florets, bisexual, and characterized by a bright yellow colour. The fruit is a cotton-like ball with numerous seeds (Fig. 1). T. officinale is a common weed native to Europe, Asia, and North America that spreads worldwide [20]. In India, it is distributed throughout the sub-tropical to alpine region of Himalaya, covering from Ladakh to Arunachal Pradesh. T. officinale has been used in traditional medicine since ancient times to cure various diseases. The medicinal properties of the plant are due to the presence of health-promoting compounds belonging to phenolics, saponins, and sesquiterpenes [21]. Being a good source of nutritional and health-promoting components, T. officinale is consumed in various forms worldwide. The leaves and aerial parts are eaten raw as salad and processed as soup, tea, wine, and soft drinks [19, 20, 22]. The roots are utilized as coffee, tea, tincture, and capsule products. In contrast, flowers are popularly known to produce wines, teas, and syrups and are also used as flavouring agents in various food and dairy products [22, 23].

Taraxacum officinale plant and its different parts (a) Whole plant in natural habitat, (b) leaves, (c) roots, (d) flower, and (e) fruit.
Taraxacum officinale is also reported for various pharmacological activities such as antidiabetic, antioxidant, hepatoprotective, diuretic, anti-inflammatory, neuroprotective, antidepressant, antimicrobial, and immunostimulatory [19, 24–32]. T. officinale also possesses anticancer activity against a large variety of cancers, including colon, liver, and gastric, which has been scientifically validated by various in vitro and in vivo methods [33].
Although a few review articles are available on T. officinale, they largely lack complete analysis of existing information in specific areas of research. A review published in 2015 highlighted the traditional uses and pharmacological properties of Taraxacum genus [21]. Another review published in 2021 focused on the therapeutic properties of T. officinale and twelve therapeutic properties namely, diuretic, hepatoprotective, anticolitis, immunoprotective, antiviral, antifungal, antibacterial, antiarthritic, antidiabetic, antiobesity, antioxidant, and anticancer were described [19]. A recently published review highlighted the biologically active compounds of T. officinale and its anticancer potential. A few compounds were listed without any other detailed description [21]. As the species has been studied extensively for its therapeutic properties, phytochemistry, and pharmacological activity, there is a need to analyze systematically and discuss the complete scientific data available for the anticancer activity of this important medicinal plant. The present review aims to provide an in-depth analysis of existing research to document the utmost information on ethno-medicinal, phytochemical, anticancer potential, and toxicological aspects of T. officinale. Efforts have also been made to identify the biologically active compounds and elucidate the mechanism of action. This further leads to the identification of novel drugs for cancer treatment from natural sources with low toxicity and cost-effectiveness. In addition, the present review also aims to disclose the gaps in existing knowledge, major challenges, and further clinical applications of this promising anticancer candidate for future studies.
Methods
We conducted a literature search (1985–2021) related to the anticancer activity of T. officinale Weber according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), an internationally recognized guideline for reporting reviews [34]. The search was carried out from February 2020 to October 2020 in four databases: Google Scholar, Web of Science, Scopus, and Pubmed, and thoroughly searched cross-references of the literature. This review included the following search terms ‘Taraxacum officinale’, ‘ethnomedicinal uses’, OR ‘traditional uses’, ‘phytochemicals’, OR ‘secondary metabolites’, ‘anticancer activity’, ‘in vitro anticancer activity’, and ‘in vivo anticancer activity’, ‘anticancer activity of bioactive compounds’, ‘toxicological activity’. Initially, 1006 references were searched for the study, of which 504 duplicates were excluded. The authors thoroughly read the papers for eligibility criteria, and 250 articles were retrieved. These findings were further assessed for inclusion (full papers, investigation associated with ethnomedicinal, phytochemical, and anticancer activity of T. officinale) and exclusion (only abstracts, posters, presentations, not experimental, qualitative data only, and dissertation) criteria. Finally, a total of 122 studies were selected for this review. Fig. 2 shows the procedure for selecting and screening literature for PRISMA analysis.
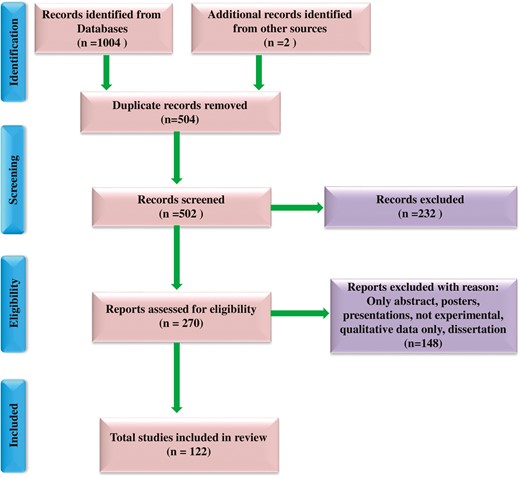
Flow chart describing selection and screening of literature using preferred reporting items for systematic reviews and meta-analyses (PRISMA).
Traditional/ethnomedicinal uses of T. officinale
Different plant parts (root, leaves, flowers, latex, aerial parts, seeds, and whole plant) and their formulations (paste, poultice, decoction, and juice) of T. officinale are used by traditional health healers to cure several diseases (Supplementary Table S1). In Cameroon, the decoction of the whole plant is used to treat liver disorders, kidney problems, and spleen problems [35]. The local inhabitants of Pakistan use flowers and leaves of the species for jaw swelling and toothache [36]. They also use the powder of T. officinale roots to cure tumours and wounds [37]. The Hakims (elderly and experienced people) of Kashmir Himalaya (India) use paste prepared from boiled leaves of T. officinale mixed with salt and turmeric for bone fracture [38]. Infusion of an aerial part to treat bile and vesicular disorders, kidney and liver pain, and stomach ulcers used by the medicinal and magic plant sellers in Bolivian Andes [39]. In Uttarakhand Himalaya (India), the traditional health healers (Vaidyas) use various medicinal preparations of roots such as decoction, extract, and powder, and the paste against migraine, cardiac complaints, jaundice, abdominal complaints, fever, hepatitis, and headache, liver trouble, and kidney problem [40–47]. The latex is applied externally to cure corn, warts, and skin eruption by the local inhabitants of Uttarakhand [40, 48]. The infusion of T. officinale leaves is used to treat cancer and bacterial and viral infections by the people of Mexico [49] T. officinale is also used as a health tonic, blood purifier, urinary antiseptic, analgesic, diuretic, purgative, appetizer, depurative, laxative, aperient, and astringent in different regions of Bosnia and Herzegovina, India, and Pakistan [50–55].
Phytochemical and nutritional screening of Taraxacum officinale
Taraxacum officinale possesses a variety of biologically active phyto-constituents, viz. alkaloids, saponins, flavonoids, glycosides, phenols, tannins, terpenoids, steroids, anthracenosides, phlobatanins, and anthraquinones in different plant parts, including leaves, roots, flowers, and whole plant [56–60]. Various tissues such as leaves, roots, flowers, and aerial parts of T. officinale contain pharmacologically active constituents (Supplementary Table S2; Fig. 3). Coumaric acid, sinapic acid, ferulic acid, monocaffeyltartaric acid, dicaffeoylquinic acid, cichoric acid, cycloartenol, taraxerol, Ψ-taraxasterol, quercetin glycosides, taraxacolide-β-D-glucoside, hesperidin, naringenin, kaempferol, gallic acid, luteolin diglycoside, luteolin-7-glycoside, luteolin glycoside II, luteolin, dicaffeoylquinic acid, inulin, phytol, lupeol, taraxasteryl acetate, and cycloartenol acetate isolated from T. officinale and few of them exert various therapeutic activity [32, 56, 57, 61–69]. Latex is a rich source of taraxinic acid, β-D-glucopyranosyl ester, α-amyrin acetate, β-amyrin acetate, cycloartenol acetate, and lupeol acetate [70]. The anticancer compounds such as taraxasterol, taraxinic acid, and taraxinic acid β-D-glucopyranosyl ester, have also been reported in this species [71–78]. The leaves and aerial parts of T. officinale are nutritionally rich and contain abundant carbohydrates, proteins, vitamins (A, E, C, and K), essential fatty acids, fibres, mineral elements, and natural antioxidants. In contrast, roots possess storage carbohydrates in inulin, which is used to develop functional foods as prebiotic and texture modifiers [79–83].
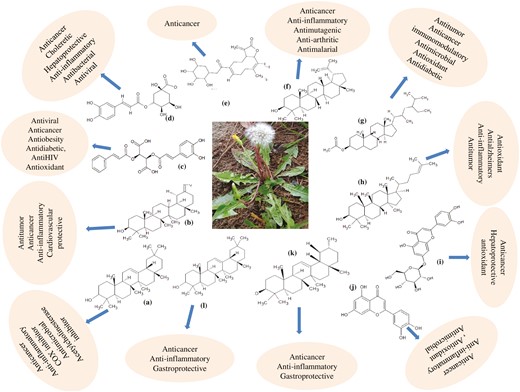
Major active compounds of T. officanale responsible for anticancer activity:(a) Taraxerol, (b) Taraxasterol, (c) Chicoric acid, (d) Chlorogenic acid, (e) Taraxinic acid β-glucopyranosyl ester, (f) Lupeol, (g) β-sitosterol, (h) Cycloartenol, (i) luteolin-7-glucoside, (j) luteolin, (k) α-amyrin, and (l) β amyrin.
Anticancer activity of Taraxacum officinale
Different parts of T. officinale, such as leaves, roots, and flowers extracted in various solvents, are used to evaluate cytotoxic activity against several cancer cell lines (Table 1).
| S. N. . | Plant part . | Extract medium . | Experimental model . | Dose/concentration . | Positive/standard . | Time of incubation . | Assay conducted . | Mechanism of action . | References . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | (TOL-AgNPs), Lf | Aqueous | In vitro: HepG2 | 10–200 μg/ml | Doxorubicin | 24 h | MTT | Cell growth inhibited | [84] |
| 2 | Lf, Fl, Rt | Aqueous | In vitro:MCF-7/ AZ; LNCaP | AgNPs and LB media | 24 h | MTT | Leaf extract decreased the growth of MCF-7/AZ cells in an ERK-dependent manner whereas the LNCaP remained unaffected, invasion of tested cell lines inhibited due to decreased phosphorylation of FAK and src and reduced activity of mMMP-2 and MMP-9 | [85] | |
| 3 | Lf, Fl, Rt, St | Methanol | In vitro:Calu-6; SNU-601 | 50–800 μg/ml | 96 h | MTT | Flower extract showed highest cytotoxicity | [86] | |
| 4 | Lf, St | Aqueous | In vitro: HL-60 | 0.25–1.25 mg/ml | Ursolic acid | 72 h | MTT | Cell growth inhibited, induction of apoptosis, fragmentation of DNA | [87] |
| 5 | Lf | Methanol, hexane, methylene chloride, ethyl acetate, butanol | In vitro: SGT; RAW 264.7 | 0.2 mg/ml and 0.4 mg/ml | Curcumin | 48 h | MTT | Cell growth inhibited, induced cytotoxicity | [88] |
| 6 | Dried prescription | Aqueous | in vitro: Hep G2 | 0.02–2 mg/ml | Dulbecco’s modified Eagle’s medium (DMEM) | 24 and 48 h | MTT, ELISA | Reduction in cell viability, induction of apoptosis; increased production of cytokines (TNF-α and IL-1 α) | [89] |
| 7 | Fl | Ethanol | in vitro: SK-OV-3 | 1.5625–100 µg/ml | 24 h | MTT, RIA | Cell proliferation inhibited, accumulation of cell population at either the S or G2/M phase, accumulation of DNA in sub-G0/G1 cells, activation of p53, significant cell-cycle arrest at S and G2/M, sub-G0 DNA fragmentation, up-regulation of bax and down-regulation of bcl-2 | [90] | |
| 8 | Fr | Ethanol | in vitro: Male Wistar rats | 1–20 μg/ml | MTT | Protect brain slices (cortex, hippocampus, and striatum) against SNP-induced decreases in cellular viability and increases in lipid peroxidation | [91] | ||
| 9 | WP | Ethanol | in vitro: human breast cancer cells; human normal liver cells WRL-68 | 6.25–400 µg/ml | 24 h | MTT | Induced cytotoxicity | [92] | |
| 10 | Lf | Methanol | In vitro: B16; HL-60; MCF-7; HCT-8 | Doxorubicin | 72 h | MTT | Induced cytotoxicity | [93] | |
| 11 | Rt | Aqueous | In vivo: Female albino rats | 500 mg/kg | Dimethylbenz(α)-anthracene (DMBA) | 1 month | Chemiluminescence, ELISA, RT-PCR | Decreased serum 15-3 CA15-3 levels, normalized up-regulated mRNA for Pdk1, Akt1, Pik3r1, Map3k1, Erbb2 and increased Pik3r1 expression, decreased tumour size, proliferation of lining epithelium of acini and ductules with hyperchromatic nuclei, increased expression of Bcl2 in the proliferated epithelium | [94] |
| 12 | WP | TOP | In vitro: HepG-2 | 0.25–4 mg/ml | Cyclo-phosphamide | 24, 48, 72 h | MTT | Cell growth inhibited | [95] |
| 13 | Lf, Fl | Triton X-100; acetone | In vitro: RK13 | 250–1000 µg/ml for MTT assay; 125–1000 µg/ml for xCELLigence Assay | 48 h | MTT, RTCA | Induced cytotoxicity | [68] | |
| 14 | WP | Aqueous fermented extract | In vitro Hela; MCF-7, SK-BR-3; MDA-MB-231; Ishikawa; AN3 Ca; NIH: OVCAR-3; SKOV-3; EJ28; RT112; SW620; PANC-1; HuH-7; A549; NHDF-C | 0.1–200 mg/ml | MTT, Mitotracker staining, Boyden chamber assay, Scratch-assay | Reduced cell viability, induced apoptosis, inhibited migration and mitochondrial integrity | [96] | ||
| 15 | Lf | Ethanol | In vitro: Cancer stem cells squamous cell; epidermoid carcinoma | 6.25–100 µg/ml | 24 h | Flow cytometry, Tunnel assay, microscopic method, PCR, agarose gel electrophoresis | Induction of apoptosis, downregulation of Sox2 gene, increased RARß2 gene expression. | [97] | |
| 16 | Lf, St | Methanol | Huh7 | MTT, Caspase-3 activity assay, ELISA, GST pull-down assay, HPLC | Induced apoptosis through JNK activation due to inhibition of the MKK7-TIPRL interaction | [98] | |||
| 17 | Rt | Aqueous | In vitro: Gastric cancer cell lines SGC7901, BGC823; normalgastric epithelium cell line GES-1 | 48 h | MTT, qRT-PCR, colony formation assay, cell transfection, wound healing assay, migration and invasion assays | Reduced cell viability, inhibited migration and invasion, down-regulated lncRNA -CCAT1 | [99] |
| S. N. . | Plant part . | Extract medium . | Experimental model . | Dose/concentration . | Positive/standard . | Time of incubation . | Assay conducted . | Mechanism of action . | References . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | (TOL-AgNPs), Lf | Aqueous | In vitro: HepG2 | 10–200 μg/ml | Doxorubicin | 24 h | MTT | Cell growth inhibited | [84] |
| 2 | Lf, Fl, Rt | Aqueous | In vitro:MCF-7/ AZ; LNCaP | AgNPs and LB media | 24 h | MTT | Leaf extract decreased the growth of MCF-7/AZ cells in an ERK-dependent manner whereas the LNCaP remained unaffected, invasion of tested cell lines inhibited due to decreased phosphorylation of FAK and src and reduced activity of mMMP-2 and MMP-9 | [85] | |
| 3 | Lf, Fl, Rt, St | Methanol | In vitro:Calu-6; SNU-601 | 50–800 μg/ml | 96 h | MTT | Flower extract showed highest cytotoxicity | [86] | |
| 4 | Lf, St | Aqueous | In vitro: HL-60 | 0.25–1.25 mg/ml | Ursolic acid | 72 h | MTT | Cell growth inhibited, induction of apoptosis, fragmentation of DNA | [87] |
| 5 | Lf | Methanol, hexane, methylene chloride, ethyl acetate, butanol | In vitro: SGT; RAW 264.7 | 0.2 mg/ml and 0.4 mg/ml | Curcumin | 48 h | MTT | Cell growth inhibited, induced cytotoxicity | [88] |
| 6 | Dried prescription | Aqueous | in vitro: Hep G2 | 0.02–2 mg/ml | Dulbecco’s modified Eagle’s medium (DMEM) | 24 and 48 h | MTT, ELISA | Reduction in cell viability, induction of apoptosis; increased production of cytokines (TNF-α and IL-1 α) | [89] |
| 7 | Fl | Ethanol | in vitro: SK-OV-3 | 1.5625–100 µg/ml | 24 h | MTT, RIA | Cell proliferation inhibited, accumulation of cell population at either the S or G2/M phase, accumulation of DNA in sub-G0/G1 cells, activation of p53, significant cell-cycle arrest at S and G2/M, sub-G0 DNA fragmentation, up-regulation of bax and down-regulation of bcl-2 | [90] | |
| 8 | Fr | Ethanol | in vitro: Male Wistar rats | 1–20 μg/ml | MTT | Protect brain slices (cortex, hippocampus, and striatum) against SNP-induced decreases in cellular viability and increases in lipid peroxidation | [91] | ||
| 9 | WP | Ethanol | in vitro: human breast cancer cells; human normal liver cells WRL-68 | 6.25–400 µg/ml | 24 h | MTT | Induced cytotoxicity | [92] | |
| 10 | Lf | Methanol | In vitro: B16; HL-60; MCF-7; HCT-8 | Doxorubicin | 72 h | MTT | Induced cytotoxicity | [93] | |
| 11 | Rt | Aqueous | In vivo: Female albino rats | 500 mg/kg | Dimethylbenz(α)-anthracene (DMBA) | 1 month | Chemiluminescence, ELISA, RT-PCR | Decreased serum 15-3 CA15-3 levels, normalized up-regulated mRNA for Pdk1, Akt1, Pik3r1, Map3k1, Erbb2 and increased Pik3r1 expression, decreased tumour size, proliferation of lining epithelium of acini and ductules with hyperchromatic nuclei, increased expression of Bcl2 in the proliferated epithelium | [94] |
| 12 | WP | TOP | In vitro: HepG-2 | 0.25–4 mg/ml | Cyclo-phosphamide | 24, 48, 72 h | MTT | Cell growth inhibited | [95] |
| 13 | Lf, Fl | Triton X-100; acetone | In vitro: RK13 | 250–1000 µg/ml for MTT assay; 125–1000 µg/ml for xCELLigence Assay | 48 h | MTT, RTCA | Induced cytotoxicity | [68] | |
| 14 | WP | Aqueous fermented extract | In vitro Hela; MCF-7, SK-BR-3; MDA-MB-231; Ishikawa; AN3 Ca; NIH: OVCAR-3; SKOV-3; EJ28; RT112; SW620; PANC-1; HuH-7; A549; NHDF-C | 0.1–200 mg/ml | MTT, Mitotracker staining, Boyden chamber assay, Scratch-assay | Reduced cell viability, induced apoptosis, inhibited migration and mitochondrial integrity | [96] | ||
| 15 | Lf | Ethanol | In vitro: Cancer stem cells squamous cell; epidermoid carcinoma | 6.25–100 µg/ml | 24 h | Flow cytometry, Tunnel assay, microscopic method, PCR, agarose gel electrophoresis | Induction of apoptosis, downregulation of Sox2 gene, increased RARß2 gene expression. | [97] | |
| 16 | Lf, St | Methanol | Huh7 | MTT, Caspase-3 activity assay, ELISA, GST pull-down assay, HPLC | Induced apoptosis through JNK activation due to inhibition of the MKK7-TIPRL interaction | [98] | |||
| 17 | Rt | Aqueous | In vitro: Gastric cancer cell lines SGC7901, BGC823; normalgastric epithelium cell line GES-1 | 48 h | MTT, qRT-PCR, colony formation assay, cell transfection, wound healing assay, migration and invasion assays | Reduced cell viability, inhibited migration and invasion, down-regulated lncRNA -CCAT1 | [99] |
Footnote: AgNPs—silver nanoparticles; Fl—flower; Fr—fruit; Lf—leaf; Rt—root; St—stem; WP—whole plant; TOL-AgNPs—Taraxacum officinale leaf—silver nanoparticles; in vitro: HepG2—human liver cancer cells; MCF-7/AZ—breast cancer cells; LNCaP—prostate cancer cells; Calu-6—human pulmonary carcinoma cells; SNU-601, SGC7901, BGC823—gastric carcinoma cells; HL-60—human promyelocytic leukemia cells; SGT—oral cancer cells; RAW 264.7—macrophage cells; Hep G2—human hepatoma cells; HCT-116—colon carcinoma cells; SK-OV-3—ovarian cancer cells; B16—murine skin cells; HL-60—human leukemia cells; MCF-7—breast cancer cells; HCT-8—human colon cells; SH-SY5Y—human neuroblastoma cells; WISH—epithelial cells; RK13—rabbit epithelial kidney cell line; Hela—human cervical cancer cell lines; MCF-7, SK-BR-3, MDA-MB-231—mamma carcinoma; Ishikawa, AN3 Ca—endometrial-carcinoma; NIH:OVCAR-3, SKOV-3—ovarian carcinoma; EJ28, RT112—urinary bladder carcinoma cell lines; SW620—colon carcinoma cell line; PANC-1—pancreas carcinoma; HuH-7—hepatocellular carcinoma; A549—lung carcinoma; NHDF-C—human fibroblast cell line, SH-SY5Y, Kelly—neuroblastoma cell lines; GES-1—normalgastric epithelium cell line.
| S. N. . | Plant part . | Extract medium . | Experimental model . | Dose/concentration . | Positive/standard . | Time of incubation . | Assay conducted . | Mechanism of action . | References . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | (TOL-AgNPs), Lf | Aqueous | In vitro: HepG2 | 10–200 μg/ml | Doxorubicin | 24 h | MTT | Cell growth inhibited | [84] |
| 2 | Lf, Fl, Rt | Aqueous | In vitro:MCF-7/ AZ; LNCaP | AgNPs and LB media | 24 h | MTT | Leaf extract decreased the growth of MCF-7/AZ cells in an ERK-dependent manner whereas the LNCaP remained unaffected, invasion of tested cell lines inhibited due to decreased phosphorylation of FAK and src and reduced activity of mMMP-2 and MMP-9 | [85] | |
| 3 | Lf, Fl, Rt, St | Methanol | In vitro:Calu-6; SNU-601 | 50–800 μg/ml | 96 h | MTT | Flower extract showed highest cytotoxicity | [86] | |
| 4 | Lf, St | Aqueous | In vitro: HL-60 | 0.25–1.25 mg/ml | Ursolic acid | 72 h | MTT | Cell growth inhibited, induction of apoptosis, fragmentation of DNA | [87] |
| 5 | Lf | Methanol, hexane, methylene chloride, ethyl acetate, butanol | In vitro: SGT; RAW 264.7 | 0.2 mg/ml and 0.4 mg/ml | Curcumin | 48 h | MTT | Cell growth inhibited, induced cytotoxicity | [88] |
| 6 | Dried prescription | Aqueous | in vitro: Hep G2 | 0.02–2 mg/ml | Dulbecco’s modified Eagle’s medium (DMEM) | 24 and 48 h | MTT, ELISA | Reduction in cell viability, induction of apoptosis; increased production of cytokines (TNF-α and IL-1 α) | [89] |
| 7 | Fl | Ethanol | in vitro: SK-OV-3 | 1.5625–100 µg/ml | 24 h | MTT, RIA | Cell proliferation inhibited, accumulation of cell population at either the S or G2/M phase, accumulation of DNA in sub-G0/G1 cells, activation of p53, significant cell-cycle arrest at S and G2/M, sub-G0 DNA fragmentation, up-regulation of bax and down-regulation of bcl-2 | [90] | |
| 8 | Fr | Ethanol | in vitro: Male Wistar rats | 1–20 μg/ml | MTT | Protect brain slices (cortex, hippocampus, and striatum) against SNP-induced decreases in cellular viability and increases in lipid peroxidation | [91] | ||
| 9 | WP | Ethanol | in vitro: human breast cancer cells; human normal liver cells WRL-68 | 6.25–400 µg/ml | 24 h | MTT | Induced cytotoxicity | [92] | |
| 10 | Lf | Methanol | In vitro: B16; HL-60; MCF-7; HCT-8 | Doxorubicin | 72 h | MTT | Induced cytotoxicity | [93] | |
| 11 | Rt | Aqueous | In vivo: Female albino rats | 500 mg/kg | Dimethylbenz(α)-anthracene (DMBA) | 1 month | Chemiluminescence, ELISA, RT-PCR | Decreased serum 15-3 CA15-3 levels, normalized up-regulated mRNA for Pdk1, Akt1, Pik3r1, Map3k1, Erbb2 and increased Pik3r1 expression, decreased tumour size, proliferation of lining epithelium of acini and ductules with hyperchromatic nuclei, increased expression of Bcl2 in the proliferated epithelium | [94] |
| 12 | WP | TOP | In vitro: HepG-2 | 0.25–4 mg/ml | Cyclo-phosphamide | 24, 48, 72 h | MTT | Cell growth inhibited | [95] |
| 13 | Lf, Fl | Triton X-100; acetone | In vitro: RK13 | 250–1000 µg/ml for MTT assay; 125–1000 µg/ml for xCELLigence Assay | 48 h | MTT, RTCA | Induced cytotoxicity | [68] | |
| 14 | WP | Aqueous fermented extract | In vitro Hela; MCF-7, SK-BR-3; MDA-MB-231; Ishikawa; AN3 Ca; NIH: OVCAR-3; SKOV-3; EJ28; RT112; SW620; PANC-1; HuH-7; A549; NHDF-C | 0.1–200 mg/ml | MTT, Mitotracker staining, Boyden chamber assay, Scratch-assay | Reduced cell viability, induced apoptosis, inhibited migration and mitochondrial integrity | [96] | ||
| 15 | Lf | Ethanol | In vitro: Cancer stem cells squamous cell; epidermoid carcinoma | 6.25–100 µg/ml | 24 h | Flow cytometry, Tunnel assay, microscopic method, PCR, agarose gel electrophoresis | Induction of apoptosis, downregulation of Sox2 gene, increased RARß2 gene expression. | [97] | |
| 16 | Lf, St | Methanol | Huh7 | MTT, Caspase-3 activity assay, ELISA, GST pull-down assay, HPLC | Induced apoptosis through JNK activation due to inhibition of the MKK7-TIPRL interaction | [98] | |||
| 17 | Rt | Aqueous | In vitro: Gastric cancer cell lines SGC7901, BGC823; normalgastric epithelium cell line GES-1 | 48 h | MTT, qRT-PCR, colony formation assay, cell transfection, wound healing assay, migration and invasion assays | Reduced cell viability, inhibited migration and invasion, down-regulated lncRNA -CCAT1 | [99] |
| S. N. . | Plant part . | Extract medium . | Experimental model . | Dose/concentration . | Positive/standard . | Time of incubation . | Assay conducted . | Mechanism of action . | References . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | (TOL-AgNPs), Lf | Aqueous | In vitro: HepG2 | 10–200 μg/ml | Doxorubicin | 24 h | MTT | Cell growth inhibited | [84] |
| 2 | Lf, Fl, Rt | Aqueous | In vitro:MCF-7/ AZ; LNCaP | AgNPs and LB media | 24 h | MTT | Leaf extract decreased the growth of MCF-7/AZ cells in an ERK-dependent manner whereas the LNCaP remained unaffected, invasion of tested cell lines inhibited due to decreased phosphorylation of FAK and src and reduced activity of mMMP-2 and MMP-9 | [85] | |
| 3 | Lf, Fl, Rt, St | Methanol | In vitro:Calu-6; SNU-601 | 50–800 μg/ml | 96 h | MTT | Flower extract showed highest cytotoxicity | [86] | |
| 4 | Lf, St | Aqueous | In vitro: HL-60 | 0.25–1.25 mg/ml | Ursolic acid | 72 h | MTT | Cell growth inhibited, induction of apoptosis, fragmentation of DNA | [87] |
| 5 | Lf | Methanol, hexane, methylene chloride, ethyl acetate, butanol | In vitro: SGT; RAW 264.7 | 0.2 mg/ml and 0.4 mg/ml | Curcumin | 48 h | MTT | Cell growth inhibited, induced cytotoxicity | [88] |
| 6 | Dried prescription | Aqueous | in vitro: Hep G2 | 0.02–2 mg/ml | Dulbecco’s modified Eagle’s medium (DMEM) | 24 and 48 h | MTT, ELISA | Reduction in cell viability, induction of apoptosis; increased production of cytokines (TNF-α and IL-1 α) | [89] |
| 7 | Fl | Ethanol | in vitro: SK-OV-3 | 1.5625–100 µg/ml | 24 h | MTT, RIA | Cell proliferation inhibited, accumulation of cell population at either the S or G2/M phase, accumulation of DNA in sub-G0/G1 cells, activation of p53, significant cell-cycle arrest at S and G2/M, sub-G0 DNA fragmentation, up-regulation of bax and down-regulation of bcl-2 | [90] | |
| 8 | Fr | Ethanol | in vitro: Male Wistar rats | 1–20 μg/ml | MTT | Protect brain slices (cortex, hippocampus, and striatum) against SNP-induced decreases in cellular viability and increases in lipid peroxidation | [91] | ||
| 9 | WP | Ethanol | in vitro: human breast cancer cells; human normal liver cells WRL-68 | 6.25–400 µg/ml | 24 h | MTT | Induced cytotoxicity | [92] | |
| 10 | Lf | Methanol | In vitro: B16; HL-60; MCF-7; HCT-8 | Doxorubicin | 72 h | MTT | Induced cytotoxicity | [93] | |
| 11 | Rt | Aqueous | In vivo: Female albino rats | 500 mg/kg | Dimethylbenz(α)-anthracene (DMBA) | 1 month | Chemiluminescence, ELISA, RT-PCR | Decreased serum 15-3 CA15-3 levels, normalized up-regulated mRNA for Pdk1, Akt1, Pik3r1, Map3k1, Erbb2 and increased Pik3r1 expression, decreased tumour size, proliferation of lining epithelium of acini and ductules with hyperchromatic nuclei, increased expression of Bcl2 in the proliferated epithelium | [94] |
| 12 | WP | TOP | In vitro: HepG-2 | 0.25–4 mg/ml | Cyclo-phosphamide | 24, 48, 72 h | MTT | Cell growth inhibited | [95] |
| 13 | Lf, Fl | Triton X-100; acetone | In vitro: RK13 | 250–1000 µg/ml for MTT assay; 125–1000 µg/ml for xCELLigence Assay | 48 h | MTT, RTCA | Induced cytotoxicity | [68] | |
| 14 | WP | Aqueous fermented extract | In vitro Hela; MCF-7, SK-BR-3; MDA-MB-231; Ishikawa; AN3 Ca; NIH: OVCAR-3; SKOV-3; EJ28; RT112; SW620; PANC-1; HuH-7; A549; NHDF-C | 0.1–200 mg/ml | MTT, Mitotracker staining, Boyden chamber assay, Scratch-assay | Reduced cell viability, induced apoptosis, inhibited migration and mitochondrial integrity | [96] | ||
| 15 | Lf | Ethanol | In vitro: Cancer stem cells squamous cell; epidermoid carcinoma | 6.25–100 µg/ml | 24 h | Flow cytometry, Tunnel assay, microscopic method, PCR, agarose gel electrophoresis | Induction of apoptosis, downregulation of Sox2 gene, increased RARß2 gene expression. | [97] | |
| 16 | Lf, St | Methanol | Huh7 | MTT, Caspase-3 activity assay, ELISA, GST pull-down assay, HPLC | Induced apoptosis through JNK activation due to inhibition of the MKK7-TIPRL interaction | [98] | |||
| 17 | Rt | Aqueous | In vitro: Gastric cancer cell lines SGC7901, BGC823; normalgastric epithelium cell line GES-1 | 48 h | MTT, qRT-PCR, colony formation assay, cell transfection, wound healing assay, migration and invasion assays | Reduced cell viability, inhibited migration and invasion, down-regulated lncRNA -CCAT1 | [99] |
Footnote: AgNPs—silver nanoparticles; Fl—flower; Fr—fruit; Lf—leaf; Rt—root; St—stem; WP—whole plant; TOL-AgNPs—Taraxacum officinale leaf—silver nanoparticles; in vitro: HepG2—human liver cancer cells; MCF-7/AZ—breast cancer cells; LNCaP—prostate cancer cells; Calu-6—human pulmonary carcinoma cells; SNU-601, SGC7901, BGC823—gastric carcinoma cells; HL-60—human promyelocytic leukemia cells; SGT—oral cancer cells; RAW 264.7—macrophage cells; Hep G2—human hepatoma cells; HCT-116—colon carcinoma cells; SK-OV-3—ovarian cancer cells; B16—murine skin cells; HL-60—human leukemia cells; MCF-7—breast cancer cells; HCT-8—human colon cells; SH-SY5Y—human neuroblastoma cells; WISH—epithelial cells; RK13—rabbit epithelial kidney cell line; Hela—human cervical cancer cell lines; MCF-7, SK-BR-3, MDA-MB-231—mamma carcinoma; Ishikawa, AN3 Ca—endometrial-carcinoma; NIH:OVCAR-3, SKOV-3—ovarian carcinoma; EJ28, RT112—urinary bladder carcinoma cell lines; SW620—colon carcinoma cell line; PANC-1—pancreas carcinoma; HuH-7—hepatocellular carcinoma; A549—lung carcinoma; NHDF-C—human fibroblast cell line, SH-SY5Y, Kelly—neuroblastoma cell lines; GES-1—normalgastric epithelium cell line.
In vitro anticancer activity
Aqueous fermented extract of T. officinale exhibited antitumorigenic effects on 14 cancer cell lines, including cervix carcinoma (Hela), mamma carcinoma (MCF-7, SK-BR-3, MDA-MB-231), endometrial-carcinoma (Ishikawa, AN3 Ca), ovarian carcinoma (NIH: OVCAR-3, SKOV-3), urinary bladder carcinoma cell lines (EJ28, RT112), colon carcinoma cell line (SW620), pancreas carcinoma (PANC-1), hepatocellular carcinoma (HuH-7), lung carcinoma (A549), and one non-tumorigenic human fibroblast cell line (NHDF-C) with reduction in cell viability (IC50) ranging from 12 to 160 mg/ml. In addition, apoptosis induction, cell viability inhibition, migration, and mitochondrial integrity were also observed in ovarian cancer cell lines (NIH: OVCAR-3, SKOV-3), whereas the non-tumorigenic cell (NHDF-C) remained unaffected [96]. Taraxacum officinale extracts of leaves and flowers prepared in two solvents nonionic Triton X-100 and organic solvent aqueous acetone, evaluated by two methods, namely, real-time cell analyzer (xCELLigence) and MTT, showed moderately cytotoxic activity at higher dose (125 µg/ml–1000 µg/ml) against epithelial kidney cells (RK13) of rabbits indicating that the cytotoxicity of the extracts increases with increasing concentration [100]. The polysaccharides extracted from T. officinale also inhibited (52%) the proliferation of human hepatocellular carcinoma cells (HepG-2) at higher doses (2000 µg/ml) [95]. The effect of aqueous extract of T. officinale different plant parts showed cytotoxicity and production of cytokines is also reported in Hep G2, breast (MCF-7/AZ), prostate (LNCaP C4-2B), pulmonary carcinoma cells (Calu-6), colon carcinoma cells (HCT-116), and gastric carcinoma cells (SNU-601) cancer cells in time and dose-dependent manner (40%–50% inhibition at 200 μg/ml for 48h). The extract increased the production of cytokines (TNF-α and IL-1 α) [85,86,89].
The ethanol extract of T. officinale flowers exhibited antiproliferative activity against cell proliferation and induction of apoptosis in human ovarian cancer cells (SK-OV-3) in a concentration-dependent manner (1.56–100 µg/ml). The cells treated with ethanol extract accumulated cell population at the S or G2/M phase caused induction of apoptosis in a dose-dependent manner. The extract also accumulated DNA in the sub-G0/G1 phase. Furthermore, p53 (antitumour suppressor gene) activation was also investigated, resulting in cell cycle arrest at S and G2/M and DNA fragmentation at the sub-G0 phase. Up-regulation of Bax (pro-apoptotic proteins) and downregulation of BCL-2 (anti-apoptotic protein) were also observed concentration-dependent [90]. The different solvent fractions (hexane, methylene chloride, ethyl acetate, and butanol) and methanol extract of T. officinale leaves exhibited antiproliferative effects against oral cancer cells (SGT) and non-cancerous macrophage cells (RAW 264.7). Methylene chloride showed significantly higher antiproliferative activity against SGT cells with 97% inhibition than others at 200 μg/ml [93]. Ethanolic extract of T. officinale leaves induced apoptosis on cancer stem cells from squamous cell cervical carcinoma through increased expression of RARß2 gene, which leads to retinoic acid-dependent or retinoic acid-independent apoptosis and growth arrest cell growth of certain cancer cells and down-regulation of Sox2 gene, which is a cell-fate determining transcription that maintains pluripotency of iPS and ES cells, multipotent lineage-committed progenitors, and tissue stem cells [88]. These genes play crucial roles in regulating embryonic development, stem cell maintenance, evading apoptotic signals, growth regulation of epithelial cells, and tumourigenesis [97, 101].
Methanol fraction of T. officinale used as a novel TNF-related apoptosis-inducing ligand (TRAIL) sensitizer against Huh7 cell lines. The combination treatment of TRAIL and T. officinale exhibited a reduction in cell viability (52%) and induced apoptosis through inhibition of MKK7-TIPRL interaction and consequent activation of MKK7-JNK phosphorylation. In addition, combined treatment of chicoric acid and TRAIL-induced cell apoptosis in Huh7 cells via JNK activation due to inhibition of MKK7-TIPRAL interaction [102]. The root extract of T. officinale was investigated against two gastric cancer cell lines (SGC7901 and BGC823) and a noncancerous gastric epithelium cell line (GES-1). The results indicated that cell viability, migration, and invasion of gastric cell lines were inhibited without inducing toxicity in noncancerous cells. It was also observed that long noncoding RNAs (lncRNA)—colon cancer-associated transcript-1 (CCAT1) was significantly down-regulated in the root extract of T. officinale treated gastric cancer cells, inhibiting proliferation and migration of gastric cells [98]. These lncRNAs noncoding RNAs play a central role in carcinogenesis, including DNA transcription, chromosomal looping, chromatin modification, interactions with proteins, and mRNAs [99]. lncRNAs are thus considered as therapeutic, diagnostic, and prognostic factors in cancer. Downregulation of the lncRNAs in cancer chemo-resistance decreases proliferation and metastasis with reduced viability and increased apoptosis [103].
The stem extract of T. officinale revealed significantly higher cytotoxic activity (IC50 71 μg/ml) than its leaf extract (IC50 540 μg/ml) in HL-60 cells. These extracts induced apoptosis, evidenced by morphological changes such as cell shrinkage, formation of apoptotic bodies, and DNA fragmentation of tested cell lines [104]. The aqueous extract of T. officinale leaves for the synthesis of silver nanoparticles (TOL-AgNPs) exhibited cytotoxic activity against HepG2 cell lines in a dose-dependent manner, and 95% cell growth inhibition was achieved % at the higher concentration (200 μg/ml) [87]. The human breast cancer cell line (MCF-7) undergoes significantly higher cytotoxicity with an IC50 value at 190.5 µg/ml of T. officinale whole plant extract. [84]. Overall, further fractionation of extract and isolation of compounds might lead to the discovery of new potent anticancerous molecules from the species.
In vivo anticancer activity
Ethanolic extract T. officinale fruits were investigated to determine (SNP)-cellular viability and lipid peroxidation. The extract at 1–20 µg/ml concentration significantly protected sodium nitroprusside SNP-induced decrease in cellular viability and increase in lipid peroxidation in rats’ brain slices (cortex, hippocampus, and striatum). Moreover, the extract also exerted potent scavenger activity against DPPH, NO, and H2O2 and protected against Fe2+-induced deoxyribose oxidation [92]. Aqueous extract of T. officinale roots administered to female albino mice reduced the elevated CA15-3 levels in DMBA-induced breast cancer. Administration of DMBA increased the expression of Pdk1, Akt1, Pik3r1, Map3k1, Erbb2, and PIk3ca genes in mRNA. The extract normalized the up-regulated m-RNA for Pdk1, Akt1, Pik3r1, Map3k1, and Erbb2, while increasing the expression of Pik3r1. In addition, the extract showed weak expression of Bcl2 in acini and ductules in mammary tissue of DMBA-treated animals.[91] The Pdk1 gene is responsible for the phosphorylation of many other AGC kinases, including p90 ribosomal protein S6 kinase (p90RSK), p70 ribosomal protein S6 kinase (p70S6K), serum/glucocorticoid regulated kinase (SGK), and the members of protein kinase C (PKC) family [94]. The abnormal activation of these genes has been reported to cause uncontrolled cell replication, apoptosis escape, aberrant angiogenesis, invasion and dissemination, metastasis, and metabolic reprogramming [105]. Akt1, Erbb2, PIK3R1, and Map3k1 also play a crucial role in cell proliferation, migration, invasion, metabolism, apoptosis, and survival parameters [106–111]. PIk3ca represents the most common mutationally activated gene in the cancer genome and participates in cell proliferation and survival without growth factors [112].
Anticancer activity of bioactive compounds
Various isolated compounds from T. officinale were reported to have cytotoxic activity in different cancers, including liver, papillary thyroid, promyelocytic leukemia, cervical carcinoma, and many others (Fig. 4). Taraxasterols are the major biological compounds of T. officinale reported to have anticarcinogenic activity against colon and breast cancer by inhibiting invasion of tumour cells and metastasis [71]. Taraxasterol a major active compound of T. officinale inhibited the cell proliferation of liver cancer cells. This activity is characterized by the induction of apoptosis, and cell cycle arrest at G0/G1 phase by up-regulating Hint1 and Bax, down-regulating Bcl2, and cyclin D1 expression along with promoting the demethylation in the Hint1 promoter region [113]. Taraxasterol also investigated against papillary thyroid cancer cell migration, invasion, and epithelial to mesenchymal transition induced by TGF-β. Taraxasterol significantly suppressed TGF-β induced migration by decreasing MMP-2 and MMP-9 levels and reversing the EMT process via Wnt/β-catenin pathway [114]. Taraxinic acid exhibited antiproliferative activity against human leukemia cell lines HL-60 via induction of cell differentiation, down-regulation of c-myc expression, and upregulation of p21(CIP1) and p27(KIP1) cyclin-dependant kinase inhibitors suggested that taraxinic acid could be utilized as a potential therapeutic agent for the treatment of human leukemia [72]. Michalska et al. reported that taraxinic acid and its derivatives 11β,13-dihydrotaraxinic acid, taraxinic acid β-glucopyranosyl ester, 11β,13-dihydro derivative showed cytotoxic activity against human melanoma cells (A375, HTB140, and WM793), human normal prostate epithelial cells (PNT-2), human prostate carcinoma cell line (DU145), and normal human keratinocytes (HaCaT) [73]. Taraxinic acid β-D-glucopyranosyl ester extracted from the leaf extract of T. officinale also showed cytotoxic activity against human liver cells (Huh7) by induction of Nrf2 and Hmox1 [113].
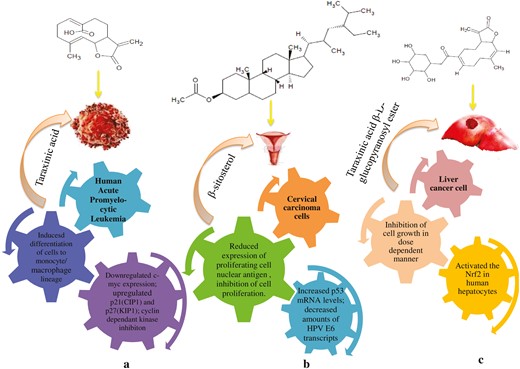
Representation of underlying mechanism of different compounds: (a) antiproliferative activity of taraxinic acid against human leukemia cells, (b) antiproliferative activity of β-sitosterol against cervical carcinoma cancer cells, and (c) antiproliferative activity of taraxinic acid β-D-glucopyranosyl ester against human liver cells.
Among other compounds present in the species, β-sitosterol reduced the action of proliferating cell nuclear antigen and inhibited cell proliferation in cervical carcinoma cancer cells (Caski and HeLa cells) with increased levels of p53 mRNA and decreased amounts of HPV E6 transcripts [115]. Chlorogenic acid exhibited an inhibitory effect against hepatocellular carcinoma cell (HepG2) proliferation using in vitro assays through inactivation of ERK1/2 [74]. It inhibited osteosarcoma cell lines (U2OS, Saos-2, and MG-63 OS) proliferation by induction of apoptosis and activating ERK1/2 [114]. Chicoric acid showed cell viability reduction and apoptosis induction in gastric cancer cell lines (GC7901, MGC803) in dose-dependent manner (5–100 μM) mediated by induction of autophagy through the activation of AMPK [75]. Inulin, α and β-amyrins, isolated from T. officinale root reported to have potent cytotoxic activity [116, 117]. Cycloartenol, luteolin, α-amyrin, and β-amyrin inhibited cell growth proliferation of different cancer cell lines (IC50 25 µM) attributed to induction of apoptosis, G2/M cycle arrest, activation of p38, and JNK signalling pathways [11, 77, 78, 118, 119].
Toxicological studies
The toxicity effect of dried powder of T. officinale was administered to Sprague–Dawley rats, and albino rabbits showed no sign of acute toxicity and changes in behavioural patterns for up to 7 days [120]. In two successive phases, mice treated with Nω-nitro-L-arginine methyl ester (L-Name) (40 mg/kg bw) assessed the acute toxicity of 70% ethanolic extract of T. officinale root and leaves via the oral route (p.o). In the first phase (doses 10, 100, and 1000 mg/kg BW), the extract did not cause mortality up to the highest dose. Similarly, in the second phase (1600, 2900, and 5000 mg/kg BW.), no mortality was observed (LD50 > 5000 mg/kg). Furthermore, both extracts showed no changes in behaviour and physical appearance up to 14 days after administration [121]. A 70% ethanolic extract of T. officinale roots exerts low acute toxicity (LD50 500–5000 mg/kg), but shows no renal, lipid, or hematological parameters [122].
Conclusion and future prospective
Medicinal plants are important in disease prevention and have been used for centuries for different healthcare practices worldwide. The effect of different compounds present in T. officinale in different types of cancer in laboratory conditions might lead to the discovery of novel anticancer agents. However, developing a chemotherapy drug still has many challenges, such as a poor understanding of molecular mechanisms due to unknown molecular targets of the active molecules and insufficient efforts in clinical trials. Moreover, identified potential extracts, fractions, and active compounds in different in vitro screening might be targeted for clinical trials to harness the potential of species right perspectives.
Further, there is a need for characterization and toxicological investigation of active constituents of T. officinale responsible for anticancer activity. The isolation of different compounds for the species and their bioassay-guided fractionation analysis will further help identify potential compounds for curing a specific type of cancer. Identification of an effective drug delivery system is also an important aspect for future research as identified molecules in the species such as taraxerol, taraxasterol, lupeol, luteolin, and α and β-amyrin have either low or unknown bioavailability in the human body. The synergistic effects of multiple constituents present in herbal drugs on different molecular targets increase more systematic investigations following the system biology approach. Research must continue to identify other potential active constituents and their optimization for developing potential analogs with improved safety and efficacy. Advanced molecular biology, genetics, bioinformatics, nanotechnological approaches, and biotechnology technologies can improve biological constituents by isolation, purification, characterization, and development of plant-derived natural products, which can be effective in novel drug discovery systematically and cost-effectively.
Acknowledgments
The authors thank the director, G. B. Pant National Institute of Himalayan Environment, Kosi-Katarmal, Almora, Uttarakhand, India, for providing necessary facilities and encouragement. Members of the Centre for Biodiversity Conservation and Management are greatly acknowledged for their support and cooperation during the study. Support from In-house Project 4 is also greatly acknowledged.
Author contributions
I.D.B. and D.T. conceived the idea and designed the study. D.T., P.K., and L.S. compiled the database and wrote the manuscript. S.R., R.C.S., and V.P. reviewed and edited the MS. I.D.B. critically reviewed and improved the MS. All authors contributed to the editing and critical revision of the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Funding
Support for this research work provided by In-house Project 4 is greatly acknowledged.
Data availability
In this review paper, no primary data was generated.



