-
PDF
- Split View
-
Views
-
Cite
Cite
Chetan B Mukhtyar, Clare Beadsmoore, Georgina Ducker, Sarah Fordham, Katherine Sisson, Colin Jones, Ultrasonography-led multimodal diagnostic pathway for giant cell arteritis, Rheumatology, Volume 64, Issue 4, April 2025, Pages 2077–2082, https://doi.org/10.1093/rheumatology/keae493
Close - Share Icon Share
Abstract
This study aims to establish the sensitivity and negative predictive value of a multimodal pathway incorporating ultrasonography, 18-fluorodeoxyglucose labelled PET-CT and temporal artery biopsy for the diagnosis of giant cell arteritis.
In total, 1000 consecutive referrals for a new diagnosis of giant cell arteritis were analysed. All patients had a protocolized examination. Patients with a negative ultrasonography and a CRP of ≥20 mg/L received an extended ultrasound examination. If that was negative, and there was no other explanation for their presentation, a second test in the form of either a temporal artery biopsy or an 18-fluorodeoxyglucose labelled PET-CT was offered. We calculated the sensitivity and negative predictive value of the interventions for diagnosing giant cell arteritis.
279/1000 patients had positive ultrasonography for giant cell arteritis. 202 had bilateral superficial temporal arterial involvement. Ultrasonography of the axillary artery and other head/neck arteries increased the yield by 53 and 24 patients, respectively. 181 patients were referred for a second test. 24/139 temporal artery biopsies and 7/42 18-fluorodeoxyglucose labelled PET-CT scans were positive. The sensitivity and negative predictive value rise from 62.3% and 84.7%, respectively, for imaging superficial temporal arteries alone, to 95.7% and 98.0%, respectively, for extended ultrasonography plus a second test.
This is the first real-world evidence of the utility of ultrasonography for the diagnosis of giant cell arteritis as part of a multimodal diagnostic pathway.
Ultrasonography-led diagnostic pathways have a place in the real world when accompanied by clinical filtering and supplemented by additional diagnostic modalities.
Ultrasonographic imaging of superficial temporal arteries should be complemented by imaging of the axillary arteries and other head and neck arteries to reduce dependence on biopsies.
A negative ultrasonography, when accompanied by a C-reactive protein of <20 mg/L, has a high sensitivity and negative predictive value for giant cell arteritis.
Introduction
GCA is a large vessel vasculitis that has an affinity for branches of the external carotid artery. To a lesser extent, it can affect the aorta and the subclavian system of arteries, including the vertebral artery [1]. GCA is the most common primary systemic vasculitis, with an annual incidence of 80–100 million over the age of 50 in Norfolk County, UK [2]. The technique of imaging the superficial temporal arteries using ultrasonography was first described in the mid-1990s [3]. Since then, there has been an international consensus on the definitions of normal and abnormal ultrasonography images [4]. The EULAR has endorsed the use of ultrasonography as a first-line diagnostic test for giant cell arteritis [5]. The ACR recommends temporal artery biopsy in preference to ultrasonography as a first-line diagnostic test [6]. There have been several small studies examining the diagnostic accuracy of ultrasonography [7]. In the most recent meta-analysis of eight studies with a low risk of bias, including 584 patients, the pooled sensitivity of ultrasonography with clinical diagnosis as a reference standard was 88% [8]. There is a complete absence of large volume real-world data that establishes the value of ultrasonography as a first-line diagnostic test. Our centre has provided an ultrasonography service since 2017, established after a formal validation process [9]. Between 2017 and 2023, we received 1000 referrals for ultrasonography to assess for a new diagnosis of giant cell arteritis. We use these data to report the utility of this investigation in the real world.
Aim
The primary aim of this study was to test the sensitivity and negative predictive value of our pathway. The secondary aims were to: (a) measure the contribution of imaging arteries beyond the superficial temporal artery to the diagnostic yield and (b) measure the contribution of stratified diagnostic tests to improve the diagnostic performance of the pathway.
Methods
Centre
All referrals were made to the vasculitis service in the rheumatology department of the Norfolk and Norwich University Hospital.
Patients
In total, 1000 consecutive referrals for new diagnosis of giant cell arteritis from January 2017 to July 2023 were included for analysis. Repeat referrals and referrals for relapsing disease were not included. All data were recorded at the time of examination. No specific ethical approval was sought to analyse these data, which were acquired as part of routine care for our patients.
Protocol
All referrals were first assessed by either an ophthalmology or rheumatology doctor. Those suspected to have giant cell arteritis had blood tests done as per a hospital-approved protocol and referred for ultrasonography. Patients with cranial symptoms were all started on high-dose prednisolone. At the ultrasonography appointment, the superficial temporal arteries and the axillary arteries were scanned. The superficial temporal artery was scanned from the neck of the mandible to its bifurcation into the frontal and parietal rami. Both rami were scanned as distally as possible. All three parts of the axillary artery from the clavicle to its termination as the brachial artery were scanned. If the ultrasonography did not demonstrate vasculitis, we stratified the patients by CRP results. Patients with a CRP of ≥20 mg/L (normal levels <5 mg/L) prior to the use of glucocorticoids (or if it was not available) received an extended manifestation-based ultrasonography of other cranial and extracranial arteries. If that was also negative, further review of history and investigations took place. In the absence of another explanation, they were referred for a second test. The choice of the second test was a temporal artery biopsy for patients with cranial symptoms or an 18-fluorodeoxyglucose labelled PET-CT if the symptoms were predominantly constitutional (weight loss >2 kg, fever >38°C, drenching night sweats, loss of appetite). In the circumstance that a second test was not performed when indicated, either because of patient preference or on the advice of the referring physician, for the purposes of the analysis it was assumed that the second test would have been negative and the pathway would not have identified the case.
Ultrasonography
The ultrasound machines used during this study were Toshiba Viamo using a linear 4–14 MHz linear probe, GE Logiq e using 10–22 MHz linear probe for temporal arteries OR 8–18 MHz hockey stick probe for other cranial arteries OR 4–12 linear MHz probe for extracranial arteries, GE Logiq P9 using 10–22 MHz linear probe for temporal arteries OR 8–18 MHz hockey stick probe for other cranial arteries OR 4–12 linear MHz probe for extracranial arteries OR curvilinear 1–5 MHz probe for the arch of aorta. The examinations were performed by C.B.M. or G.D. The validation of their skills has been published elsewhere [9, 10].
Manifestations-based ultrasonography
The extended ultrasonography depended on clinical manifestations. Posterior headache led to occipital artery scan; dental pain, jaw claudication, diplopia led to maxillary artery scan; dysphagia led to facial artery scan; taste perversion or tongue claudication led to lingual artery scan; carotidynia led to common carotid and external carotid artery scan; neck pain with or without dizziness or ataxia led to vertebral artery scan; proximal shoulder girdle pain or stiffness led to scan of the subclavian arteries. The expertise in scanning these arteries grew progressively throughout the timeframe of the study. The development of the maxillary artery technique was developed in our unit [11]. Supplementary Table 1, available at Rheumatology online, has the details of how the arteries were scanned.
Case definition
The presence of concentric homogeneous hypoechoic thickening of the intima-media complex was considered to be diagnostic of vasculitis—the ‘halo’ sign. For the superficial temporal artery and its frontal and parietal rami, the inability to compress the halo was also necessary—the ‘compression’ sign. These definitions are as agreed upon through international collaborative work [4]. The involvement of at least two arteries was necessary for the ultrasonography to be considered diagnostic for giant cell arteritis. The parietal and frontal rami were considered to be part of the superficial temporal artery; unilateral positivity in all three segments was not sufficient to be considered diagnostic. Involvement of a single artery always prompted extended ultrasonography, irrespective of the CRP level.
Temporal artery biopsy
Unilateral temporal artery biopsy was performed by an ophthalmic surgeon. The course of the temporal artery was marked out by palpating the pulse, but in some cases where manual palpation of the temporal artery was not possible, a pencil-type Doppler ultrasound with water-based lubrication was used to identify the artery course. Subcutaneous block was performed around the incision site using lidocaine 2% with adrenaline 1:80 000. Incision was made with a No. 15 blade scalpel across the artery, penetrating only skin and subcutaneous tissue to avoid injuring the underlying vessel. Blunt dissection through the subcutaneous fat and temporoparietal fascia allowed exposure of 3–5 cm of the vessel. 5-0 vicryl was used to ligate the vessel both proximally and distally, isolating a 3 cm section of the artery. This intervening segment of the artery was then removed using sharp scissors and sent to pathology in formalin. Demonstration of intramural inflammation was considered as a positive biopsy. Degenerative changes suggestive of ‘healed arteritis’ were not considered to be positive. The ophthalmologist and the pathologist were not blinded to the ultrasonography results.
18-Fluorodeoxyglucose PET-CT
All scans were done on a GE Discovery MI Gen 2 machine. The imaging was performed about 1 h after the injection of the radionuclide. The standardized uptake values are based on Q-Clear images. Radionuclide uptake in the walls of the large vessels greater than the background uptake in the liver was considered positive. The radiologist was not blinded to the ultrasonography results.
Follow-up of negative tests
Patients with a CRP of <20 mg/L with an initial negative ultrasonography, and those whose second test was also negative were advised a rapid taper of prednisolone. Those who had been on prednisolone for <2 weeks were advised to stop the drug immediately. The others had their prednisolone reduced immediately to prednisolone 20 mg daily and subsequent taper by 5 mg every week. All patients received instructions to call the vasculitis service if they or their primary care physician felt that giant cell arteritis was still a possibility. On further contact, the patients were investigated as new presentation. If they had positive tests for giant cell arteritis, the pathway was considered to have failed them. After 6 months of no contact, the patient was considered to not have giant cell arteritis and discharged. Norfolk, UK, does not have another vasculitis service where the patient could be lost to follow-up. For the purposes of this analysis, the final diagnosis was therefore made at 6 months.
Results
The median (interquartile range) age of the patients referred for ultrasonography was 73 (13). Patients were seen at a median (interquartile range) of 4 (4) calendar days after referral and a median (interquartile range) of 4 (5) calendar days after commencing prednisolone. Of the 1000 referrals, 324 had a final diagnosis of GCA. Figure 1 shows the journey of patients through our pathway.
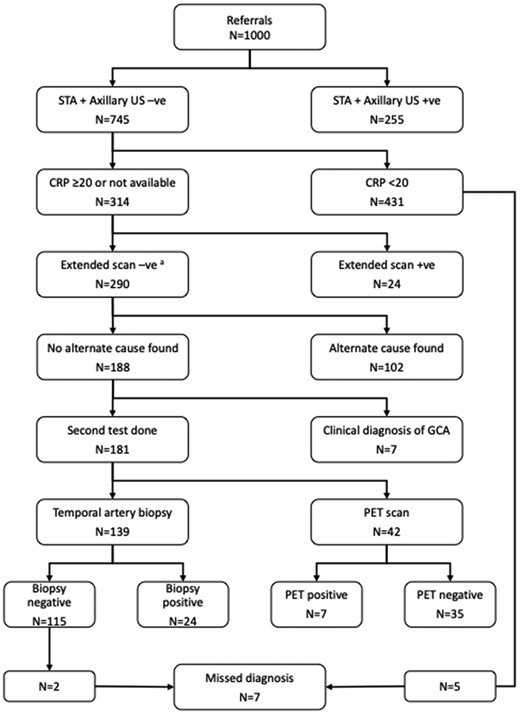
Results of the 1000 referrals for ultrasonography. aSeven cases had missing CRP levels, and 283 had a level ≥20 mg/L. STA: superficial temporal artery; US: ultrasonography; PET: 18-fluorodeoxyglucose PET-CT
255/1000 ultrasound scans of the superficial temporal and axillary arteries were diagnostic for giant cell arteritis. 202 cases had bilateral superficial temporal artery involvement. 15 cases had unilateral superficial temporal arterial involvement with evidence of involvement in at least one other artery in the head or neck. Imaging of the axillary artery increased the yield in an additional 53 cases, 5 of whom had unilateral superficial temporal arterial involvement.
Of the 745 cases, the pre-steroid CRP was unavailable in 7 cases and was ≥20 mg/L in 307 cases. Treatment was stopped in 431 patients with a CRP <20 mg/L. Extended ultrasonography performed in 314 patients identified 24 further cases, including 10 that had unilateral superficial arterial involvement.
Of the remaining 290 cases, an alternate explanation for the CRP (and the presentation) was found in 102 cases. 7 received a clinical diagnosis of giant cell arteritis without a second test, and 181 were referred for a second test.
139/181 were referred for a temporal artery biopsy, and 42/181 were referred for an 18-fluorodeoxyglucose-labelled PET-CT. The second test was diagnostic for giant cell arteritis in 31/181 cases. 24/139 temporal artery biopsies were positive, and 7/42 18-fluorodeoxyglucose labelled PET-CT scans were positive. Treatment was stopped in 115 biopsy-negative patients and 35 PET (positron emission tomography) scan-negative patients.
In all, 581 patients were advised to stop or rapidly taper their prednisolone—431 patients had a CRP <20 mg/L, and 150 patients had a negative second test. 24 patients requested reassessment within 6 months of the date of the original presentation, and 7 were found to have giant cell arteritis. Details of those seven cases are shown in Table 1. None of them suffered permanent visual loss. If our triage criterion for CRP had been lowered to 10 mg/L, we may have picked up 3/7 cases, but we would have referred 85 more patients for a temporal artery biopsy. The pathway failed to diagnose giant cell arteritis in these seven cases, and the second test was deemed to have been negative in the seven cases where a clinical diagnosis was made without resort to a second test.
| Age . | Gender . | Duration of prednisolone prior to ultrasonography (days) . | CRP level (mg/L) . | Second test . | Diagnostic delay (days) . | Details of representation on follow-up . | |
|---|---|---|---|---|---|---|---|
| 1 | 66 | Female | 6 | 12 | Not offered | 7 | Presented with headache; temporal artery biopsy was positive. |
| 2 | 80 | Female | 112 | Not available | Temporal artery biopsy—negative | 135 | Presented with visual symptoms; repeat ultrasonography was positive. |
| 3 | 71 | Female | 3 | 95 | Temporal artery biopsy—negative | 111 | Presented with headache, neck pain, jaw claudication, and constitutional symptoms; repeat ultrasonography was positive. |
| 4 | 80 | Female | 0 | 19 | Not offered | 59 | Presented with scalp tenderness, jaw claudication, transient visual symptoms; repeat ultrasonography was positive. |
| 5 | 67 | Female | 8 | 10 | Not offered | 21 | Presented with bitemporal scalp tenderness, shoulder girdle stiffness; temporal artery biopsy was positive. |
| 6 | 75 | Female | 3 | 1 | Not offered | 33 | Details of presentation not recorded; temporal artery biopsy was positive. |
| 7 | 71 | Male | 9 | 9 | Not offered | 17 | Presented with recurrence of headaches; repeat ultrasonography was positive. |
| Age . | Gender . | Duration of prednisolone prior to ultrasonography (days) . | CRP level (mg/L) . | Second test . | Diagnostic delay (days) . | Details of representation on follow-up . | |
|---|---|---|---|---|---|---|---|
| 1 | 66 | Female | 6 | 12 | Not offered | 7 | Presented with headache; temporal artery biopsy was positive. |
| 2 | 80 | Female | 112 | Not available | Temporal artery biopsy—negative | 135 | Presented with visual symptoms; repeat ultrasonography was positive. |
| 3 | 71 | Female | 3 | 95 | Temporal artery biopsy—negative | 111 | Presented with headache, neck pain, jaw claudication, and constitutional symptoms; repeat ultrasonography was positive. |
| 4 | 80 | Female | 0 | 19 | Not offered | 59 | Presented with scalp tenderness, jaw claudication, transient visual symptoms; repeat ultrasonography was positive. |
| 5 | 67 | Female | 8 | 10 | Not offered | 21 | Presented with bitemporal scalp tenderness, shoulder girdle stiffness; temporal artery biopsy was positive. |
| 6 | 75 | Female | 3 | 1 | Not offered | 33 | Details of presentation not recorded; temporal artery biopsy was positive. |
| 7 | 71 | Male | 9 | 9 | Not offered | 17 | Presented with recurrence of headaches; repeat ultrasonography was positive. |
| Age . | Gender . | Duration of prednisolone prior to ultrasonography (days) . | CRP level (mg/L) . | Second test . | Diagnostic delay (days) . | Details of representation on follow-up . | |
|---|---|---|---|---|---|---|---|
| 1 | 66 | Female | 6 | 12 | Not offered | 7 | Presented with headache; temporal artery biopsy was positive. |
| 2 | 80 | Female | 112 | Not available | Temporal artery biopsy—negative | 135 | Presented with visual symptoms; repeat ultrasonography was positive. |
| 3 | 71 | Female | 3 | 95 | Temporal artery biopsy—negative | 111 | Presented with headache, neck pain, jaw claudication, and constitutional symptoms; repeat ultrasonography was positive. |
| 4 | 80 | Female | 0 | 19 | Not offered | 59 | Presented with scalp tenderness, jaw claudication, transient visual symptoms; repeat ultrasonography was positive. |
| 5 | 67 | Female | 8 | 10 | Not offered | 21 | Presented with bitemporal scalp tenderness, shoulder girdle stiffness; temporal artery biopsy was positive. |
| 6 | 75 | Female | 3 | 1 | Not offered | 33 | Details of presentation not recorded; temporal artery biopsy was positive. |
| 7 | 71 | Male | 9 | 9 | Not offered | 17 | Presented with recurrence of headaches; repeat ultrasonography was positive. |
| Age . | Gender . | Duration of prednisolone prior to ultrasonography (days) . | CRP level (mg/L) . | Second test . | Diagnostic delay (days) . | Details of representation on follow-up . | |
|---|---|---|---|---|---|---|---|
| 1 | 66 | Female | 6 | 12 | Not offered | 7 | Presented with headache; temporal artery biopsy was positive. |
| 2 | 80 | Female | 112 | Not available | Temporal artery biopsy—negative | 135 | Presented with visual symptoms; repeat ultrasonography was positive. |
| 3 | 71 | Female | 3 | 95 | Temporal artery biopsy—negative | 111 | Presented with headache, neck pain, jaw claudication, and constitutional symptoms; repeat ultrasonography was positive. |
| 4 | 80 | Female | 0 | 19 | Not offered | 59 | Presented with scalp tenderness, jaw claudication, transient visual symptoms; repeat ultrasonography was positive. |
| 5 | 67 | Female | 8 | 10 | Not offered | 21 | Presented with bitemporal scalp tenderness, shoulder girdle stiffness; temporal artery biopsy was positive. |
| 6 | 75 | Female | 3 | 1 | Not offered | 33 | Details of presentation not recorded; temporal artery biopsy was positive. |
| 7 | 71 | Male | 9 | 9 | Not offered | 17 | Presented with recurrence of headaches; repeat ultrasonography was positive. |
The sensitivity and the negative predictive value of the diagnostic pathway improve as we add more parameters (Table 2). For ultrasonography of superficial temporal arteries alone, the sensitivity is 62.3%, improving to 78.7% with the addition of imaging the axillary arteries. The extended manifestation-based approach improves the sensitivity to 86.1%. A multimodal diagnostic approach with the addition of a second test optimizes the sensitivity to 95.7%.
Stepwise sensitivity and negative predictive value of diagnostic interventions
| Diagnostic intervention . | Sensitivity . | Negative predictive value . |
|---|---|---|
| Ultrasonography of superficial temporal arteries alone | 202/324 = 62.3% | 676/798 = 84.7% |
| Ultrasonography of superficial temporal and axillary arteries | 255/324 = 78.7% | 676/744 = 90.1% |
| Ultrasonography of superficial temporal, axillary and other arteries based on clinical manifestations | 279/324 = 86.1% | 676/721 = 93.8% |
| Ultrasonography plus second test (temporal artery biopsy or 18-fluorodeoxyglucose labelled PET-CT) | 310/324 = 95.7% | 676/690 = 98.0% |
| Diagnostic intervention . | Sensitivity . | Negative predictive value . |
|---|---|---|
| Ultrasonography of superficial temporal arteries alone | 202/324 = 62.3% | 676/798 = 84.7% |
| Ultrasonography of superficial temporal and axillary arteries | 255/324 = 78.7% | 676/744 = 90.1% |
| Ultrasonography of superficial temporal, axillary and other arteries based on clinical manifestations | 279/324 = 86.1% | 676/721 = 93.8% |
| Ultrasonography plus second test (temporal artery biopsy or 18-fluorodeoxyglucose labelled PET-CT) | 310/324 = 95.7% | 676/690 = 98.0% |
Stepwise sensitivity and negative predictive value of diagnostic interventions
| Diagnostic intervention . | Sensitivity . | Negative predictive value . |
|---|---|---|
| Ultrasonography of superficial temporal arteries alone | 202/324 = 62.3% | 676/798 = 84.7% |
| Ultrasonography of superficial temporal and axillary arteries | 255/324 = 78.7% | 676/744 = 90.1% |
| Ultrasonography of superficial temporal, axillary and other arteries based on clinical manifestations | 279/324 = 86.1% | 676/721 = 93.8% |
| Ultrasonography plus second test (temporal artery biopsy or 18-fluorodeoxyglucose labelled PET-CT) | 310/324 = 95.7% | 676/690 = 98.0% |
| Diagnostic intervention . | Sensitivity . | Negative predictive value . |
|---|---|---|
| Ultrasonography of superficial temporal arteries alone | 202/324 = 62.3% | 676/798 = 84.7% |
| Ultrasonography of superficial temporal and axillary arteries | 255/324 = 78.7% | 676/744 = 90.1% |
| Ultrasonography of superficial temporal, axillary and other arteries based on clinical manifestations | 279/324 = 86.1% | 676/721 = 93.8% |
| Ultrasonography plus second test (temporal artery biopsy or 18-fluorodeoxyglucose labelled PET-CT) | 310/324 = 95.7% | 676/690 = 98.0% |
Discussion
There is no accepted gold standard for the diagnosis of giant cell arteritis. Histopathology could be considered specific but is reliant on adequate surgical technique, appropriate sampling, prior length of glucocorticoid exposure, and variations in reporting [12]. A meta-analysis of 32 studies including 3092 patients reported a sensitivity of 77.3% for temporal artery biopsy against the reference standard of classification criteria for giant cell arteritis [13]. But we know that classification criteria function poorly when used for diagnostic purposes [14]. In this paper, we demonstrate that ultrasonography-led multimodal diagnostic pathway has a high sensitivity and high negative predictive value for giant cell arteritis. Like for all investigations, the results of these tests only make sense in the appropriate clinical context. All 1000 referrals to our service had been seen by a primary care physician and a secondary care doctor who both thought that this was a valid differential diagnosis. It is extremely important to note that this pathway cannot exist without that level of prior clinical filtering. We are aware that risk models have been created for predicting the results of tests [15, 16]. They are of academic value, but we do not use them. It did not feel justifiable to restrict access to clinical opinion and diagnostics when at least two clinicians considered giant cell arteritis to be a diagnostic possibility. We have deliberately not reported specificity or positive predictive value because that would be a spurious exercise. In patients with significant prior clinical filtering, a positive test by any modality was diagnostic for giant cell arteritis. We did not consider any positive result to be a false-positive result. If we had to report specificity and positive predictive value, they would both be 100%. But that could be misrepresented as the specificity of the technologies and not the pathway.
This paper demonstrates that an ultrasonography-led multimodal diagnostic pathway has a very high certainty of ruling out giant cell arteritis. There are other important considerations to note. EULAR recommends a referral to a specialist team or centre that has access to requisite clinical expertise and access to diagnostic imaging [17]. Our centre has undergone a developmental journey to build this expertise, the details of which have been published elsewhere [18]. To a certain extent, this paper is a continuation of our journey to improving patient care. During the timeline of this study, we continued developing techniques for other branches of the external carotid artery. The facial, occipital, and posterior auricular arteries are superficial and can be scanned without significant modification of technique. But, of the deeper branches of the external carotid artery, the maxillary artery is the most significant one in giant cell arteritis. Jaw claudication is thought to be a signature sign that has few differential diagnoses [19]. The cause of the claudication is due to ischaemia of the masseter and the pterygoids, which are mainly nourished by the maxillary artery. The ability to reliably apply the definitions of ‘halo sign’ to the maxillary artery was developed at our centre during this study [11]. The aortic arch ultrasonography is the latest addition to our service and has required investment for a new C1–5 MHz probe [20]. We hope that in time we will be able to add techniques to image the ascending aorta as described by Schmidt [20]. The subclavian and vertebral arteries do not need significant modification beyond depth adjustment from the technique of scanning the axillary artery. We identified 24 cases where arteries besides the superficial temporal and axillary arteries needed imaging. It is likely that this number would have been higher if the skill set was higher at the outset, resulting in a lower number of patients needing a second test.
We have assumed that when patients did not contact us, they had come off their prescribed glucocorticoids and had not gone to a different centre. This is a valid assumption given our rural location and the close working relationships with our primary care services. Patients have access to the vasculitis clinic via a helpline telephone number, and our primary care colleagues can refer patients for same-day assessments. Our ability to offer diagnostic appointments within a median of 4 days also allows us the confidence to make that assumption.
We have stratified access to the extended ultrasonography and the second test using CRP level of 20 mg/L. We are aware that giant cell arteritis can occur with normal CRP levels [21]. Between 2011 and 2020, 27/227 patients with giant cell arteritis in Norfolk, UK, had a CRP level of <20 mg/L [2]. After scanning a sizeable arterial tree that we would expect to be involved, we had to make a pragmatic decision on which patients to offer a second test. Kermani et al. had estimated the optimal cut-off of a CRP level of 26.9 mg/L to make a diagnosis of giant cell arteritis [22]. Previously, the American College of Rheumatology has used an erythrocyte sedimentation rate of 50 mm/h as a cut-off [23]. Hayreh et al. had estimated that a CRP level of 24.5 mg/L equates to an erythrocyte sedimentation rate of 47 mm/h [24]. Looking at these data, we made a pragmatic decision to use 20 mg/L as our cut-off value. If we had used a CRP level of 10 mg/L as our cut-off, we may have picked up three of the seven missed cases in Table 1, but it would have resulted in 85 more biopsies.
At a time interval of 6 months after the original suspicion of giant cell arteritis, we missed seven individuals who had gone through the diagnostic pathway. 2/7 had a negative ultrasonography and a negative temporal artery biopsy. Patient 2 in Table 1 had been diagnosed clinically while on a holiday and therefore presented late to the vasculitis service. With her agreement, we investigated and rapidly tapered the prednisolone. Her relapse resulted in a quick diagnosis. This was done under full supervision of the vasculitis clinic, and while it may have been a higher short-term risk as compared with long-term continuation of glucocorticoids, in the long term, it will allow her access to higher cost drugs in a public healthcare system where access to tocilizumab is restricted to patients with a definite diagnosis. These seven patients also illustrate that giant cell arteritis can be difficult to diagnose. The presentations may be atypical, and the low CRP may be misleading. Having a protocolized approach to diagnostics minimizes the risk and prevents the accumulation of damage.
In conclusion, our study is the first real-world evidence of the merit and value of ultrasonography in the diagnostics of giant cell arteritis. This strategy was first suggested after statistical modelling in a clinical trial comparing ultrasonography with temporal artery biopsy [25]. We demonstrate that ultrasonography performs well in a pathway which includes clinical filtering, stratification using CRP, and highly specific second tests like temporal artery biopsy and 18-fluorodeoxyglucose labelled PET-CT in a select patient population. In our centre, we have trained clinician sonographers whose performance has been validated against other measures [9, 10]. Likewise, we have dedicated surgeons for temporal artery biopsies, dedicated pathologists for reporting the biopsies, and dedicated radiologists for reporting nuclear medicine images. The presence of a skilled multidisciplinary team allows minimization of observer variation. We have demonstrated that a multimodal diagnostic pathway performed by trained clinicians results in high sensitivity for a diagnosis of giant cell arteritis.
Supplementary material
Supplementary material are available at Rheumatology online.
Data availability
Data can be made available on reasonable request to collaborators.
Funding
Norfolk and Norwich Hospitals Charity for funding the GE Logiq e machine with two probes. C.B.M. is funded by the Norfolk and Norwich University Hospital NHS Foundation Trust to do research for 1 day of the week.
Disclosure statement: The authors have declared no conflicts of interest.




Comments