-
PDF
- Split View
-
Views
-
Cite
Cite
Krishan Pratap, Ross Penglase, Edward Roper, Alina Stoita, Raynaud’s of the gut? Systemic sclerosis–associated microvascular ischaemic colitis responsive to intravenous iloprost, Rheumatology, Volume 63, Issue 8, August 2024, Pages e219–e221, https://doi.org/10.1093/rheumatology/keae052
Close - Share Icon Share
Targeting the microvasculature may have a role in impeding progression of systemic sclerosis–related gastrointestinal dysfunction.
Dear Editor, SSc is a severe autoimmune condition characterized by vasculopathy and end-organ fibrosis. Gastrointestinal (GI) manifestations are mostly characterized by dysmotility and are very common, with 90% of patients developing these manifestations and 8% of patients having severe GI involvement [1, 2]. GI involvement is attributed to 7.6% of all-cause mortality in SSc [3]. Vasculopathy, fibrosis, neural damage and dysbiosis may contribute individually or in combination with the GI manifestations of SSc, which can occur along the entire length of the GI tract. As the specific mechanism of pathogenesis is yet to be elucidated, management of SSc-related GI dysfunction is currently limited to symptom management. We describe a case of ischaemic colitis that we hypothesize was secondary to microvascular dysfunction related to the patient’s SSc that resolved with iloprost infusion.
A 47-year-old woman was reviewed in an outpatient setting for worsening constipation and reflux. This was in the background of diffuse SSc with interstitial lung disease, gastro-oesophageal reflux and microvascular disease, requiring an autologous haematopoietic stem cell transplant (AHSCT) in 2017. She had an excellent cutaneous and pulmonary response to AHSCT and remained in remission. The patient was ANA positive with a 1:160 titre with a homogeneous and speckled pattern and positive Scl-70 autoantibodies. Her comorbid conditions included osteoporosis and a previous pulmonary embolism after AHSCT. Her regular medications were mycophenolate 1 g twice a day, hydroxychloroquine 200 mg daily, prednisolone 1 mg daily, nifedipine 30 mg daily, nizatidine 300 mg daily, pantoprazole 40 mg daily, denosumab, cholecalciferol, paracetamol 1 g daily, coloxyl and senna. At review, she reported chronic constipation, with her bowels not opening for up to 7 days at a time.
Gastroscopy and colonoscopy were performed to investigate her symptoms. Gastroscopy was unremarkable; however, the colonoscopy found a very large necrotic ulcer in the proximal rectum extending into the rectosigmoid junction and the procedure was abandoned due to safety concerns (see Fig. 1A). Histology showed an ischaemic colitis pattern of injury. Occasional crypt apoptotic bodies were noted, active inflammation was not prominent, granulomas and parasites were absent and a CMV immunohistochemical stain was negative (see Fig. 1B). A subsequent mesenteric CT angiogram and mesenteric Doppler US demonstrated no macrovascular arterial occlusions. Thrombophilia and aPL antibody screening demonstrated that she has a heterozygous prothrombin gene mutation; the remainder of the tests were negative and the ischaemic colitis ulceration was attributed to microvascular ischaemic colitis in the setting of SSc.
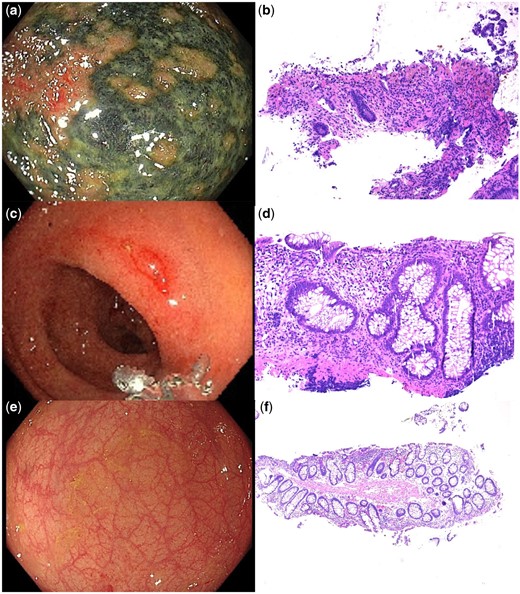
(A) Endoscopic image of necrotic rectal ulceration from the initial colonoscopy and (B) corresponding histopathology showing an ischaemic colitis pattern of injury with erosion, hyalinization of the lamina propria and superficial crypt withering. (C) Endoscopic image of the area of previous necrotic ulceration at the second colonoscopy and (D) corresponding histopathology showing erosion, crypt architectural distortion indicating chronicity and prominent crypt apoptotic bodies. (E) Endoscopic image of the area of previous necrotic ulceration with complete macroscopic resolution and (F) corresponding histopathology showing mild crypt architectural distortion only
Shortly after the colonoscopy the patient was admitted for a scheduled iloprost infusion for treatment of RP and digital ulcer prophylaxis prior to winter. She received a 5-day course of intravenous iloprost, titrated to patient tolerability. A repeat colonoscopy was performed 2 days after the infusion was completed and 26 days after the first colonoscopy, which found macroscopic resolution of the ulcer with an area of mild erythema in the centre of the previous ulcer (see Fig. 1C). Histology of the erythematous area showed apoptotic proctitis with erosion, crypt architectural distortion and prominent apoptotic bodies, which was clinically interpreted as changes in a healing ischaemic ulcer (see Fig. 1D). The rest of the colonic biopsies were normal. Given the positive response to iloprost, the maintenance dose of nifedipine was increased to 90 mg daily with a view to adding sildenafil if required.
A third colonoscopy was performed ≈4 months following the second procedure and found complete macroscopic and microscopic healing of the ischaemic ulceration previously seen (see Fig. 1E, F). The patient’s constipation had symptomatic improvement with intermittent use of laxatives.
In summary, we demonstrated the first case of microvascular colonic ischaemia suspected to represent SSc-associated microscopic vasculopathy of the gastrointestinal tract that responded to iloprost without any other identifiable secondary causes. This would support the hypothesis that SSc causes microvascular dysfunction in the GI tract similar to RP, which conceptually may result in secondary fibroproliferative changes and neural damage, manifesting as GI dysmotility and dysfunction. Hence, therapies targeting the microvasculature may have a role in treating and impeding progression of SSc-related GI dysfunction.
Data availability
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Authors’ contributions
Krishan Pratap: Lead author responsible for case review and and development of the manuscript. Ross Penglase: Supervisor and editor. Edward Roper: Editor, provided histopathology images and interpretation. Alina Stoita: Editor, supervisor and endoscopist providing interpretation of endoscopic images.
Funding
No funding was received.
Disclosure statement: The author has declared no conflicts of interest.




Comments